Abstract
Red blood cell (RBC) transfusions treat and prevent severe complications of sickle cell disease (SCD) and can be delivered as a simple or exchange transfusion. During an exchange, some of the patient's abnormal hemoglobin (Hb) S (HbS) RBCs are removed. An apheresis device can accomplish an automated RBC exchange, simultaneously removing patient’s RBCs while returning other blood components along with normal RBCs. Automated RBC exchange is therefore an isovolemic transfusion that can efficiently decrease HbS RBCs while limiting iron loading and hyperviscosity. However, specialized equipment, trained personnel, appropriate vascular access, and increased RBC exposure are required compared to simple or manual RBC exchange. Therefore, risks and benefits must be balanced to make individualized decisions for patients with SCD who require transfusion.
Learning Objectives
Define appropriate venous access options for automated RBC exchange based on size of patient
List 3 benefits of automated RBC exchange compared to simple transfusion
CLINICAL CASE
A 16-year-old Black male weighing 65 kg with homozygous SS sickle cell disease (SCD) developed an abnormal transcranial doppler at age 8 while treated with hydroxyurea (HU). He has been treated with monthly transfusions and HU for 8 years. His pretransfusion hemoglobin on HU is about 9-10 g/dL. He has received a mixture of simple and manual exchange transfusions (depending on pretransfusion hemoglobin). He is prescribed iron chelation but frequently misses doses and has evidence of iron overload. His peripheral access has been difficult, and some attempts at manual exchange have been aborted due to failed access, resulting in higher-than-target hemoglobin S (HbS) levels. Automated RBC exchange (aRCE) has been discussed, but patient has been resistant to surgery for placement of a central venous catheter (CVC). While he is trying to make his decision, he asks if he could stop chelation if he transitioned to aRCE and what changes in his HbS he could expect. This review will discuss the logistics, risks, and benefits of aRCE in SCD in order to address these and other clinical questions.
Introduction
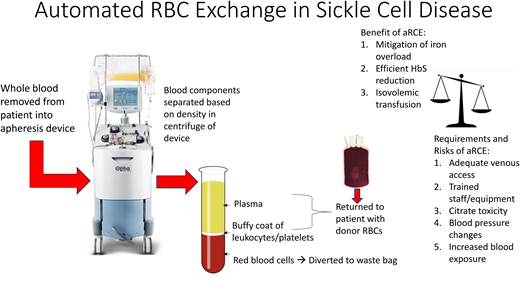
Red blood cell transfusion was the first disease-modifying therapy for individuals with SCD and remains an integral component of the management of SCD today, both acutely to treat and prophylactically to prevent severe SCD complications. Transfusions provide benefit by increasing Hb and, therefore, oxygen delivery to tissues, but also by decreasing abnormal HbS RBCs that contribute to SCD vaso-occlusive pathophysiology. Transfusions can be administered either as a simple transfusion by infusing normal HbA RBCs to dilute the HbS cells or as an exchange transfusion to remove HbS RBCs and replace them with HbA RBCs. Exchange transfusions can be accomplished manually through alternating cycles of phlebotomy and transfusion or as an automated procedure using an apheresis device. Whole blood is drawn from the patient into the device where blood components are separated in a centrifuge according to density. The most dense RBCs will layer on the bottom, followed by white blood cells, platelets, and then plasma. The machine will divert a proportion of the RBC layer to a waste bag to remove RBCs while simultaneously returning most of the patient's white blood cells, platelets, and plasma along with donor RBCs to replace the removed RBCs. There is some loss of other components in the waste bag, and transient decreases in white cells and platelets are expected.1
Logistics of performing an aRCE
Red blood cell volume to exchange
In order to calculate the total volume/number of RBC units required for aRCE, the patient's data and goals of an individual procedure can be entered into the apheresis device software (or an application downloaded to a smartphone2 ). The patient's height, weight, and sex are needed to calculate total blood volume (TBV); in addition, the patient's hematocrit as well as an estimated hematocrit of the RBC units that will be used for the exchange are entered. Citrate-phosphate-dextrose-adenine units typically have a hematocrit of ~75%, while units with additive solutions such as AS-1 usually have a hematocrit of ~55%; however, institution-specific averages should be obtained from local blood banks.3 Next, the desired post-aRCE hematocrit as well as the pre- and target post-aRCE HbS levels are entered. With this information, the software can calculate the exact mL of RBCs required to achieve the desired final hematocrit and HbS. The machine cannot differentiate between HbS and HbA RBCs; therefore, the machine actually calculates the fraction of patient's cells remaining (FCR). Assuming the units are sickle cell negative with no HbS, HbS reflects the patient's RBCs and HbA reflects the transfused RBCs. Therefore, FCR is proportional to HbS:
Pre-aRCE HbS = 75%
Post-aRCE HbS = 30%
FCR = 30/75 = 40%
If some data inputs are unknown or access to the software is not possible, the volume of RBC needed for an exchange can also be estimated by calculating 1.5 times the patient's RBC volume that would be removed/replaced. Over 1 RBC volume is used in the estimate because over the course of the exchange, HbA RBCs will be removed as well. At the beginning of the exchange, primarily HbS RBCs are removed. However, because the machine cannot differentiate HbS from HbA, HbA RBCs are removed as well, and as the exchange progresses, a larger proportion of the RBC volume removed will include the HbA RBCs transfused earlier in the procedure. Estimating 1.5 RBC volume to be removed/replaced accounts for this dynamic. For children, TBV can be estimated to be 70 mL/kg, with the exception of neonates/infants, who have larger blood volume per unit of weight, and obese patients, who have smaller blood volume per unit of weight. Formulas that include height and sex, such as the Nadler equation, can be used to calculate TBV for adults.4
An example calculation of 1.5 RBC volume for a 30 kg child with a hematocrit of 25% (therefore RBC volume is 25% of TBV) with no recent transfusion follows.
TBV = 70 mL/kg × 30 kg = 2100 mL
RBC volume = 2100 mL × 0.25 = 525 mL
1.5 × RBC volume = 787.5 mL
RBC units have an average volume of approximately 300 mL. Assuming adsol units are available for this exchange, the RBC volume of available units would be approximately 165 mL (300 mL * hematocrit 55%).
1.5 × patient's RBC volume is estimated to remove the majority of patient's RBCs to decrease HbS from ~100% (no recent transfusion) to as low as possible. For a 30-kg patient on chronic aRCE with a lower pre-transfusion HbS, 3 units are likely sufficient.
Venous access
The success of aRCE depends on adequate intravenous (IV) access. Two lines are required: 1 draw line to remove whole blood from the patient and 1 return line. The draw line must be capable of supporting adequate flow rates without collapsing against the negative pressure created to pull blood into the device. The flow rate is dependent upon the patient's size. A rate of only 20-35 mL/min is likely sufficient for smaller children (~25-35 kg), whereas a larger adult (80 kg) might require ≥50 mL/min. Our policy is to require a CVC for all acute/urgent aRCE and to attempt peripheral access for all chronic/prophylactic aRCE. We prefer peripheral access for chronic aRCE to avoid complications related to CVCs. In order to optimize chances for success via peripheral IV (PIV) access, we educate all patients about adequate hydration in the 1-2 days prior to aRCE and ensure that patients are warm and calm prior to attempting PIV placement. In addition, apheresis nurses are trained to use ultrasound to guide PIV placement, allowing slightly deeper peripheral veins to be accessed and overall improving the rate of successful PIV placement.
For the draw line, a larger bore PIV capable of supporting the flow and negative pressure is required. A slightly smaller PIV can be placed for the return, but it must be sufficient to withstand the positive pressure of the returning blood. The exact size will depend on the patient size (Table 1). We are able to establish peripheral access in approximately 60% of our chronic aRCE patients, which includes both children and adults. For patients with inadequate peripheral access, a variety of options for chronic aRCE are available. Most commonly, a double lumen implanted port is used. At the time of this writing, only 2 double lumen ports capable of apheresis are available in the US, the 9.5 Fr Bard Power Port Duo and the 11.4 Fr Angiodynamics Vortex, both of which can be used in adults or children whose weight >~45 kg (depending on expertise of surgeon) but not in smaller children. Other options include a single lumen port and PIV return, 2 single lumen implanted ports, a temporary CVC placed and removed on the day of an aRCE, and arterio-venous fistulas or grafts. No option has been demonstrated to be superior, and the choice depends on the patient's preference and anatomy as well as local resources and expertise of the multi-disciplinary team caring for the patient.
Appropriate PIV/CVC for aRCE according to patient weight
| Weight of patient (kg) . | Size of PIV for draw* (Gauge) . | Size of PIV for return (Gauge) . | Size of CVC (French) . |
|---|---|---|---|
| <10** | 5-6 | ||
| 10-30 | 22 | 22 | 6-7 |
| 30-40 | 18-20 | 20-22 | 7-9 |
| 40-50 | 18 | 18-20 | 8-10 |
| >50 | 16-18 | 18 | 9-11.5 |
| Weight of patient (kg) . | Size of PIV for draw* (Gauge) . | Size of PIV for return (Gauge) . | Size of CVC (French) . |
|---|---|---|---|
| <10** | 5-6 | ||
| 10-30 | 22 | 22 | 6-7 |
| 30-40 | 18-20 | 20-22 | 7-9 |
| 40-50 | 18 | 18-20 | 8-10 |
| >50 | 16-18 | 18 | 9-11.5 |
A range of PIV sizes is suggested; however note that some patients may require smaller or larger PIVs based on their individual anatomy and the flow rates achieved.
We have not performed apheresis procedures in patients <10 kg with PIVs.
For patients who require urgent aRCE, we require a temporary CVC, as acutely ill patients may be dehydrated and thus peripheral access could be more difficult to obtain. The CVC must be an appropriate size for the patient (Table 1) and also rigid enough to withstand pressure and flow rates during the procedure. There are a variety of appropriate rigid lines specifically designed for apheresis or dialysis, typically called power lines. The package insert will confirm flow rates supported by a line to ensure appropriateness for aRCE.
One caveat to this discussion of 2 lines for aRCE is the recent introduction of software and tubing connectors to allow a single intravenous access line to function as both draw and return. Rather than simultaneously removing/returning blood, which occurs in the double-needle procedure, the single- needle (SN) procedure involves alternating cycles of first drawing whole blood and exchanging RBCs in the device and then returning blood to the patient through the same line. These discontinuous cycles repeat multiple times until the target parameters are met, resulting in a longer procedure. One group has reported that in their experience with SN procedures using a single lumen port, no difference was seen in pre- or post-HbS levels when compared to double-needle procedures. The group was able to increase flow rates to avoid longer procedure times.5 In our experience using the SN procedure in an adult with 1 PIV, the procedure times are longer than those for the patient's historical double-needle aRCEs. Because SN procedures are not yet widely used, further experience is necessary to define outcomes.
Anticoagulation
An anticoagulant must be used during aRCE to prevent clotting of blood in the machine. Citrate is the most commonly used anticoagulant, as it primarily functions as an anticoagulant in the machine with minimal systemic effects. Citrate binds the calcium ions necessary for activation of calcium-dependent coagulation proteins and is added to the draw line so whole blood is anticoagulated as it enters the machine.6 Upon return of blood to the patient, citrate is quickly metabolized by the liver, kidneys, and skeletal muscle.6
Benefits of aRCE
The benefits of aRCE are due to the removal of patient RBCs, rather than only dilution of patient RBCs, as well as the simultaneous return of blood with the removal.
Isovolemic transfusion
Blood viscosity increases with increasing hemoglobin and patients with SCD have increased viscosity compared to non-SCD controls even at the same hemoglobin level due to lower deformability of HbS RBCs.7,8 Therefore transfusion to a hemoglobin level significantly higher than patient’s baseline can result in symptoms of increased viscosity including hypertension, hemorrhagic stroke and even death.9-14 The ability to remove blood (and decrease HbS) while transfusing RBCs allows an isovolemic transfusion and limits the risk of viscosity.15,16
Mitigation of iron loading
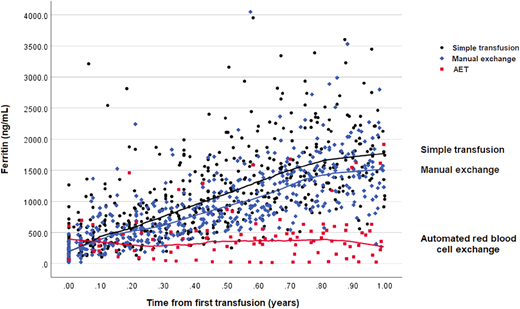
An RBC unit contains approximately 250 mg of iron,17 which accumulates in the liver and, to a lesser degree, in the heart, pituitary and pancreas over time.18 Without chelation, excessive iron can result in liver or heart failure and contribute to mortality in SCD.18-21 Removal of iron-containing RBCs with aRCE can mitigate iron loading, and multiple studies have shown less iron overload with aRCE than with simple transfusion or manual RCE.22-25 Most of these studies were retrospective, and the ability to control for compliance with chelation medication was difficult or impossible. A secondary analysis of the Silent Cerebral Infarct Trial data was able to compare iron loading, as measured by rise in ferritin, that occurred prospectively in the first year of chronic transfusions before chelation was necessary.26 This analysis clearly showed the ability of aRCE to attenuate rise in ferritin (Figure 1). It is important to note that iron balance depends on the difference between the pre- and post-aRCE hemoglobin/hematocrit. If the ordered post-aRCE hematocrit is significantly higher than the pre-aRCE hematocrit, the patient will remain in positive iron balance and chelation will likely be required.
Automated exchange compared to manual and simple blood transfusion attenuates rise in ferritin level after 1 year of regular blood transfusion therapy in chronically transfused children with sickle cell disease.26 Change in ferritin levels in participants of the Silent Cerebral Infarct Trial (n = 83) randomized to transfusions. The median (IQR) ferritin levels after 1 year of transfusion were as follows: 1800 ng/mL (IQR, 1426 to 2204 ng/mL) in simple transfusion participants, 1530 ng/mL (IQR, 1205 to 1805 ng/mL) in manual exchange participants, and 355 ng/mL (IQR, 179 to 579 ng/mL) in automated RBC exchange participants. Figure reprinted from Kelly et al.26
Automated exchange compared to manual and simple blood transfusion attenuates rise in ferritin level after 1 year of regular blood transfusion therapy in chronically transfused children with sickle cell disease.26 Change in ferritin levels in participants of the Silent Cerebral Infarct Trial (n = 83) randomized to transfusions. The median (IQR) ferritin levels after 1 year of transfusion were as follows: 1800 ng/mL (IQR, 1426 to 2204 ng/mL) in simple transfusion participants, 1530 ng/mL (IQR, 1205 to 1805 ng/mL) in manual exchange participants, and 355 ng/mL (IQR, 179 to 579 ng/mL) in automated RBC exchange participants. Figure reprinted from Kelly et al.26
HbS reduction
Exchange transfusion can decrease HbS to a significantly greater extent than can simple transfusion. An apheresis device can accomplish a larger volume exchange and therefore more efficiently lowers HbS than can manual RCE.27 As described earlier in this article, a specific posttransfusion HbS level can be targeted with an individual aRCE procedure. For patients receiving an acute transfusion, the post-HbS level can be more efficiently and reliably decreased with aRCE. For patients treated with chronic transfusions, a specific pretransfusion HbS level is targeted, particularly for patients who undergo transfusion for stroke prevention. The Stroke Prevention Trial in Sickle Cell Anemia demonstrated transfusions that occurred approximately once a month to maintain a pretransfusion HbS of <30% were associated with a 92% reduction (P < 0.001) in stroke risk compared to standard care, making 30% HbS the standard for stroke prevention.28 While most case series have shown lower pretransfusion HbS with chronic aRCE compared to simple or manual RCE,24,25,29,30 this association has not consistently been shown.27 The pretransfusion HbS is harder to predict because it depends on (1) how low the post-HbS level was after the last transfusion, (2) the interval between transfusions, and (3) the patient's endogenous suppression/production of HbS. For an individual patient, the pretransfusion HbS must be monitored. If the HbS level is above target, consider 1 of the following modifications: (1) increase the number of RBC units during aRCE to decrease posttransfusion HbS levels, (2) decrease the transfusion interval, or (3) increase the post-aRCE hematocrit to suppress endogenous HbS production. However, option 3 might not be possible for patients with higher baseline pretransfusion hemoglobin/hematocrit due to viscosity risk. In addition, significantly increasing hemoglobin levels after the procedure with respect to preprocedure levels will negate the potential mitigation of iron loading.
Risk of aRCE
In general, aRCE is well tolerated, even in the acute setting. Possible adverse affects are summarized in Table 2 and are more likely to be related to CVCs than the actual procedure. Because aRCE exposes patients to significantly more blood than simple transfusion, there has been concern of increased risk of transfusion-related adverse events, though aRCE has actually been associated with lower risk of alloimmunization and transfusion reactions.31-33
Adverse effects of aRCE
| Risk . | Clinical comments and mitigation/treatment strategies . |
|---|---|
| Catheter-related complications | |
| Thrombosis | Catheters should be locked with heparin or citrate after the procedure. Many institutions (including ours) also perform a 30-60 minute dwell of thrombolytic medication prior to all procedures. Resistance to draw or flush with frequent procedure alarms may suggest thrombosis, and radiologic evaluation of line such as fluoroscopy should be performed. |
| Infection | Blood cultures should be obtained in any febrile SCD patient with a CVC. High suspicion of catheter related bacteremia if signs of sepsis occur after flushing CVC, and prompt antibiotics are warranted. |
| Migration of line | Tip of line should be near superior vena cava/right atrial junction. Poor flow rates, frequent alarms, or resistance to draw or flush can suggest migration of line from central location and should be evaluated with chest radiograph. |
| Communication between 2 catheters of implanted ports creating recirculation | We have experienced this 3 times over the past 10 years and only suspected recirculation based on no change in HbS and platelets in post-aRCE labs compared to pre-aRCE labs because no alarms or other indications of difficulties occurred during procedure. Communication was diagnosed by fluoroscopy exam and necessitated line replacement. |
| Transfusion reactions | Febrile nonhemolytic transfusion reactions and allergic reactions are the most commonly seen transfusion reactions, though hemolytic and other transfusion reactions are also possible. |
| Hypocalcemia due to citrate toxicity | Citrate-induced hypocalcemia can cause paresthesias or nausea/vomiting though is typically asymptomatic when detected. Ionized calcium can be monitored during the procedure, and we elect to give calcium gluconate infusions through the return line to maintain normal ionized calcium. |
| Hypotension | Changes in blood pressure can occur, though rare, and are typically responsive to normal saline boluses. |
| Symptoms related to fluid shifts | Vasovagal symptoms, abdominal pain, nausea, and vomiting despite normal blood pressure and ionized calcium can be seen, though rare, and are presumed to be due to fluid shifts during the procedure. Normal saline boluses and/or antiemetics can be administered in future procedures for patients who experience these symptoms. |
| Alloimmunization | Prophylactic phenotypically matching RBCs at a minimum for Ce, Ee, and K antigens (in addition to ABO/D) is recommended for SCD.34 Despite this, alloimmunization can occur and if multiple/rare RBC antibodies develop, and it can be difficult to maintain patients on chronic aRCE programs due to the need for rare blood. Note that lower alloimmunization rates with aRCE compared to chronic simple transfusion have been reported despite significantly increased exposure with aRCE.31 |
| Risk . | Clinical comments and mitigation/treatment strategies . |
|---|---|
| Catheter-related complications | |
| Thrombosis | Catheters should be locked with heparin or citrate after the procedure. Many institutions (including ours) also perform a 30-60 minute dwell of thrombolytic medication prior to all procedures. Resistance to draw or flush with frequent procedure alarms may suggest thrombosis, and radiologic evaluation of line such as fluoroscopy should be performed. |
| Infection | Blood cultures should be obtained in any febrile SCD patient with a CVC. High suspicion of catheter related bacteremia if signs of sepsis occur after flushing CVC, and prompt antibiotics are warranted. |
| Migration of line | Tip of line should be near superior vena cava/right atrial junction. Poor flow rates, frequent alarms, or resistance to draw or flush can suggest migration of line from central location and should be evaluated with chest radiograph. |
| Communication between 2 catheters of implanted ports creating recirculation | We have experienced this 3 times over the past 10 years and only suspected recirculation based on no change in HbS and platelets in post-aRCE labs compared to pre-aRCE labs because no alarms or other indications of difficulties occurred during procedure. Communication was diagnosed by fluoroscopy exam and necessitated line replacement. |
| Transfusion reactions | Febrile nonhemolytic transfusion reactions and allergic reactions are the most commonly seen transfusion reactions, though hemolytic and other transfusion reactions are also possible. |
| Hypocalcemia due to citrate toxicity | Citrate-induced hypocalcemia can cause paresthesias or nausea/vomiting though is typically asymptomatic when detected. Ionized calcium can be monitored during the procedure, and we elect to give calcium gluconate infusions through the return line to maintain normal ionized calcium. |
| Hypotension | Changes in blood pressure can occur, though rare, and are typically responsive to normal saline boluses. |
| Symptoms related to fluid shifts | Vasovagal symptoms, abdominal pain, nausea, and vomiting despite normal blood pressure and ionized calcium can be seen, though rare, and are presumed to be due to fluid shifts during the procedure. Normal saline boluses and/or antiemetics can be administered in future procedures for patients who experience these symptoms. |
| Alloimmunization | Prophylactic phenotypically matching RBCs at a minimum for Ce, Ee, and K antigens (in addition to ABO/D) is recommended for SCD.34 Despite this, alloimmunization can occur and if multiple/rare RBC antibodies develop, and it can be difficult to maintain patients on chronic aRCE programs due to the need for rare blood. Note that lower alloimmunization rates with aRCE compared to chronic simple transfusion have been reported despite significantly increased exposure with aRCE.31 |
Indication for aRCE
There have been no randomized trials comparing simple transfusion to aRCE to define when one should be used instead of the other. Recommendations are based on case reports/series or expert opinion (Table 3). In general, in an acute setting, if the hemoglobin level is >2 g/dL lower than patient's baseline, a simple transfusion can be given to increase hemoglobin and improve tissue oxygenation. If the goal of the acute transfusion is to significantly decrease HbS, aRCE should be performed. Automated RBC exchange is most commonly indicated for acute stroke, severe acute chest syndrome, and multiorgan failure. Other indications should be evaluated on a case-by-case basis considering the risks and benefits in the individual patient. Automted RBC exchange should be considered in all patients treated with long term chronic transfusion therapy to mitigate iron loading.34,35
Indications for aRCE
| Indication* . | Relevant literature or guidelines . |
|---|---|
| Acute stroke | RBC exchange (manual or automated) during management of initial stroke was associated with a lower stroke recurrence (21% [8/38]) compared to simple transfusion (57% [8/14])39 Category 1 recommendation by ASFA35 ** |
| Severe acute chest syndrome (ACS) | aRCE shown to reverse hypoxia within 24 hours in 5 patients.40 Comparison of simple transfusion and aRCE in 81 children with ACS showed aRCE was given in more severe cases, yet similar length of stay/clinical course was achieved when compared to less severe cases treated with simple transfusion.41 Suggested by ASH 2020 guidelines for SCD: transfusion support34 and is a category 2 recommendation by ASFA35 ** |
| Multiorgan failure | No randomized trials, but small case series suggest improved outcomes with RBC exchange transfusion.42,43 Of note, recent case reports describe improvement with plasma (rather than RBC) exchange.44-46 |
| Prophylactic preoperative transfusion if hemoglobin >9 g/dL | Transfusion is indicated prior to surgery for patients with SCD due to high risk of perioperative complications in this population. A randomized trial demonstrated that a conservative regimen to achieve hemoglobin 10 g/dL was as effective as an aggressive regimen to decrease HbS <30%,47 therefore aRCE should be used only in patients with high baseline hemoglobin who cannot receive simple transfusion. Suggested by ASH 2020 guidelines for SCD: transfusion support34 |
| Long term chronic transfusion therapy | aRCE suggested by ASH 2020 guidelines for SCD: transfusion support34 and category 1 recommendation by ASFA.35 |
| Indication* . | Relevant literature or guidelines . |
|---|---|
| Acute stroke | RBC exchange (manual or automated) during management of initial stroke was associated with a lower stroke recurrence (21% [8/38]) compared to simple transfusion (57% [8/14])39 Category 1 recommendation by ASFA35 ** |
| Severe acute chest syndrome (ACS) | aRCE shown to reverse hypoxia within 24 hours in 5 patients.40 Comparison of simple transfusion and aRCE in 81 children with ACS showed aRCE was given in more severe cases, yet similar length of stay/clinical course was achieved when compared to less severe cases treated with simple transfusion.41 Suggested by ASH 2020 guidelines for SCD: transfusion support34 and is a category 2 recommendation by ASFA35 ** |
| Multiorgan failure | No randomized trials, but small case series suggest improved outcomes with RBC exchange transfusion.42,43 Of note, recent case reports describe improvement with plasma (rather than RBC) exchange.44-46 |
| Prophylactic preoperative transfusion if hemoglobin >9 g/dL | Transfusion is indicated prior to surgery for patients with SCD due to high risk of perioperative complications in this population. A randomized trial demonstrated that a conservative regimen to achieve hemoglobin 10 g/dL was as effective as an aggressive regimen to decrease HbS <30%,47 therefore aRCE should be used only in patients with high baseline hemoglobin who cannot receive simple transfusion. Suggested by ASH 2020 guidelines for SCD: transfusion support34 |
| Long term chronic transfusion therapy | aRCE suggested by ASH 2020 guidelines for SCD: transfusion support34 and category 1 recommendation by ASFA.35 |
ASH, American Society of Hematology.
For all acute indications for aRCE, the hemoglobin upon acute presentation should be considered and if >2 g/dL below patient's baseline, a simple transfusion may be given first. In particular, this may be a temporizing measure to allow time for line placement and mobilization of apheresis service.
The American Society for Apheresis (ASFA) provides evidenced based recommendation for apheresis procedures: category 1 (first line therapy), 2 (second line therapy), 3 (role of apheresis not established; decision-making should be individualized), 4 (ineffective or harmful).
Cost of aRCE
The cost of an individual aRCE compared to simple or manual RCE is higher due to the cost of the kit, anticoagulation and tubing for the machine, increased blood units required, any central line–related costs, and skilled nursing time. Cost has been reported as a barrier to implementing aRCE programs.36 However, studies that have balanced the cost savings created by decreased chelation, and SCD hospitalizations have actually shown chronic aRCE programs to be cost-effective.24,37,38
Resolution of clinical case and conclusions
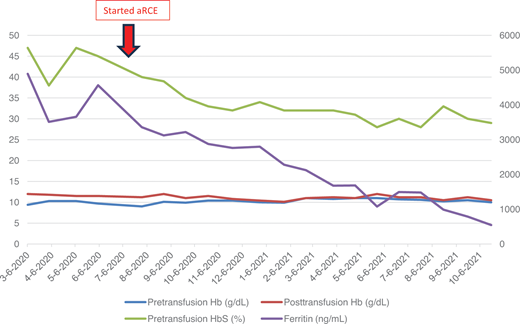
The patient presented in the initial clinical case had a double lumen port placed and was transitioned to aRCE. His course demonstrates the potential benefit of aRCE, as both his HbS and ferritin levels significantly decreased (Figure 2). He did intermittently have issues related to his port, such as repeated need for local thrombolytic medication, highlighting potential adverse effects related to the required vascular access. These risks and benefits must be weighed to make patient-specific decisions for individuals with SCD who require chronic transfusions. Areas of future investigation should include further research regarding the SN procedure; comparing the effects of aRCE with chronic simple transfusion on clinical outcomes, such as prevention of stroke and other severe SCD complications; and examining mechanisms of decreased alloimmunization despite significantly increased RBC exposure with aRCE.
Laboratory values of patient reported in clinical case before and after aRCE. Pretransfusion and posttransfusion hemoglobin (Hb) and hemoglobin S (HbS) are shown on the left axis with units g/dL and percent, respectively. Units for ferritin shown on right axis with units ng/mL. A double lumen port was placed in July 2020, and patient started aRCE in August 2020 using 6 U for each aRCE. Note prior to this, posttransfusion hemoglobin shown in red was typically 11-12 g/dL. Over the next year on aRCE, his posthemoglobin level was closer to his pretransfusion hemoglobin level, typically 10 g/dL. On aRCE, his pretransfusion HbS level decreased and was ultimately maintained closer to his target 30%. His ferritin steadily declined, and he was able to stop chelation medication within 9 months.
Laboratory values of patient reported in clinical case before and after aRCE. Pretransfusion and posttransfusion hemoglobin (Hb) and hemoglobin S (HbS) are shown on the left axis with units g/dL and percent, respectively. Units for ferritin shown on right axis with units ng/mL. A double lumen port was placed in July 2020, and patient started aRCE in August 2020 using 6 U for each aRCE. Note prior to this, posttransfusion hemoglobin shown in red was typically 11-12 g/dL. Over the next year on aRCE, his posthemoglobin level was closer to his pretransfusion hemoglobin level, typically 10 g/dL. On aRCE, his pretransfusion HbS level decreased and was ultimately maintained closer to his target 30%. His ferritin steadily declined, and he was able to stop chelation medication within 9 months.
Conflict-of-interest disclosure
Shannon Kelly: no competing financial interests to declare.
Off-label drug use
Shannon Kelly: nothing to disclose.