Abstract
Hematopoietic stem cell transplantation (HSCT) represents a consolidated therapeutic strategy for high-risk pediatric acute lymphoblastic leukemia (ALL), offering the potential for curative treatment. This manuscript delves into the debate around the more universal application of HSCT for pediatric ALL in the modern era, considering the ubiquitous availability of suitable donors. In fact, despite significant advancements in chemotherapy, targeted therapy, and immunotherapy, a subset of pediatric patients with ALL with high-risk features or relapse continue to encounter poor prognostic outcomes. For this subgroup of patients, HSCT often remains the only potentially curative measure, leveraging the graft-versus- leukemia effect for long-term disease control. Nevertheless, the procedure's complexity and associated risks have traditionally curtailed its widespread use. However, the scenario is shifting with improvements in HLA matching, availability of alternative donor sources, less toxic conditioning regimens, and improved supportive care protocols. Concurrently, emerging therapies like CD19+ CAR T cells present new considerations for definitive therapy selection in relapsed/ refractory ALL. This article reviews critical current evidence and debates the potential of HSCT as a more universal treatment for ALL, reevaluating traditional treatment stratification in light of the constant availability of stem cell donors.
Learning Objectives
To understand the current landscape of donor availability for HSCT in pediatric ALL and the reasons why HSCT is not utilized universally
To weigh the benefits and drawbacks of HSCT in pediatric ALL, including survival outcomes, risk of GvHD, and treatment-related toxicities
To explore the potential of integrating HSCT with other treatment modalities such as CAR T therapy
To understand how advances in HSCT have improved accessibility to treatment for pediatric ALL, reducing disparities in care
CLINICAL CASE 1
A 9-year-old male presented with relapsed B-lineage acute lymphoblastic leukemia (ALL), which was first diagnosed when he was 2 years old. He received ALL treatment per BFM-90 in his home country. This patient had his first relapse at the age of 7 and was treated with chemotherapy per BFM-95. He presented to our institution with a second relapse and had both bone marrow (BM) and central nervous system involvement. Cytogenetics testing showed complex chromosomal abnormalities, including additions of unknown material to chromosomes 3, 7, and 10, as well as monosomy 17. He received CD19 CAR T cells (Kymirah) and achieved complete remission by both flow cytometry and next-generation sequencing (NGS). Subsequently, the patient underwent allogeneic hematopoietic stem cell transplantation (HSCT), while still having B-cell aplasia (BCA) and in complete remission (CR) as determined by NGS minimal residual disease (MRD), with rabbit antithymocyte globulin, 1320 cGy of fractionated total body irradiation (TBI), thiotepa 10 mg/kg, fludarabine 160 mg/m2, followed by TCRαβ/CD19+ B-cell–depleted peripheral blood stem cell graft. The post-HSCT course was complicated by transplant-associated thrombotic-microangiopathy, which subsequently led to renal failure. Six months post-HSCT, he experienced a third leukemia relapse (BM only). As a result, he received institutional CD19/22 CAR T (NCT03233854) but had no response. A Heme Stanford Actionable Mutation panel performed on leukemic BM cells shortly prior to his death showed pathogenic TP53 and NRAS mutations with high variant allele frequency not identified at the diagnosis sample.
CLINICAL CASE 2
A 20-year-old patient presented with very-high-risk B-lineage ALL first diagnosed at 14 years old. Cytogenetic testing revealed hypodiploidy. He received treatment per COG AALL1131 and achieved CR with negative MRD by flow cytometry at end of the induction cycle. He relapsed 16 months after the end of his chemotherapy treatment (BM only) and was treated with institutional CD19/CD22 CAR T cells (NCT03233854) 1 month after relapse. Foundation One testing on the relapsed BM sample prior to CAR T infusion found CDKN2A/B exon 2-3 loss and RUNX1T1 A471V alteration. He achieved CR2 with CAR T cells but had loss of BCA 3 months later and relapsed 6 months post CAR T. He was then treated with an individualized protocol consisting of inotuzumab, blinatumomab, dexamethasone, and vincristine. Once in remission, he underwent HSCT using a haploidentical sibling BM graft conditioned with TBI 1200 Cy, cyclophosphamide 100 mg/kg, thiotepa 10 mg/kg, and 100 mg/kg of posttransplant cyclophosphamide. Tacrolimus and mycophenolate were administered as GvHD prophylaxis. His transplantation course was complicated by veno-occlusive liver disease with hepatorenal syndrome requiring dialysis, as well as idiopathic pneumonitis syndrome. At the last follow-up (120 days post-HSCT) the patient is alive, disease free, and off steroids and etanercept used to control the pulmonary alloimmune-mediated complication.
Introduction
Pediatric ALL, a rapidly progressive hematologic malignancy, is the most prevalent form of childhood leukemia. Current therapeutic modalities such as chemotherapy, targeted therapy, and immunotherapy have substantially improved overall survival (OS) rates.1 Despite these advancements, a subset of patients with high-risk features or those who experience a relapse after initial treatment continue to face poor outcomes, with 5-year survival rates approximately at 50%.2 For this group of patients, allogeneic HSCT often represents the only potentially curative option.2 HSCT is a long-established standard of care for these patients with long-term favorable OS outcomes.3 However, its role in the treatment paradigm of ALL remains a topic of ongoing discussion. HSCT leverages the immunologic graft-versus-leukemia effect to eradicate residual leukemic cells, offering the promise of long-term disease control. Even so, HSCT is a complex procedure with significant associated risks, including graft- versus-host disease (GvHD), infections, and transplant-related mortality (TRM), which have traditionally limited its universal application.
In recent years, improvements in donor matching, conditioning regimens, and supportive care, coupled with the advent of alternative donor sources such as haploidentical donors and umbilical cord blood, have expanded the availability of HSCT. Additionally, the recent emergence of CD19+ CAR T cell therapy as viable treatment option for relapsed and refractory (R/R) ALL offers unique considerations for the selection of definitive therapy in these patients. These advances raise a provocative question: if a suitable stem cell donor can always be found for patients with ALL, should HSCT be used more universally in the treatment of this high-risk disease? Here we will explore this question, reviewing the current evidence and providing perspectives on the optimal use of HSCT in the management of R/R ALL (Table 1).
This table describes key modern studies investigating the optimal use of HSCT in the management of R/R ALL.
| Reference . | Design . | N; Median age; Range . | Indication . | Staging . | Graft source . | Conditioning . | GVHD prophylaxis . | aGVHD . | cGVHD . | Relapse . | TRM . | Survival . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bethge et al (2022)19 | Prospective | 60; 18.5; 1-63 | ALL AML MDS MPS MM NM ST | CR1 20% >CR1 22% NR 13% | MMRD | ATG/Flu/ TT/Mel | TCRαβ/ CD19 depletion | Grade II-IV 10% Grade III-IV 0% | Overall 31% Severe 8% | 20% | 17% | 2 year OS63% DFS 50% GRFS 39% |
| Pulsipher et al (2022)17 | Prospective | 51; 35; 6-61 | ALL AML MDS | CR1 53% CR2 37.3% | MMRD PBSC | MAC-TBI 31%, MAC-Bu 22% RIC-Mel 43% RIC-TLI 4% | TCRαβ/ CD19 depletion | 2 year OS 75% DFS 75% EFS 69% | ||||
| Peters et al (2021)33 | Randomized, controlled, international, multicentered, phase 3 | 417; 4-21 | ALL | CR1 54% CR2 40% CR3 4% | MSD or MD BM, PBSC or Cord | MAC-TBI 50% MAC-Bu 24% Treo 23% Other 3% | MSD-CSA only MD-ATG/ MTX/ CSA | Grade II-IV TBI 37% Chemo29% | Overall-TBI 16% Overall-Chemo 11% | TBI-12% Chemo-33% | TBI-2% Chemo-9% | 2-year OS-TBI 91% OS-chemo 75% GRFS-TBI 72% GRFS-chemo 51% |
| Ruggeri et al (2021)16 | Retrospective | 180; 9.25 | ALL | CR1 24% CR2 45% CR3 12% NR 19% | MMRD BM or PBSC | MAC-TBI 25.6% MAC-Chemo 51.6% RIC 22.78% | PtCy | Grade II-IV 28.3% Grade III-IV 12.4% | Overall 21.9% Extensive 9.5% | CR1 25% CR2 37% CR3 50% NR 70% | 19.6% | 2 year OS 50.8% LFS 38.5% GRFS 29% |
| Symons et al (2020)44 | Prospective, single center, phase 2 | 96; 42; 1-65 | ALL AML MDS Lymphoma | CR1 41% >CR1 18% | MMRDBM | MAC-TBI MAC-Bu | PtCy | Grade II-IV 11% Grade III-IV 4% | Overall 15% Moderate to severe 6% | 43% | 11% | 3 year OS 54% EFS 48% |
| Bertaina et al (2018)43 | Retrospective | 98: 6.6; 0.1-17.3 | AL | CR1 43% CR2 47% Other CR 10% | MMRD PBSC | MAC-TBI 74% | TCRαβ/ CD19 depletion | Grade II-IV 16% Grade III-IV 0% | Overall 6%, Extensive 1% | 29% | 9% | 5 year OS 68% LFS 62% |
| Locatelli et al (2017)18 | Prospective | 80; 9.7; 0.9-20.9 | ALL AML | CR1 39% CR2 56% | MMRD PBSC | TBI/TT/Flu TBI/TT/Mel Bu/TT/Flu Bu/Cy/Mel with ATG/Ritux | TCRαβ/ CD19 depletion | Grade I-II 30% | Limited 5% | 24% | 5% | 5 year OS 72% DFS 71% |
| Reference . | Design . | N; Median age; Range . | Indication . | Staging . | Graft source . | Conditioning . | GVHD prophylaxis . | aGVHD . | cGVHD . | Relapse . | TRM . | Survival . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bethge et al (2022)19 | Prospective | 60; 18.5; 1-63 | ALL AML MDS MPS MM NM ST | CR1 20% >CR1 22% NR 13% | MMRD | ATG/Flu/ TT/Mel | TCRαβ/ CD19 depletion | Grade II-IV 10% Grade III-IV 0% | Overall 31% Severe 8% | 20% | 17% | 2 year OS63% DFS 50% GRFS 39% |
| Pulsipher et al (2022)17 | Prospective | 51; 35; 6-61 | ALL AML MDS | CR1 53% CR2 37.3% | MMRD PBSC | MAC-TBI 31%, MAC-Bu 22% RIC-Mel 43% RIC-TLI 4% | TCRαβ/ CD19 depletion | 2 year OS 75% DFS 75% EFS 69% | ||||
| Peters et al (2021)33 | Randomized, controlled, international, multicentered, phase 3 | 417; 4-21 | ALL | CR1 54% CR2 40% CR3 4% | MSD or MD BM, PBSC or Cord | MAC-TBI 50% MAC-Bu 24% Treo 23% Other 3% | MSD-CSA only MD-ATG/ MTX/ CSA | Grade II-IV TBI 37% Chemo29% | Overall-TBI 16% Overall-Chemo 11% | TBI-12% Chemo-33% | TBI-2% Chemo-9% | 2-year OS-TBI 91% OS-chemo 75% GRFS-TBI 72% GRFS-chemo 51% |
| Ruggeri et al (2021)16 | Retrospective | 180; 9.25 | ALL | CR1 24% CR2 45% CR3 12% NR 19% | MMRD BM or PBSC | MAC-TBI 25.6% MAC-Chemo 51.6% RIC 22.78% | PtCy | Grade II-IV 28.3% Grade III-IV 12.4% | Overall 21.9% Extensive 9.5% | CR1 25% CR2 37% CR3 50% NR 70% | 19.6% | 2 year OS 50.8% LFS 38.5% GRFS 29% |
| Symons et al (2020)44 | Prospective, single center, phase 2 | 96; 42; 1-65 | ALL AML MDS Lymphoma | CR1 41% >CR1 18% | MMRDBM | MAC-TBI MAC-Bu | PtCy | Grade II-IV 11% Grade III-IV 4% | Overall 15% Moderate to severe 6% | 43% | 11% | 3 year OS 54% EFS 48% |
| Bertaina et al (2018)43 | Retrospective | 98: 6.6; 0.1-17.3 | AL | CR1 43% CR2 47% Other CR 10% | MMRD PBSC | MAC-TBI 74% | TCRαβ/ CD19 depletion | Grade II-IV 16% Grade III-IV 0% | Overall 6%, Extensive 1% | 29% | 9% | 5 year OS 68% LFS 62% |
| Locatelli et al (2017)18 | Prospective | 80; 9.7; 0.9-20.9 | ALL AML | CR1 39% CR2 56% | MMRD PBSC | TBI/TT/Flu TBI/TT/Mel Bu/TT/Flu Bu/Cy/Mel with ATG/Ritux | TCRαβ/ CD19 depletion | Grade I-II 30% | Limited 5% | 24% | 5% | 5 year OS 72% DFS 71% |
AL, acute leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; Bu, busulfan; DFS, disease-free survival; Flu, fludarabine; GRFS, GVHD relapse-free survival; LFS, leukemia-free survival; MAC, myeloablative conditioning; MD, matched related or unrelated donor; MDS, myelodysplastic syndrome; Mel, melphalan; MM, multiple myeloma; MMRD, mismatched related donor; MPS, myeloproliferative syndrome; MSD, matched sibling donor; NM, nonmalignant disease; NR, not in remission; RIC, reduced intensity conditioning; Treo, treosulfan; TT, thiotepa; ST, solid tumor.
Allogeneic HSCT for pediatric ALL in 2023: an overview
HSCT has an established track record in pediatric ALL, spanning multiple decades and hundreds of pediatric patients. Studies by Pulsipher et al and others show that 5-year event-free survival (EFS) and OS rates post allogeneic HSCT in high-risk pediatric patients with ALL range between 55% and 75%, influenced by factors like conditioning regimen, donor source, and MRD.4,5 The 2022 Center of International Blood and Marrow Research registry data show a 3-year OS of ~78% in pediatric patients with ALL transplanted with HLA-matched donors (related or unrelated) in CR1, and 68% in CR2. Similarly, excellent results are observed in HSCT using unrelated mismatched donors (68% in CR1, 61% in CR2).4,6 It is important to note that these survival rates can vary significantly based on several factors, such as the patient's risk stratification, disease status at transplantation, conditioning regimen, donor type, and GvHD prophylaxis.
For instance, while HSCT has been demonstrated capable of achieving durable remission, it is mostly ineffective in patients with detectable disease at the time of HSCT.7 This observation underscores the importance of effective induction and consolidation therapy for achieving deep MRD negativity prior to proceeding with HSCT in the pediatric population with ALL. A study by Bader et al revealed that MRD positivity (≥10−4) pre-HSCT was associated with an increased relapse rate and inferior EFS and OS rates when compared to patients who achieved MRD negativity (<10−4) before HSCT.8 Pulsipher et al confirmed that MRD negativity pre-HSCT correlates with improved 5-year EFS in this setting.9 Thus, therapeutic strategies aiming to maximize the likelihood of achieving MRD negativity pre-HSCT may significantly improve long-term survival in this patient population.
Quality of life post-HSCT is an equally critical aspect. While HSCT can introduce potential complications such as chronic GvHD and long-term organ toxicities, advancements in supportive care and GvHD prophylaxis have led to significant improvements. Although HSCT recipients might experience some long-term effects, most pediatric patients exhibit acceptable to good health-related quality-of-life scores in the years following HSCT compared to patients with other cancers.10 Collectively, all available data suggest that, while HSCT has shown great benefits in terms of survival outcomes and an improved quality of life, careful patient selection, optimal conditioning regimen, meticulous GvHD prophylaxis, and comprehensive supportive care remain crucial for its success in treating pediatric ALL.
Historically, the use of HSCT has been limited by the availability of matched donors. More recently, the adoption of high-resolution HLA-typing methods has enabled more accurate matching of donors and recipients, leading to better survival and a reduction in the risk of GvHD. Of note, the likelihood of finding an optimal donor varies among racial and ethnic groups, with the probability of identifying an appropriate donor being highest among whites of European descent (75%) and lowest among blacks of South or Central American descent (16%).11 Thus, it is paramount to develop strategies that allow the use of mismatched donors, which would significantly widen the accessibility of HSCT for every patient in need. One such strategy is the introduction of alternative donor sources, such as haploidentical donors and umbilical cord blood. With haploidentical HSCT, a partially HLA-matched family member can serve as a donor, thus virtually providing a suitable donor for nearly every patient in need. A recent meta-analysis revealed no significant difference in OS between haploidentical, matched, or cord blood transplants. Interestingly, relapse was higher in matched sibling donor transplants, but haploidentical transplants had a higher GvHD incidence, suggesting their safety and a better leukemic control if GvHD is managed. Of note, the meta-analysis predominantly included T-replete haploidentical transplants with specific GvHD prophylaxis (serotherapy, calcineurin inhibitor, and methotrexate or mycophenolate).12
The successful implementation of haploidentical (haplo-) HSCT has been largely the result of the development of 2 approaches: the introduction of post-transplant cyclophosphamide (PT-Cy) and the use of ex vivo T-cell depletion strategies.13,14 The use of PT-Cy after unmanipulated HSCT has shown ability to control severe GvHD in adults.15 However, long-term outcome data in pediatric patients are still lacking. On behalf of the EBMT, Ruggeri et al analyzed the outcomes of haplo-HSCT with PT-Cy in 180 children with ALL and found that disease status, age at transplantation older than 13, and the use of peripheral blood stem cells (PBSC) were factors associated with decreased OS.16
On the flip side, in the Western world, a burst of ex vivo T-cell depletion strategies have been implemented, with the goal of reducing the risk of GvHD mediated by the mismatched alloreactive T cells, while retaining the graft-versus-leukemia effect of effector T cells. A strategy that attracted considerable interest and that is now widely used in both Europe and the US involves the selective elimination of αβ T cells and CD19+ B cells from the graft (αβhaplo-HSCT). This advanced graft manipulation approach enables the transfer of large numbers of donor hematopoietic stem cells and committed hematopoietic progenitors, as well as mature natural killer and γδ T cells to the recipient.17,18 European studies found risks following αβhaplo-HSCT in pediatric patients with leukemia comparable to HLA-identical sibling or unrelated donor-HSCT, but with lower GvHD rates and improved GvHD-free/relapse-free survival.19 A 2022 study by Pulsipher et al confirmed low GvHD and TRM rates and suggested the superiority of reduced toxicity preparative regimen in αβhaplo-HSCT settings, challenging the benefits of TBI-based regimens.17
Another source of stem cells is umbilical cord blood. Compared to BM or PBSC transplants, UCB transplantation offers advantages such as faster availability, less stringent HLA matching requirements, simpler procurement, and low chronic GvHD rates.20,21 There is evidence indicating that UCB transplantation may be considered the preferred option in situations at high risk of leukemia relapse, as recently observed in patients with pretransplant persistent MRD.22
Is HSCT a universal solution for pediatric R/R ALL? Weighing the pros and cons
As we strive to refine the best therapeutic approach in pediatric R/R ALL, the universal application of HSCT is a subject of continuous debate, especially in light of more recent advancements in cell therapy (ie, CAR T). To fully understand HSCT potential as a standard intervention, it is crucial to weigh the advantages and drawbacks that stem from its broader use in this patient population.
Pros of universal HSCT for pediatric R/R ALL
Higher long-term survival rates: several studies indicate HSCT can offer improved survival rates, especially for high-risk or relapsed patients. Leukemia-free survival and OS have been well established in hundreds of patients over decades of collective experience utilizing different donor sources.4
Potential for cure: HSCT offers the possibility of a definitive cure, rather than the management of disease remission as other therapies.23
Universal donors' availability: Improvements in HLA-matching techniques have expanded the donor pool, making HSCT a viable option virtually for every patient who needs it.24
Reduced treatment-related toxicities: Advances in conditioning regimens, supportive care, and strategies to prevent and treat GvHD have helped minimize transplant-associated morbidity and mortality.25-27
Strategic role of HSCT: HSCT can serve as a powerful alternative or adjunct treatment in patients with refractory disease unable to achieve remission.5,28
Cons of universal HSCT for pediatric R/R ALL
Risk of GvHD: Despite the implementation of modern prophylactic strategies, the incidence of acute GVHD remains significant, varying greatly depending on the donor sources and contributing to both morbidity and mortality.26 However, the implementation of graft manipulation strategies remarkably reduced this risk.29
Risk of relapse: While reduced, the risk of relapse post-HSCT is still present and ranges between 20% and 50%, mostly based on the disease status at the time of HSCT (see also Table 1).30
Long-term side effects: HSCT recipients may suffer from long-term sequelae, including endocrine, cardiovascular, and psychosocial complications.31 This risk is mostly associated with the use of myeloablative conditioning regimens. TBI constitutes a key component of myeloablative conditioning for ALL.32,33 One mitigating strategy is the recent use of volumetric modulated arc therapy TBI, an innovative and precise radiation delivery strategy that has showed favorable toxicity profiles, excellent disease control, and improved organ sparing in children and young adults.34-36
TRM: Despite tremendous improvements, the risk of TRM remains and accounts for about 5%-20% of patients across donor sources. Infections and severe GvHD still represent the main causes.37
Cost and resource-intensive: HSCT is a complex procedure requiring significant healthcare resources and can be cost prohibitive in certain settings.38 Strategizing about cost-effective care models and optimizing resource allocation is crucial to increase the accessibility and affordability of HSCT, ensuring that all children with ALL who could benefit from it can do so.
Look to the future: the expert's perspective
While allogeneic HSCT stands as the current gold standard for pediatric ALL, the revolutionary allure of CAR T-cell therapies cannot be denied. Boasting impressive remission rates (80% CR MRD at 100 days39-42 ) and a tantalizing glimpse at a potential cure for ALL, CAR T-cell therapies beckon as a game-changing contender in the therapeutic landscape. However, recent data from the pivotal ELIANA trial showed 3-year OS of 63% and relapse-free survival around 50%, suggesting HSCT may be needed for long-term control.40
Currently, the convergence of HSCT and immunotherapy represents the most captivating frontier in the field, and we can foresee that the combination of HSCT with innovative cell therapies will lead the wave of future pediatric cancer immunotherapy clinical trials. Identifying biomarkers and other parameters that determine the response to CAR T treatment (ie, anticipate antigen-escape relapse) vs the need for immune and myeloablation followed by HSCT is crucial for successfully pinpointing the need and the optimal timing for HSCT. As described in the clinical case 1, using HSCT as consolidative therapy after CAR T might not always be the right choice. On the other hand, watch and wait for CAR T and/or BCA loss inevitably means significantly increasing the risk of transplant-related toxicity as a result of the need of reinducing leukemia remission (as in the clinical case 2).
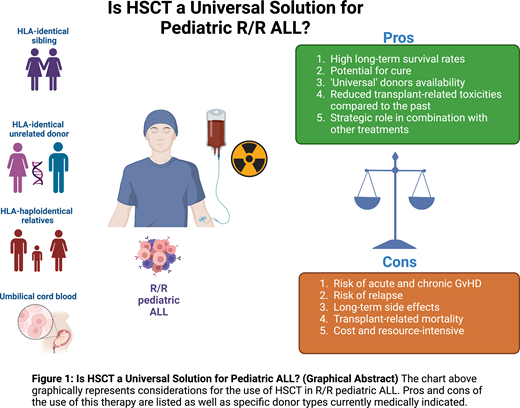
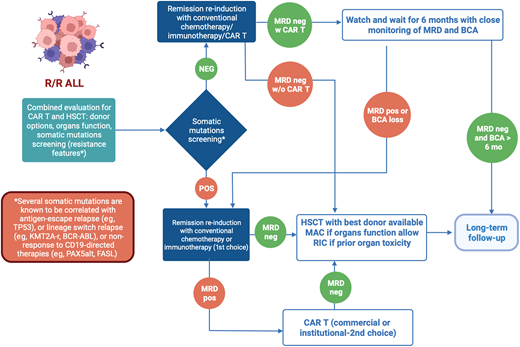
In conclusion, in managing pediatric R/R ALL, HSCT and CAR T both present unique strengths and challenges. The selection of treatment must consider a multitude of factors specific to the patient and treatment center, beyond just reported outcomes (Figure 1). To enhance therapeutic decision making, guidelines incorporating disease risk stratification, leukemia genetic profile, ultrasensitive NGS MRD measurement to assess remission depth, organ function, complications history, donor options, conditioning regimens, and cellular therapy access need development. Here we propose a cascade of clinical considerations to decide whether HSCT should be always offered as curative treatment for pediatric R/R ALL or not (Figure 2). While current evidence supporting the use of new diagnostic tools (ie, the role of somatic mutations) for clinical management remains inadequate, their potential to influence treatment outcomes is significant. Determining the optimal treatment for R/R ALL patients continues to be challenging. However, well-designed prospective clinical trials hold promise for more informed decision making, using a range of treatment strategies tailored to the individual patient's needs.
Factors influencing the selection of definitive therapy in R/R pediatric ALL. The chart graphically represents the many factors that contribute to the cost-benefit analysis of the selection of definitive therapy with the highest chance of clinical success in R/R pediatric patients with ALL.
Factors influencing the selection of definitive therapy in R/R pediatric ALL. The chart graphically represents the many factors that contribute to the cost-benefit analysis of the selection of definitive therapy with the highest chance of clinical success in R/R pediatric patients with ALL.
Proposed clinical decision-making flowchart for R/R pediatric ALL. This figure proposes a novel schema for clinical decision making regarding the selection of definitive therapy for R/R pediatric ALL. Given the vast number of considerations that influence the selection of definitive therapy in these difficult cases, this schema provides a framework to guide clinical decision making.
Proposed clinical decision-making flowchart for R/R pediatric ALL. This figure proposes a novel schema for clinical decision making regarding the selection of definitive therapy for R/R pediatric ALL. Given the vast number of considerations that influence the selection of definitive therapy in these difficult cases, this schema provides a framework to guide clinical decision making.
Conflict-of-interest disclosure
David Shyr: no competing financial interests to declare.
Kara L. Davis: no competing financial interests to declare.
Alice Bertaina: no competing financial interests to declare.
Off-label drug use
David Shyr: Nothing to disclose in terms of off-label drug use outside of standard of care practice for SCT.
Kara L. Davis: Nothing to disclose in terms of off-label drug use outside of standard of care practice for SCT.
Alice Bertaina: Nothing to disclose in terms of off-label drug use outside of standard of care practice for SCT.