Abstract
Myeloproliferative neoplasms (MPNs) are characterized by clonal myeloproliferation in 1 or more of the hematopoietic stem cell lineages. Primary myelofibrosis (MF), post–polycythemia vera MF, and post–essential thrombocythemia MF have the worst prognosis and are characterized by the presence of cytokine-mediated symptom complex, splenomegaly, progressive marrow failure, and clonal instability, leading to leukemic transformation. The key therapeutic aims encompass the management of symptoms, splenomegaly, and anemia and the improvement of survivals. These therapeutic aims have evolved with the availability of Jak inhibitors and novel agents, making disease modification potentially achievable. Novel agents may potentially target MPN stem cells, epigenetic alterations, signaling pathways, and apoptotic pathways. In this case-based review, we outline our approach to the management of MF and discuss the therapeutic landscape of MF, highlighting the utility of Jak inhibitors and novel Jak inhibitor–based combinations.
Learning Objectives
Cite the efficacies of Jak inhibitors and novel agents in the first- and second-line settings
Discuss the therapeutic approach to MF, taking into consideration patient characteristics and clinical needs
CLINICAL CASE
A 71-year-old woman diagnosed with polycythemia vera (PV) in 1999 had received regular venesections, aspirin, and hydroxyurea. Since 2015 the number of venesections and the dosage of hydroxyurea required for optimal hemoglobin (Hb) control had gradually decreased. From September 2022 onward, she had developed progressive weight loss. Physical examination showed mild pallor and an 8-cm splenomegaly. A complete blood count showed an Hb level of 8.5 g/dL, a leukocyte count of 8.2 × 109/L, and a platelet count of 226 × 109/L. There was a leukoerythroblastic blood picture with 1% blasts and teardrop poikilocytes. The bone marrow (BM) aspirate was a “dry tap.” The BM trephine biopsy showed a diffuse increase in reticulin (MF-3) and collagen fibrosis (grade 2). Blasts were not obviously increased. Features were consistent with post-PV myelofibrosis (MF). Next-generation sequencing showed JAK2 V617F with a variant allele frequency (VAF) of 0.89, a DNMT3A p.R749L missense variant (VAF, 0.45), and a TET2 splicing variant (VAF, 0.43). Karyotyping showed t(6;11)(q23;q23) in 4 of 20 metaphases. According to the Myelofibrosis Secondary to PV and ET-Prognostic Model, the risk was intermediate-2. At the latest follow-up, her Myelofibrosis Symptom Assessment from (MFSAF) Version 4.0 Total Symptom Score (TSS) was 27.
Introduction and treatment needs in MF
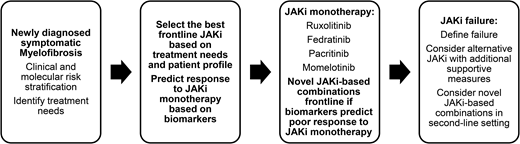
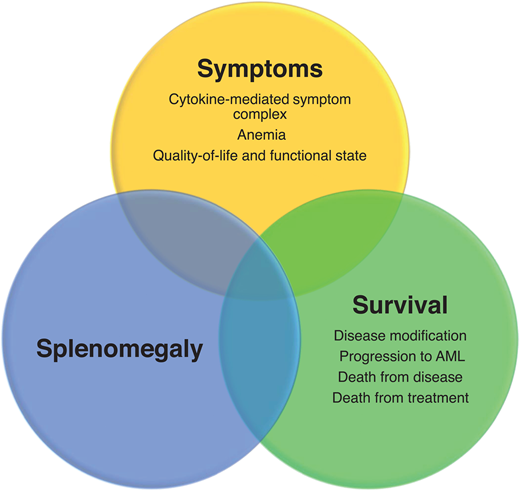
The classical Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs) comprise PV, essential thrombocythemia (ET), and primary MF (PMF), which are associated with mutations of the driver genes JAK2, CALR, and MPL. PMF is subclassified into prefibrotic/early PMF and overt PMF. Overt PMF is characterized by marrow fibrosis, cytokine-mediated systemic symptoms, anemia, hepatosplenomegaly, and a propensity for progression to acute myeloid leukemia. PV and ET may progress to post-PV and post-ET—MF with symptoms resembling those of overt PMF. The key treatment needs in MF include managing symptoms deriving from constitutional problems, insufficient quality of life, and anemia; controlling splenomegaly; and improving survival via disease modification (the 3 Ss) (Figure 1). Given the current understanding of the disease's biology, disease modification in MF is defined as therapy resulting in a clinically meaningful impact on survival outcome and/or the restoration of normal hematopoiesis with improvement in marrow fibrosis and durable reduction in the clonal burden.1 Allogeneic hematopoietic stem cell transplantation is currently the only potentially curative therapy, although applicability is limited by significant mortality and morbidity. Ruxolitinib, a first-in-class Jak inhibitor, is the most widely used, effectively improving symptoms and splenomegaly and possibly modestly improving survival.2,3 However, ruxolitinib may not adequately address the underlying disease biology, showing generally modest effects on mutant allele burdens, BM fibrosis (BMF), and the prevention of leukemic transformation. Three other Jak2 inhibitors, fedratinib, pacritinib, and momelotinib, have also been developed, but they, too, do not address all the unmet needs in MF, especially in the second-line setting and disease modification.4 To address these problems, multiple “non–Jak inhibitor” molecules have been developed and are being tested in phase 2 and 3 studies, either as monotherapy or in combination with Jak2 inhibitors (Table 1) (Table 2).
Results of selected studies of Jak inhibitors as monotherapy in MF
| Jak inhibitor/ targets . | Study/phase . | Population . | Treatment/ sample size . | Control/ sample size . | TSS50 at wk 24 . | SVR35 at wk 24 . | Anemia response . | Molecular responses/BMF reduction . | Relevant toxicities . |
|---|---|---|---|---|---|---|---|---|---|
| Ruxolitinib Jak1, Jak2 | COMFORT-1 phase 3 | Int-2/high-risk MF Platelets ≥100 × 109/L Intolerant/resistant to available therapy | 15 mg twice a day for platelets 100-200 × 109/L; 20 mg twice a day for platelets >200 × 109/L N = 155 | Placebo N = 154 | 45.9% | 41.9% | TI: 41% (14/34 TI patients) | JAK2 V617F VAF: 8% reduction at 24 wk; 17% at 48 wk BMF reduction: NR | Anemia, thrombocytopenia, headache, opportunistic infections |
| COMFORT-2 phase 3 | Int-2/high-risk MF Platelets ≥100 × 109/L | Same as COMFORT-1 N = 146 | BAT N = 73 | NR | 32% | NR | JAK2 V617F VAF: 38% with >20% reduction at 168 wk BMF reduction: 16% after median of 26 mo | ||
| Fedratinib Jak1, Jak2, Jak3, TYK3 | JAKARTA phase 3 | Int-2/high-risk MF Platelets ≥50 × 109/L Jak inhibitor naive | 400 mg or 500 mg/d N = 193 | Placebo N = 96 | 36% with 400 mg/d; 34% with 500 mg/d | 36% with 400 mg/d; 40% with 500 mg/d | TI: 88% (7/8 TD patients) | JAK2 V617F VAF: 0.4% increase at 24 wk/ BMF: NR | Anemia, thrombocytopenia, gastrointestinal toxicity, transaminitis, raised amylase and lipase, Wernicke's encephalopathy (black box warning) |
| JAKARTA-2 phase 2 | Int-1 MF with symptoms Int-2/high-risk MF Platelets ≥50 × 109/L Ruxolitinib failure/ intolerance | 400 mg/d N = 97 | NA | 26% | 55% | NR | NR | ||
| Pacritinib Jak2, FLT3,IRAK1, CSF1R, ACVR1 | PERSIST-1 phase 3 | Int-1/int-2/high-risk MF Any platelet count Jak inhibitor naive | 400 mg/d N = 220 | BAT N = 107 (excluded ruxolitinib) | 19% | 19% | TI: 25% (9/36 TD patients) | JAK2 V617F VAF: 15.8% reduction at 24 wk | Thrombocytopenia, anemia, diarrhea and gastrointestinal toxicity, fluid retention, heart failure, squamous cell skin cancer |
| PERSIST-2 phase 3 | Int-1/int-2/high-risk MF Platelets <100 × 109/L Jak inhibitor exposed or naive | 400 mg/d or 200 mg twice a day N = 211 | BAT N = 100 (45% on ruxolitinib) | 25% | 18% | TI ≥8 wk or ≥2 g/dL increase in Hb: 25% (11/44 with Hb <10 g/dL) | NR | ||
| PAC203 phase 2 | Int-1/int-2/high-risk MF Any platelet count Ruxolitinib failure/ intolerance | 100 mg/d or 100 mg twice a day or 200 mg twice a day N = 165 | NA | 7.5% | 9.3% for 200 mg twice a day; 1.8% for 100 mg twice a day; 0% for 100 mg/d | ≥1 g/dL increase in Hb: 10% (4/42 with Hb <10 g/dL) | NR | ||
| Momelotinib Jak1, Jak2, ACVR1 | SIMPLIFY-1 phase 3 | Int-1 MF with symptoms Int-2/high-risk MF Platelets ≥50 × 109/L Jak inhibitor naive | 200 mg/d N = 215 | Ruxolitinib N = 217 | 28.4% | 26.5% | TI at 24 wk: 66.5% | NR | Anemia, thrombocytopenia, neutropenia, transaminitis, raised amylase/lipase, peripheral neuropathy, first-dose effect (transient hypotension, flushing, dizziness, and nausea) |
| SIMPLIFY-2 phase 3 | Int-1 MF with symptoms Int-2/high-risk MF Any platelet count Suboptimal response/intolerance to ruxolitinib | 200 mg/d N = 104 | BAT N = 52 (89% ruxolitinib) | 26.2% | 7% | TI at 24 wk: 43% | NR | ||
| MOMENTUM phase 3 | Int-1/Int-2/high-risk MF with symptoms Platelets ≥25 × 109/L Hb <10 g/dL Jak inhibitor exposed | 200 mg/d N = 130 | Danazol N = 65 | 25% | 23% | TI at 24 wk: 31% | NR | ||
| Jaktinib Jak1, Jak2, ACVR1, TYK2 | NCT03886415 phase 2 | Int-1/Int-2/high-risk MF with symptoms Platelets ≥75 × 109/L | 100 mg twice a day or 200 mg/d N = 118 | NA | 69.6% with 100 mg twice a day; 57.5% with 200 mg/d | 54.8% with 100 mg twice a day; 31.3% with 200 mg/d | Hb increase: 36% (in patients with Hb <10 g/dL) | NR | Anemia, thrombocytopenia |
| Jak inhibitor/ targets . | Study/phase . | Population . | Treatment/ sample size . | Control/ sample size . | TSS50 at wk 24 . | SVR35 at wk 24 . | Anemia response . | Molecular responses/BMF reduction . | Relevant toxicities . |
|---|---|---|---|---|---|---|---|---|---|
| Ruxolitinib Jak1, Jak2 | COMFORT-1 phase 3 | Int-2/high-risk MF Platelets ≥100 × 109/L Intolerant/resistant to available therapy | 15 mg twice a day for platelets 100-200 × 109/L; 20 mg twice a day for platelets >200 × 109/L N = 155 | Placebo N = 154 | 45.9% | 41.9% | TI: 41% (14/34 TI patients) | JAK2 V617F VAF: 8% reduction at 24 wk; 17% at 48 wk BMF reduction: NR | Anemia, thrombocytopenia, headache, opportunistic infections |
| COMFORT-2 phase 3 | Int-2/high-risk MF Platelets ≥100 × 109/L | Same as COMFORT-1 N = 146 | BAT N = 73 | NR | 32% | NR | JAK2 V617F VAF: 38% with >20% reduction at 168 wk BMF reduction: 16% after median of 26 mo | ||
| Fedratinib Jak1, Jak2, Jak3, TYK3 | JAKARTA phase 3 | Int-2/high-risk MF Platelets ≥50 × 109/L Jak inhibitor naive | 400 mg or 500 mg/d N = 193 | Placebo N = 96 | 36% with 400 mg/d; 34% with 500 mg/d | 36% with 400 mg/d; 40% with 500 mg/d | TI: 88% (7/8 TD patients) | JAK2 V617F VAF: 0.4% increase at 24 wk/ BMF: NR | Anemia, thrombocytopenia, gastrointestinal toxicity, transaminitis, raised amylase and lipase, Wernicke's encephalopathy (black box warning) |
| JAKARTA-2 phase 2 | Int-1 MF with symptoms Int-2/high-risk MF Platelets ≥50 × 109/L Ruxolitinib failure/ intolerance | 400 mg/d N = 97 | NA | 26% | 55% | NR | NR | ||
| Pacritinib Jak2, FLT3,IRAK1, CSF1R, ACVR1 | PERSIST-1 phase 3 | Int-1/int-2/high-risk MF Any platelet count Jak inhibitor naive | 400 mg/d N = 220 | BAT N = 107 (excluded ruxolitinib) | 19% | 19% | TI: 25% (9/36 TD patients) | JAK2 V617F VAF: 15.8% reduction at 24 wk | Thrombocytopenia, anemia, diarrhea and gastrointestinal toxicity, fluid retention, heart failure, squamous cell skin cancer |
| PERSIST-2 phase 3 | Int-1/int-2/high-risk MF Platelets <100 × 109/L Jak inhibitor exposed or naive | 400 mg/d or 200 mg twice a day N = 211 | BAT N = 100 (45% on ruxolitinib) | 25% | 18% | TI ≥8 wk or ≥2 g/dL increase in Hb: 25% (11/44 with Hb <10 g/dL) | NR | ||
| PAC203 phase 2 | Int-1/int-2/high-risk MF Any platelet count Ruxolitinib failure/ intolerance | 100 mg/d or 100 mg twice a day or 200 mg twice a day N = 165 | NA | 7.5% | 9.3% for 200 mg twice a day; 1.8% for 100 mg twice a day; 0% for 100 mg/d | ≥1 g/dL increase in Hb: 10% (4/42 with Hb <10 g/dL) | NR | ||
| Momelotinib Jak1, Jak2, ACVR1 | SIMPLIFY-1 phase 3 | Int-1 MF with symptoms Int-2/high-risk MF Platelets ≥50 × 109/L Jak inhibitor naive | 200 mg/d N = 215 | Ruxolitinib N = 217 | 28.4% | 26.5% | TI at 24 wk: 66.5% | NR | Anemia, thrombocytopenia, neutropenia, transaminitis, raised amylase/lipase, peripheral neuropathy, first-dose effect (transient hypotension, flushing, dizziness, and nausea) |
| SIMPLIFY-2 phase 3 | Int-1 MF with symptoms Int-2/high-risk MF Any platelet count Suboptimal response/intolerance to ruxolitinib | 200 mg/d N = 104 | BAT N = 52 (89% ruxolitinib) | 26.2% | 7% | TI at 24 wk: 43% | NR | ||
| MOMENTUM phase 3 | Int-1/Int-2/high-risk MF with symptoms Platelets ≥25 × 109/L Hb <10 g/dL Jak inhibitor exposed | 200 mg/d N = 130 | Danazol N = 65 | 25% | 23% | TI at 24 wk: 31% | NR | ||
| Jaktinib Jak1, Jak2, ACVR1, TYK2 | NCT03886415 phase 2 | Int-1/Int-2/high-risk MF with symptoms Platelets ≥75 × 109/L | 100 mg twice a day or 200 mg/d N = 118 | NA | 69.6% with 100 mg twice a day; 57.5% with 200 mg/d | 54.8% with 100 mg twice a day; 31.3% with 200 mg/d | Hb increase: 36% (in patients with Hb <10 g/dL) | NR | Anemia, thrombocytopenia |
CSF1R, colony stimulating factor 1 receptor; Int-1, intermediate-1 risk by Dynamic International Prognostic Scoring System; Int-2, intermediate-2 risk by Dynamic International Prognostic Scoring System; IRAK1, interleukin 1 receptor associated kinase 1; NA, not available; NR, not reported.
Results of selected Jak inhibitor-based combinations for MF
| Agent . | Setting . | Regimen . | Phase . | Study population/sample size . | Clinical responses . | Molecular responses . | BMF reduction . |
|---|---|---|---|---|---|---|---|
| Targeting hematopoietic stem cells | |||||||
| INF-α | First-line | Ruxolitinib + PEG-IFN-α2A | 1/2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 37 (phase 1, N = 18; phase 2, N = 19) | ≥50% reduction in spleen length at 24 wk: 70% | JAK2 V617F VAF decreased from a median of 84% (range, 23%-96%) at baseline to 65% (range, 16-95) and 53% (range, 16-92) after 6 and 12 mo | NR |
| Targeting epigenetic regulators | |||||||
| HMAs | First-line | Ruxolitinib + azacitidine 25-75 mg/m2/d days 1-5 every 4 wk | 2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 46 | >50% reduction in spleen length at 24 wk: 62% (21/34); best TSS50: 54% (25/46); TI: 20% (1/5) | 81% (13/16) had reduction in JAK2 V617F VAF at 24 wk | 57% (8/14) had BM reticulin fibrosis reduction at 24 wk |
| BET inhibition | First-line | Ruxolitinib + pelabresib | 2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 84 | SVR35 at 24 wk: 68%; TSS50 at 24 wk: 56%; ≥1.5 g/dL over 12 wk: 24% | NR | 28% evaluable patients had reduction in BMF at 24 wk |
| Second-line | Ruxolitinib + pelabresib | 2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 86 | SVR35 at 24 wk: 20% (16/81); TSS50 at 24 wk: 37% (30/81) | NR | 26% evaluable patients had reduction in BM reticulin fibrosis at 24 wk | |
| Targeting apoptotic pathways | |||||||
| BCLXL/BCL2 inhibition | First-line | Ruxolitinib + navitoclax | 2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 32 | SVR35 at 24 wk: 52%; TSS50 at 24 wk: 31%; TI: 55% | 50% and 36% of patients had >20% reduction in JAK2 V617F VAF | 35% had reduction in BM reticulin fibrosis at any time |
| Second-line | Ruxolitinib + navitoclax | 2 | Int-1, int-2, and high-risk MF Suboptimal response to ruxolitinib N = 34 | SVR35 at 24 wk: 26.5%; TSS50 at 24 wk: 30%; TI: 64% | 46% had >10% reduction in VAF of driver gene mutations | 33% had reduction in BM reticulin fibrosis at any time | |
| Selective inhibition of nuclear export | First-line | Ruxolitinib + selinexor | 1/2 | Int-1, int-2, and high-risk MF N = 22 | SVR35 at 24 wk: 64% overall; 79% (11/14) in the 60-mg group and 38% (3/8) in the 40-mg group, respectively; TSS50 at 24 wk: 45% overall; 58% (7/12) in the 60-mg group and 25% (2/8) in the 40-mg group; | 50% (4/8) had >10% reduction in VAF, and 25% (2/8) had >20% reduction in VAF in the 60-mg group | NR |
| Targeting bone marrow microenvironment | |||||||
| Activin receptor IIB ligand trap | Second-line (“add-on”) | Ruxolitinib + luspatercept | 2 | Patients on ruxolitinib ≥16 wk prior to enrollment and TD; N = 38 | 31.6% achieved TI for ≥12 wk over entire treatment period; 50% had ≥50% reduction in transfusions (≥4 units) over 12 wk | NR | NR |
| Agent . | Setting . | Regimen . | Phase . | Study population/sample size . | Clinical responses . | Molecular responses . | BMF reduction . |
|---|---|---|---|---|---|---|---|
| Targeting hematopoietic stem cells | |||||||
| INF-α | First-line | Ruxolitinib + PEG-IFN-α2A | 1/2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 37 (phase 1, N = 18; phase 2, N = 19) | ≥50% reduction in spleen length at 24 wk: 70% | JAK2 V617F VAF decreased from a median of 84% (range, 23%-96%) at baseline to 65% (range, 16-95) and 53% (range, 16-92) after 6 and 12 mo | NR |
| Targeting epigenetic regulators | |||||||
| HMAs | First-line | Ruxolitinib + azacitidine 25-75 mg/m2/d days 1-5 every 4 wk | 2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 46 | >50% reduction in spleen length at 24 wk: 62% (21/34); best TSS50: 54% (25/46); TI: 20% (1/5) | 81% (13/16) had reduction in JAK2 V617F VAF at 24 wk | 57% (8/14) had BM reticulin fibrosis reduction at 24 wk |
| BET inhibition | First-line | Ruxolitinib + pelabresib | 2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 84 | SVR35 at 24 wk: 68%; TSS50 at 24 wk: 56%; ≥1.5 g/dL over 12 wk: 24% | NR | 28% evaluable patients had reduction in BMF at 24 wk |
| Second-line | Ruxolitinib + pelabresib | 2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 86 | SVR35 at 24 wk: 20% (16/81); TSS50 at 24 wk: 37% (30/81) | NR | 26% evaluable patients had reduction in BM reticulin fibrosis at 24 wk | |
| Targeting apoptotic pathways | |||||||
| BCLXL/BCL2 inhibition | First-line | Ruxolitinib + navitoclax | 2 | Int-1, int-2, and high-risk MF Jak inhibitor naive/ N = 32 | SVR35 at 24 wk: 52%; TSS50 at 24 wk: 31%; TI: 55% | 50% and 36% of patients had >20% reduction in JAK2 V617F VAF | 35% had reduction in BM reticulin fibrosis at any time |
| Second-line | Ruxolitinib + navitoclax | 2 | Int-1, int-2, and high-risk MF Suboptimal response to ruxolitinib N = 34 | SVR35 at 24 wk: 26.5%; TSS50 at 24 wk: 30%; TI: 64% | 46% had >10% reduction in VAF of driver gene mutations | 33% had reduction in BM reticulin fibrosis at any time | |
| Selective inhibition of nuclear export | First-line | Ruxolitinib + selinexor | 1/2 | Int-1, int-2, and high-risk MF N = 22 | SVR35 at 24 wk: 64% overall; 79% (11/14) in the 60-mg group and 38% (3/8) in the 40-mg group, respectively; TSS50 at 24 wk: 45% overall; 58% (7/12) in the 60-mg group and 25% (2/8) in the 40-mg group; | 50% (4/8) had >10% reduction in VAF, and 25% (2/8) had >20% reduction in VAF in the 60-mg group | NR |
| Targeting bone marrow microenvironment | |||||||
| Activin receptor IIB ligand trap | Second-line (“add-on”) | Ruxolitinib + luspatercept | 2 | Patients on ruxolitinib ≥16 wk prior to enrollment and TD; N = 38 | 31.6% achieved TI for ≥12 wk over entire treatment period; 50% had ≥50% reduction in transfusions (≥4 units) over 12 wk | NR | NR |
Int-1, intermediate-1 risk by Dynamic International Prognostic Scoring System; Int-2, intermediate-2 risk by Dynamic International Prognostic Scoring System; NR, not reported; PI3K, phosphatidylinositol 3-kinase; RBC, red blood cell.
CLINICAL CASE (continued)
The patient was started on ruxolitinib at 20 mg twice per day. She responded well with an improvement of MFSAF-TSS to 4 after 8 weeks of therapy. The splenomegaly decreased to 5 cm. Her Hb level remained stable, between 8 to 9 g/dL. Twenty-eight months later, her symptoms worsened, with the MFSAF-TSS increasing to 25 and the splenomegaly, to 12 cm. The latest complete blood count showed a Hb level of 7.1 g/dL, a leukocyte count of 12.5 × 109/L, and a platelet count of 120 × 109/L. The ruxolitinib was reduced to 15 mg twice a day in view of the worsening anemia and thrombocytopenia.
Jak inhibitors as monotherapy
Ruxolitinib—what have we learned so far?
Ruxolitinib is a nonselective Jak1/2 inhibitor that controls cellular proliferation and splenomegaly by inhibiting constitutively activated Jak/STAT signaling and constitutional symptoms by reducing the production of pro-inflammatory cytokines. Pooled analysis and long-term follow-up of the COMFORT-I and COMFORT-II studies showed that ruxolitinib treatment significantly prolonged overall survival (OS).2 Patients receiving ruxolitinib for 12 months or more from diagnosis had better spleen responses, longer OS, and fewer hematologic toxicities.2
Approximately 50% of patients discontinue ruxolitinib after 3 years, mostly due to disease progression, suboptimal response, or cytopenia. Definitions of “ruxolitinib failure” vary and are largely based on studies in the second-line setting, generally including disease progression to accelerated or blast phase, suboptimal response of spleen or constitutional symptoms, increases in splenomegaly or constitutional symptoms after initial response, and the development of transfusion-dependent (TD) anemia or grade 3/4 thrombocytopenia or hemorrhagic events while on ruxolitinib.5 Outcome after ruxolitinib discontinuation is generally poor, with a median OS of approximately 14 months. Patients with 3 or more nondriver gene mutations generally have a shorter time to discontinuation. A clinical prognostic model was developed to determine survival after 6 months of treatment with ruxolitinib in patients with MF.6 Risk factors for worse OS after 6 months included a ruxolitinib dose less than 20 mg twice a day at baseline, 3 months, and 6 months; a palpable spleen length reduction from baseline of 30% or less at 3 and 6 months; and transfusion need at 3 and/or 6 months or at any time point. The “response to ruxolitinib after 6 months” model effectively stratified patients into 3 prognostic risk groups (low risk: median OS not reached; intermediate risk: median OS equal to 61 months, and high-risk: median OS equal to 33 months).6
Fedratinib, pacritinib, and momelotinib—what can they offer?
Fedratinib is a potent Jak2–fms-like tyrosine kinase 3 (FLT3)– bromodomain 4 (BRD4) inhibitor approved for intermediate-2 and high-risk MF irrespective of prior ruxolitinib use. In the JAKARTA studies,7,8 fedratinib effectively reduced splenomegaly and symptom burden in patients naive or exposed to Jak inhibitor. In Jak inhibitor–naive patients, a 35% reduction in spleen volume (SVR35) and a 50% reduction in MF TSS (TSS50) was achieved in 47% and 40% of patients during week 24.8 Fedratinib-treated patients also achieved clinically meaningful improvement in health-related QOL.9
Pacritinib is a Jak2/FLT3 inhibitor approved for intermediate-2 or high-risk MF with platelet counts equal to or less than 50 × 109/L. In the pooled analysis of the PERSIST-1 and PERSIST-2 studies comprising 189 patients with platelet counts of equal to or less than 50 × 109/L (median platelet count, 28 × 109/L; 63.5% with a Hb level less than 10 g/dL; 35% with prior Jak inhibitor treatment), SVR35 and modified TSS50 was achieved in 23.1% and 25% of cases at week 24.
Momelotinib is a Jak1/Jak2 inhibitor that has additional inhibitory effects against activin A receptor type 1 (ACVR1).10 ACVR1 is an important mediator of SMAD2/3 signaling that upregulates hepcidin production and results in iron-restricted erythropoiesis. SMAD2/3 signaling is particularly implicated in the inhibition of terminal erythroid maturation and ineffective erythropoiesis. In the SIMPLIFY-1 study,11 which uniquely compared head-to-head momelotinib with ruxolitinib, the rate of SVR35 was similar between the 2 arms at week 24 (momelotinib, 26.5%; ruxolitinib, 29%), while symptom score reduction at week 24 was higher in the ruxolitinib arm (momelotinib, 28.4; ruxolitinib, 42.2%). However, the rate of red cell transfusion independence (TI) at week 24 was remarkably different (momelotinib, 66.5%; ruxolitinib, 49.3%).11 The achievement of TI with momelotinib was associated with superior 3-year OS at 77.2%.11,12 In the SIMPLIFY-2 study, momelotinib was evaluated in patients with a suboptimal response or intolerance to ruxolitinib.13 At week 24, SVR35 was achieved in 7% in the momelotinib arm compared with 6% in patients on best available therapy (BAT, with 89% receiving ruxolitinib).13 TI was achieved in 49.3% in the momelotinib arm and 21% in the BAT arm.13 The phase 3 MOMENTUM study evaluated Jak inhibitor–exposed patients with intermediate- or high-risk MF with an Hb level lower than 10 g/dL, a symptom score of 10 of above, and a platelet count of 25 × 109/L or higher.14 SVR35 and symptom score response rates at week 24 were achieved in 23% and 24.6% in the momelotinib arm and 3% and 9.2% in the danazol arm.14 The rates of TI at week 24 were 31% for momelotinib and 20% for danazol.14
CLINICAL CASE (continued)
The patient was concerned about her long-term survival and risk of leukemic progression. After counseling, the patient was referred to a clinical trial involving ruxolitinib in combination with a novel agent.
Promising “partners” of Jak inhibitors
Targeting hematopoietic stem cells
Interferon alfa (IFN-α)
In a phase 1/2 study of ruxolitinib in combination with pegylated IFN-α2A (PEG-IFN-α2A) in 37 patients with MF, 70% of patients achieved a reduction of 50% or better in palpable spleen length with significant reductions of JAK2 V617F VAF.15 ROPEG-IFN-α2b, with more potent activity against MPN hematopoietic stem cells (HSCs), is being evaluated as a single agent in early/prefibrotic and low- and intermediate-1 risk MF, with significant clinical and molecular responses achieved.16 In an interim analysis of 56 patients, 36 (92%) of 39 JAK2 V617F–mutated patients had stable or improved JAK2 V617F VAF by droplet digital polymerase reaction.16
Telomerase inhibition
Human telomerase reverse transcriptase (hTERT) activity is overexpressed in MPN HSCs, leading to uncontrolled myeloproliferation.
Imetelstat is a first-in-class telomerase inhibitor that binds to the RNA component of telomerase in MPN HSCs, suppressing telomerase activity. In a randomized phase 2 study, 107 patients with Jak inhibitor relapsed/refractory MF were treated with imetelstat at 9.4 mg/kg or 4.7 mg/kg given intravenously every 3 weeks.17 SVR35 and TSS50 were achieved in 10.2% and 32.2% in the 9.4 mg/kg arm and 0% and 6.3% in the 4.7 mg/kg arm. In patients treated with imetelstat at 9.4 mg/kg, BMF reduction was observed in 40.5% of patients, with a median OS of 29.9 months.17 A confirmatory phase 3 study is ongoing (NCT04576156).
Targeting epigenetic regulators
Hypomethylating agents (HMAs)
In a phase 2 study of azacitidine in combination with ruxolitinib as the first-line treatment of 46 patients with intermediate-risk and high-risk MF, 62% and 54% achieved a reduction of 50% or higher in palpable spleen length and TSS50 at week 24.18 A reduction in JAK2 V617F VAF was observed in 81% of patients at 24 weeks, with 54% of evaluable patients showing a reduction in marrow fibrosis. Ruxolitinib in combination with decitabine achieved an overall response rate of 42.9% (9/21) in patients with accelerated or blast phase MPNs.19
BRD and extraterminal (BET) protein inhibition
BET proteins (BRD2, BRD3, BRD4) are a family of chromatin-reader proteins binding to acetylated lysine residues on histones to initiate oncogene transcription and proinflammatory NF-κB activation.20 The BET inhibitor pelabresib in combination with ruxolitinib in the frontline resulted in SVR35 and TSS50 of 68% and 56% at week 24 in 84 patients with intermediate- and high-risk MF.21 In addition, 36% of patients showed improvement in Hb level. A reduction in BMF and a reduction greater than 25% in JAK2 V617F VAF was achieved in 28% and 29.5% of patients. In 86 patients with relapsed/refractory MF on ruxolitinib, pelabresib as an “add-on” therapy achieved SVR35 of 20% and TSS50 of 37% at 24 weeks.22 Beyond 24 weeks, a BMF reduction of 1 grade or more was observed in 26% of evaluable patients.22
Lysine-specific demethylase-1 (LSD1) inhibition
In MPNs, LSD1 is overexpressed and maintains self-renewal of MPN HSCs. The LSD1 inhibitor bomedemstat (IMG-7289) was evaluated in 89 patients with MF failing prior to janus kinase inhibitor (Jak) and achieved SVR35 and TSS50 in 37% and 6% of patients with prior ruxolitinib exposure.23 In TI patients (N = 41), 90% had stable or improved Hb levels. In TD patients, 14% became TI. In 59 evaluable patients, 85% experienced stable or improved BM reticulin fibrosis by at least 1 grade or more.23 Fifty-three percent of patients with improved BMF also demonstrated improvement in Hb levels or a reduced transfusion requirement.23 In 43 patients with follow-up genomic data, the VAF in one or more alleles was reduced in 42%. ASXL1 mutations were the most commonly reduced, with most consistent declines in truncating ASXL1 mutations clustered around codon 642.23 A phase 2 study of ruxolitinib in combination with bomedemstat in the frontline and second-line setting is ongoing (NCT05569538).
Targeting apoptotic pathways
B-cell lymphoma extra-large (BCLXL) inhibition
BCLXL is a member of the B-cell lymphoma-2 (BCL2) family protein that is highly expressed in MF regardless of JAK2 mutational status. Navitoclax is a novel BCL2/BCLXL inhibitor being evaluated in combination with ruxolitinib. In the phase 2 REFINE study, navitoclax as an “add-on” treatment in 34 patients with suboptimal responses to ruxolitinib achieved SVR35 and TSS50 in 26.5% and 30% of patients, respectively, at week 24.24 In addition, 64% of TD patients achieved TI.24 Forty-six percent of patients had a decrease of more than 10% in the VAF of driver gene mutations, and 21% had improvement in marrow fibrosis at 24 weeks.24 In the frontline setting evaluating 32 patients with MF, navitoclax in combination with ruxolitinib achieved SVR35 in 52% at week 24, with benefits seen across all risk groups.25 A reduction in JAK2 V617F VAF greater than 20% from baseline was demonstrated in 50% (14/28) of patients at week 12 or 24.
Human double-minute 2 (HDM2) inhibition
HDM2 negatively regulates TP53 by ubiquitination and is overexpressed in MF HSCs. HDM2 can be targeted to restore TP53 activity.26 Following earlier studies, the MDM2 inhibitor navtemadlin (KRT-232) was evaluated in a proof-of-concept phase 2 study as a single agent in patients refractory or resistant to Jak inhibitors. In 113 patients treated with navtemadlin, SVR35 and TSS50 were achieved in 16% and 30% of patients at week 24.27 Thirty-four percent experienced a reduction of 20% or more in VAF of driver or high-molecular-risk gene mutations, and 27% achieved improvement in marrow fibrosis.27 Based on these findings, the phase 3 BOREAS study has been initiated to evaluate the efficacy and safety of navtemadlin vs BAT for patients with MF refractory or resistant to Jak inhibitors (NCT03662126).
Selecting inhibition of nuclear export
The inhibition of nuclear-cytoplasmic transport by selective inhibitors of nuclear export leads to the nuclear accumulation of p53 and induces apoptosis of JAK2 V617F+ cell lines resistant to Jak inhibitors.28 In addition, selective inhibitors of nuclear export act synergistically with ruxolitinib in vitro and in vivo.28 In a phase 1/2 study of ruxolitinib in combination with selinexor (40 mg or 60 mg/wk) in the frontline setting, 64% (14/22) overall achieved SVR35 at week 24 (79% in the 60-mg group vs 38% in the 40-mg group).29 At week 24, TSS50 was achieved in 45% (9/20) overall (58% in the 60-mg group and 25% in the 40-mg group.29 In the 60-mg group, the reduction of JAK2 V617F VAF at week 24 was greater than 10% in 50% (4/8) and greater than 20% in 25% (2/8).29
Targeting of the BM microenvironment
Depleting cytokine production via transforming growth factor β (TGF-β) inhibition
The activin receptor IIA ligand trap luspatercept binds to TGF-β superfamily ligands to stimulate terminal erythroid maturation and improve anemia.30 Luspatercept was evaluated in patients with MF in a phase 2 study.31 In a subgroup of 38 patients on ruxolitinib who were TD, 26.3% achieved TI during the primary treatment period.31 Nineteen patients (50.0%) experienced a 50% or more reduction in transfusion burden during the primary treatment period, with 8 (21.1%) achieving a mean increase in Hb level of 1.5 g/dL or greater from baseline throughout the entire treatment period.31
Conclusions and future perspectives
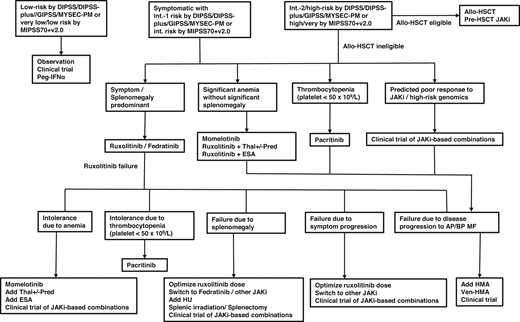
The availability of multiple Jak inhibitors and novel agents has made feasible an individualized therapeutic approach to MF based on unique patient characteristics and treatment needs (Figure 2). Furthermore, the application of novel agents has realized the possibility of disease modification even in hematopoietic stem cell transplantation–ineligible patients. Results of ongoing phase 2 and 3 studies of novel agents and Jak inhibitor– based combinations are eagerly awaited and will add to the evolving management algorithm of MF.
Treatment algorithm in patients with MF. alloHSCT, allogeneic hematopoietic stem cell transplantation; AP/BP, accelerated phase/blast phase; DIPSS, Dynamic International Prognostic Scoring System; ESA, erythropoiesis-stimulating agents; GIPSS, Genetically Inspired Prognostic Scoring System for Primary Myelofibrosis; HU, hydroxyurea; MIPSS70+v2.0, Mutation-Enhanced International Prognostic Scoring System 70 plus version 2.0; MYSEC-PM, Myelofibrosis Secondary to PV and ET Prognostic Model; Pred, prednisolone; Thal, thalidomide; Ven, venetoclax.
Treatment algorithm in patients with MF. alloHSCT, allogeneic hematopoietic stem cell transplantation; AP/BP, accelerated phase/blast phase; DIPSS, Dynamic International Prognostic Scoring System; ESA, erythropoiesis-stimulating agents; GIPSS, Genetically Inspired Prognostic Scoring System for Primary Myelofibrosis; HU, hydroxyurea; MIPSS70+v2.0, Mutation-Enhanced International Prognostic Scoring System 70 plus version 2.0; MYSEC-PM, Myelofibrosis Secondary to PV and ET Prognostic Model; Pred, prednisolone; Thal, thalidomide; Ven, venetoclax.
Conflict-of-interest disclosure
Harinder Gill: consultancy: Bristol Myers Squibb, GSK, Novartis, Pfizer, PharmaEssentia; advisory board: Bristol Myers Squibb, GSK, Novartis, Pfizer, PharmaEssentia; investigator-initiated study collaboration: Imago Biosciences, Novartis, PharmaEssentia.
Garret M. K. Leung: no competing financial interests to declare.
Yok-Lam Kwong: no competing financial interests to declare.
Off-label drug use
Harinder Gill: not applicable.
Garret M. K. Leung: not applicable.
Yok-Lam Kwong: not applicable.