Abstract
CD19-specific chimeric antigen receptor (CAR) T-cell therapy has become an integral part of our treatment armamentarium for pediatric patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL). However, despite initial remission rates of greater than 80%, durable remission occurs in only 40% to 50% of patients. In this review we summarize our current knowledge of the role of consolidative hematopoietic cell transplantation in the management of pediatric patients who achieved a minimal residual disease-negative complete response post CD19 CAR T-cell therapy. In addition, we review approaches to enhance effector function CD19 CAR T cells, focusing on how to improve persistence and prevent the emergence of CD19− B-ALL blasts.
Learning Objectives
Understand the role of hematopoietic stem cell transplantation post CD19 CAR T-cell therapy
Explain common mechanisms of ALL recurrence post CD19 CAR T-cell therapy
Explain approaches to enhance the anti-ALL activity of CD19 CAR T cells
Introduction
CD19-redirected chimeric antigen receptor (CAR) T-cell therapy is an effective therapeutic modality for pediatric patients with relapsed and/or refractory (R/R) acute lymphoblastic leukemia (B-ALL), a patient cohort that historically was largely incurable.1,2 However, despite initial remission rates of greater than 80% across studies, durable remission occurs in only 40% to 50% of patients. This includes the use of the US Food and Drug Administration–approved product tisagenlecleucel, as well as other CD19-directed CAR T-cell products evaluated in clinical trials.3-8 While data suggesting risk factors for disease nonresponse or relapse have been reported, including high leukemic disease burden and a history of nonresponse to other CD19-directed therapies, it is currently unknown for which patients stand-alone CD19 CAR T-cell therapy is curative.8-12
Outcomes after CAR T-cell relapse are dismal,13 and it is therefore critical to identify patients at high risk of relapse through close monitoring for CAR T-cell persistence in conjunction with frequent disease evaluations in the first year post CAR T-cell infusion. While many factors have to be taken into consideration when making post–CAR T-cell therapy decisions, a univeral algorithm is yet to be developed. Therefore, a key focus in the field includes efforts to better identify patients at higher risk of disease recurrence post CAR T-cell therapy, as well as investigation of novel treatment approaches aimed to enhance CAR T-cell efficacy.14
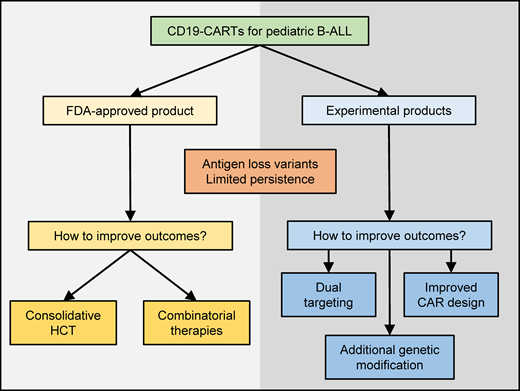
In this educational review, we present 2 cases highlighting the current challenges and then review the role of hematopoietic cell transplantation (HCT) post CD19 CAR T-cell therapy and approaches to enhance the anti-ALL activity of CD19 CAR T cells. Thus, the broad learning objectives are to i) understand the role of HCT post CD19 CAR T-cell therapy and ii) explain common mechanisms of therapeutic failure and approaches to enhance the anti-ALL activity of CD19 CAR T cells.
CLINICAL CASE 1
A 6-year-old boy with standard-risk B-ALL was treated with standard chemotherapy, achieved remission at the end of induction therapy, and then suffered a relapse during maintenance therapy. He had persistent CD19+ B-ALL after 2 cycles of intensive reinduction chemotherapy. He received CD19-redirected CAR T-cell therapy and achieved remission, with no detectable clonal cells by next-generation sequencing (NGS) testing. He subsequently proceeded to a consolidative HCT with a matched related donor and remains in remission 2 years post HCT.
CLINICAL CASE 2
An 18-year-old man with primary refractory Philadelphia chromosome–like B-ALL received CD19-redirected CAR T-cell therapy and achieved remission. At 8 months post-CAR infusion, a loss of B-cell aplasia (BCA) was noted. A bone marrow biopsy performed at that time revealed detectable disease by NGS testing, with a rising copy number on a short interval repeat marrow. In the setting of loss of BCA and rising NGS, the patient was considered at risk for impending relapse, received treatment with blinatumomab, proceeded to HCT, and remains in remission without excessive HCT-related toxicity.
HCT post CD19 CAR T-cell therapy: experience to date
Data on the use of consolidative HCT post CD19 CAR T-cell therapy in pediatric patents are limited and come largely from single-center experience using varying CAR T-cell products (Table 1).3,4,6-8,10,15-17 While at present it is difficult to draw overarching conclusions about the role of consolidative HCT in this patient population, the current experience with HCT post CD19 CAR T-cell therapy can be used to better understand potential predictors of relapse and identify patients who might benefit from HCT.
Selected studies reporting outcomes of CD19 CAR T-cell therapy +/− consolidative HCT
| Center/consortium/study . | Trial phase . | Costim . | Patients (no.) . | Initial CR (%) . | HCT in CR#a . | Relapse post HCT vs no HCT . |
|---|---|---|---|---|---|---|
| CHOP3 | 1 | 41BB | 30 | 90 | 3 | Relapse: NR vs 8/23 no HCT |
| ELIANA4,16 | 2 | 79 | 81 | 11 | Relapse: 0/8b vs NR | |
| Seattle5 | 1/2 | 64 | 23 | Relapse: 5/23 vs 19/27 no HCT | ||
| St Jude8 | 1 | 12 | 75 | 5 | Relapse: 0/5 vs 4/4 no HCT | |
| PRWCC10 | N/a | 185 | 85 | 20 | NR | |
| CIBMTR17 | N/a | 255 | 86 | 34 | NR | |
| NCI6 | 1 | CD28 | 50 | 62 | 21 | Relapse: 2/21 vs 7/7 no HCT |
| MSKCC15 | post HCT | 15c | N/a | 15 | Relapse: 3/15 | |
| SMC7 | 2 | 30 | 86 | 25 | Relapse: 8/25 vs 4/5 no HCT |
| Center/consortium/study . | Trial phase . | Costim . | Patients (no.) . | Initial CR (%) . | HCT in CR#a . | Relapse post HCT vs no HCT . |
|---|---|---|---|---|---|---|
| CHOP3 | 1 | 41BB | 30 | 90 | 3 | Relapse: NR vs 8/23 no HCT |
| ELIANA4,16 | 2 | 79 | 81 | 11 | Relapse: 0/8b vs NR | |
| Seattle5 | 1/2 | 64 | 23 | Relapse: 5/23 vs 19/27 no HCT | ||
| St Jude8 | 1 | 12 | 75 | 5 | Relapse: 0/5 vs 4/4 no HCT | |
| PRWCC10 | N/a | 185 | 85 | 20 | NR | |
| CIBMTR17 | N/a | 255 | 86 | 34 | NR | |
| NCI6 | 1 | CD28 | 50 | 62 | 21 | Relapse: 2/21 vs 7/7 no HCT |
| MSKCC15 | post HCT | 15c | N/a | 15 | Relapse: 3/15 | |
| SMC7 | 2 | 30 | 86 | 25 | Relapse: 8/25 vs 4/5 no HCT |
HCT while in CR.
Data available for 8 out of 11 patients.
One patient received a CAR/41BB T-cell product.
CHOP, Children's Hospital of Philadelphia; CIBMTR, Center for International Blood and Marrow Transplant Research; costim, costimulatory domain; hu, human; St Jude, St Jude Children's Research Hospital; MSKCC, Memorial Sloan Kettering Cancer Center; mus, murine; N/A, not applicable; NCI, National Cancer Institute; NR, not reported; Pat, patients; PRWCC, Pediatric Real World CAR Consortium; Seattle, Seattle Children's Hospital; SMC, Sheba Medical Center.
Tisagenlecleucel (Kymriah) is the only commercially available CAR T-cell product for pediatric patients with R/R CD19+ ALL. It consists of ex vivo activated and expanded autologous T cells genetically modified with a lentiviral vector encoding a CD19 CAR with a 41BB.zeta signaling domain (CD19/41BB). With a follow-up of 38.8 months, the seminal trial and the phase 2 global registration trial (ELIANA) reported high rates of initial complete response (CR) with relapse-free survival of 76% and 59%, respectively.3,4,16 Analyses of real-world use of tisagenlecleucel by the Center for International Blood and Marrow Transplant Research and the Pediatric Real-World CAR Consortium demonstrated similar response rates.13,17 Notably, very few patients in these studies proceeded to a consolidative HCT, and there are limited data on those who did.
Other CD19/41BB-CAR T-cell products were evaluated in pediatric patients with R/R B-ALL in early-phase clinical trials. The PLAT-02 study, a phase 1/2 trial, utilized an institutional product infused with a defined CD4/CD8 ratio.5 Among 64 treated patients, 50 were considered eligible for potential HCT post CAR T-cell therapy. Of these, 23 proceeded to HCT (second HCT, n = 10) at a median of 3 months post infusion. Rates of relapse were lower in the consolidative HCT group (5/23 patients) compared to the non-HCT group (19/27 patients). One patient died post HCT secondary to treatment complications. HCT-naive patients had a significantly improved leukemia-free survival compared to non-HCT-naive patients. Regardless of prior HCT status, patients with early loss of BCA (≤63 days post infusion) who underwent consolidative HCT had improved leukemia-free survival compared to those who did not proceed to HCT.18 Another study with an institutional CD19/41BB-CAR T-cell product demonstrated CRs in 9 of 12 patients treated. Post CR, 5 patients (all HCT naive, including clinical case 1) went on to consolidative HCT at a median of 2.7 months post-CAR infusion. All these patients remain in remission, with 1 patient dying secondary to transplant-related complications. Conversely, the 4 who did not proceed with HCT all subsequently relapsed. Three of these patients were not considered good candidates for HCT due to prior HCT status or a history of extramedullary disease, and 1 relapsed prior to planned HCT.8
The benefit of consolidative transplant in pediatric patients treated with T-cell products that express CD19 CARs with a CD28.zeta signaling domain (CD19/CD28) has been demonstrated by several groups. In one study, 15 of 18 responding patients proceeded to consolidative HCT at a median of 57 days post infusion. Post HCT, 2 patients suffered disease relapse, and 3 died secondary to HCT complications. Investigators found that patients who received a CD34-selected, T-cell depleted graft or proceeded to HCT fewer than 80 days from CAR infusion had better post-HCT outcomes.15 In a second phase 1 trial in which 28 patients achieved a CR after CAR T-cell therapy, 21 proceeded to a consolidative HCT, at a median of 54 days post CAR (second HCT, n = 4). After transplant, 2 patients experienced subsequent disease relapse, compared to 7 of 7 patients in the nonconsolidative HCT cohort.6 Additionally, outcomes of a third early-phase clinical study with a CD19/CD28 CAR T-cell product highlighted the benefit of consolidative HCT post CD19 CAR T-cell therapy. Among 30 patients who achieved a CR post CAR T-cell therapy, 25 (17 HCT naive) proceeded to consolidative HCT (median, 71 days). Of these, 8 patients relapsed post HCT, and 2 died secondary to treatment toxicity, with all but 1 patient relapsing in the non-HCT group.19 In the setting of loss of BCA and rising NGS post CD19 CAR T-cell therapy, a bridging therapy prior to HCT that is readily available (eg, blinatumomab for CD19+ leukemia) might be critical prior to HCT to ensure a disease-free long-term outcome, as illustrated by clinical case 2.
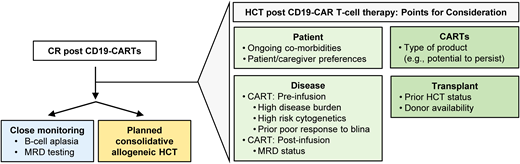
These data collectively indicate that regardless of the utilized CD19 CAR T-cell product, patients who are HCT naive benefit from a consolidative HCT in the setting of limited CAR T-cell persistence. The benefit of a consolidative second HCT is less well established and, given the risk of significant toxicities, should be considered carefully and individualized based on the patient's risk of relapse and ability to tolerate this therapy. In conclusion, based on current literature, it is difficult to give clear recommendations regarding which patients should be considered for HCT post CD19 CAR T-cell therapy. Points for consideration are discussed in the next section and summarized in Figure 1.
Management approach for patients who achieve remission after CD19 CAR T-cell therapy. The scheme highlights key factors to consider. blina, blinatumomab; CARTS, CAR T cells; MRD, minimal residual disease.
Management approach for patients who achieve remission after CD19 CAR T-cell therapy. The scheme highlights key factors to consider. blina, blinatumomab; CARTS, CAR T cells; MRD, minimal residual disease.
HCT post CD19 CAR T-cell therapy: points for consideration
Prospective studies, albeit difficult to conduct, are ultimately needed to inform the critical question on the role of consolidative HCT after CD19 CAR T-cell therapy, identify high-risk pediatric patients, and support the development of evidence-based clinical-decision algorithms. In lieu of such studies, current approaches to this question weigh the risks and benefits of pursuing HCT, considering i) risk factors prior to CD19 CAR T-cell therapy and ii) monitoring for persistence and response post infusion (Figure 1).
Risk factors pre CD19 CAR T-cell therapy
Across several studies, the presence of a high disease burden prior to CD19 CAR T-cell therapy has been associated with a higher risk of relapse after treatment. While a universal cutoff of high burden has not yet been determined, some data suggest a cutoff of as little as greater than or equal to 5% blasts.6,8,9,11,19 Prior poor response to blinatumomab has also been associated with a higher relapse risk post CAR.8 Other traditional risk factors for treatment failure have not been recapitulated after treatment with CD19 CAR T-cell therapy, including subgroups with high-risk cytogenetics,20 Down syndrome,21 infants,22,23 and extramedullary disease.24,25 Thus, CD19 CAR T-cell therapy has the potential to redefine treatments for patients who were historically high risk.
Monitoring post CD19 CAR T-cell therapy
Longer CD19 CAR T-cell persistence is associated with improved relapse-free survival. Post CAR, close monitoring of ongoing BCA and detection of recurrent and/or persistent disease by NGS, polymerase chain reaction, and/or flow cytometry serves as a surrogate of CAR persistence.5,8,18,21,26 Notably, relapse risk decreases with time postCAR T-cell therapy, and most relapses occur within the initial year after infusion.6,10,17,21 Even after treatment with CD19/41-BB CAR T-cell products, the loss of BCA within 6 months or less and/or detectable disease post CAR T-cell therapy, including by NGS testing, place patients at high risk of relapse, and these patients may be considered for HCT prior to disease progression.26 It is important to note that BCA is not a perfect surrogate for relapse risk, as patients may relapse with antigen loss variants or antigen-positive disease concurrent to findings of loss of BCA. Additional considerations include patient/caregiver preferences, provider experience, and often provider assessment of the availability of additional viable treatment options if the patient were to relapse post CAR T-cell therapy. Importantly, as CAR T-cell therapies continue to evolve and the number of patients treated with such therapies increases, continued investigation and reevaluation of such predictors will be necessary. Finally, besides patient selection the preferred HCT approach remains elusive. Ideally, the attainment of deep remission with CD19 CAR T-cell therapy would potentially allow for conditioning regimens that do not use total-body irradiation.27
Enhanced CAR T cells
Prevention of CD19− relapse post CAR T-cell therapy
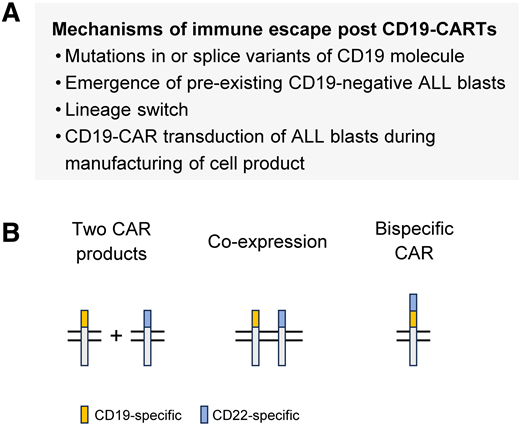
Multiple mechanisms of CD19− relapse have been described, including mutations in CD19 that lead to shedding of the extracellular domain, lineage switch with the recurring leukemia having an acute myeloid leukemia phenotype, and the emergence of a preexisting CD19− clone (Figure 2A).28-31 Likewise, the transduction of contaminating ALL blasts in the T-cell products with the viral vector encoding the CD19 CAR can lead to masking of CD19 on the cell surface, resulting in ALL blasts that are resistant to CD19 CAR T cells.32 The incidence of CD19− ALL relapse varies post CD19 CAR T-cell therapy and has been reported to be between 18% and 25%.3-8
Mechanism and prevention of antigen loss variants post CD19 CAR T-cell therapy. (A) Mechanism of CD19-targeted immune escape. (B) CAR T-cell products to enable dual targeting of CD19 and CD22.
Mechanism and prevention of antigen loss variants post CD19 CAR T-cell therapy. (A) Mechanism of CD19-targeted immune escape. (B) CAR T-cell products to enable dual targeting of CD19 and CD22.
Targeting additional antigens is actively being pursued to prevent the emergence of CD19− ALL blasts. Most efforts are focused on targeting CD22 based on the encouraging results of CD22-CAR T cells as monotherapy for pediatric ALL.33,34 Conceptually, dual targeting of CD19 and CD22 can be achieved with 3 approaches (Figure 2B): i) sequential or coadministration of 2 CAR T-cell products, 1 expressing a CD19 and the other a CD22 CAR, ii) engineering T cells to simultaneously express a CD19 CAR and a CD22 CAR, and iii) engineering T cells to express a bispecific CAR that recognizes CD19 and CD22. All 3 approaches have been evaluated in early-phase clinical studies, with the largest cohort of patients receiving 2 CAR T-cell products.35-37 The results of these studies indicate that all 3 approaches are safe; however, it remains to be determined which approach is best to prevent the emergence of antigen loss variants. In addition to CD22, other targets are actively being pursued to develop bispecific or trispecific CAR T-cell products for pediatric ALL.38-40
Enhancing persistence of functional CD19 CAR T cells
The functional persistence of CD19 CAR T cells is routinely tracked by enumerating normal CD19+ B cells in the peripheral blood post CD19 CAR T-cell infusion, with the loss of BCA either indicating the limited persistence of CD19 CAR T cells or the persistence of dysfunctional CD19 CAR T cells. Several studies have shed light on the desired characteristics of the leukapheresis product used for CD19 CAR T-cell manufacturing and the product itself associated with the functional persistence of CD19 CAR T cells.41-44 These include infusing CD19 CAR T cells that are derived from naive T cells and are less differentiated at the end of CD19 CAR T-cell production. However, none of the identified characteristics have been validated prospectively. Likewise, 2 recent studies have highlighted that long-term persisting CD19 CAR T cells have a unique transcriptional profile,45,46 opening up the opportunity to develop CD19 CAR T-cell products that promote the development of these gene signatures.
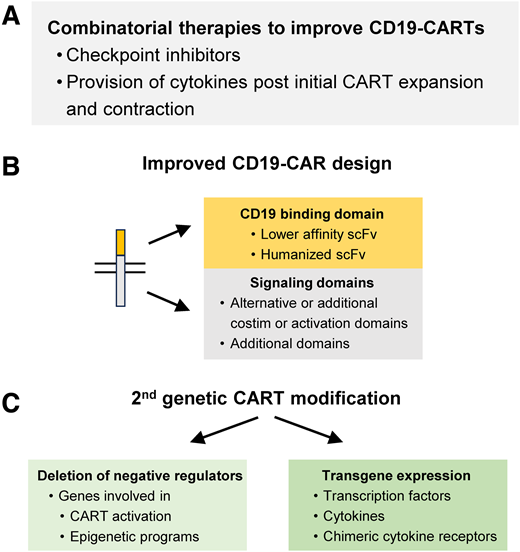
Combinatorial therapies
CD19 CAR T cells might routinely undergo exhaustion reprogramming as highlighted for 1 investigational CD19 CAR T-cell product, and combinatorial therapies represent 1 approach to counteract this (Figure 3A). Combining checkpoint inhibitors with CD19 CAR T cells is actively being pursued for pediatric R/R ALL, and early clinical data suggest that this approach is safe and may augment the effector function and persistence of CD19 CAR T cells.47 In addition to checkpoint inhibitors, the provision of cytokines after initial CD19 CAR T-cell expansion and contraction has demonstrated benefit in preclinical studies, and clinical studies in adults with B-cell lymphoma post CD19 CAR T-cell therapy are ongoing.
Strategies to enhance the effector function of CD19 CAR T cells. Examples of (A) combinatorial therapies, (B) strategies to improve CAR design, and (C) second genetic modifications of CAR T cells. costim, costimulation.
Strategies to enhance the effector function of CD19 CAR T cells. Examples of (A) combinatorial therapies, (B) strategies to improve CAR design, and (C) second genetic modifications of CAR T cells. costim, costimulation.
CAR design
Tisagenlecleucel as well as most investigator-initiated CD19 CAR T cells have employed a single-chain Fv that is derived from the monoclonal antibody FMC63. It has a high affinity, and studies have indicated that CAR T cells expressing a CAR that utilizes a CD19-specific scFv with a lower affinity have improved effector function.48,49 In addition, utilizing a humanized CD19-specific scFv has the potential to reduce CAR-specific immune responses, improving persistence.50 Currently, the choice of costimulatory domain is the most well-established factor that determines CAR T-cell persistence, with CD19/41BB CARs having longer persistence than CD19/CD28 CARs (see the section “HCT post CD19 CAR T-cell therapy: experience to date”). Finally, the incorporation of novel signaling domains holds the promise to generate CARs that endow CAR T cells with improved effector function (Figure 3B).51-53
Additional genetic modification to enhance the effector function of CD19 CAR T cells
Conceptually, there are 2 main approaches to enhance the effector function, including the persistence of CD19 CAR T cells (Figure 3C). One relies on deleting negative regulators and the other on transgenic expression of transcription factors, cytokines, and chimeric cytokine receptors.54-57 Examples of deleting negative regulators include molecules that enhance CAR T-cell activation, including RASA2 and Regnase-1,58,59 and enzymes that are critical for the epigenetic reprogramming of CAR T cells, including TET2 and DNMT3A.60-62 These approaches are reviewed in detail in recently published articles.55,56,63
Conclusions
Since the infusion of the first pediatric patient with CD19 CAR T cells in 2012, CD19 CAR T-cell therapy has become an integral part of our treatment armamentarium for pediatric patients with R/R ALL. Currently, there are 2 major, complementary efforts ongoing. One focuses on increasing our understanding of how to best use tisagenlecleucel, the only US Food and Drug Administration–approved CD19 CAR T-cell product for R/R pediatric ALL, and the other focuses on developing second-generation ALL-specific CAR T-cell products with enhanced effector function. Based on our current knowledge, subsets of patients who have received tisagenlecleucel will most likely benefit from a consolidative HCT, and further studies are needed to identify these patients. Likewise, the efficacy of tisagenlecleucel might be improved with combinatorial therapies. While we know the desired characteristics of enhanced ALL-specific CAR T-cell products—namely, resistance to antigen loss variants paired with durable persistence—further preclinical and clinical studies are needed to delineate the genetic engineering approach to accomplish this.
Acknowledgment
Aimee C. Talleur was supported by a scholar grant from the American Society of Hematology.
Conflict-of-interest disclosure
Aimee C. Talleur: no competing financial interests to declare.
Swati Naik: no competing financial interests to declare.
Stephen Gottschalk: scientific advisory board: Be Biopharma, CARGO, Immatics; honoraria: TESSA Therapeutics.
Off-label drug use
Aimee C. Talleur: none are discussed.
Swati Naik: none are discussed.
Stephen Gottschalk: none are discussed.