Abstract
Advances in the management of sickle cell disease (SCD) have made it possible for most female patients (whether homozygous or compound heterozygous) to reach childbearing age and become pregnant. However, even in the less symptomatic forms of SCD a high risk of complications during pregnancy and the postpartum period can occur for both the mother (1% to 2% mortality) and the fetus. Coordinated care from the obstetrician and the sickle cell disease expert is essential, together with the active participation of the patient. Vaso-occlusive complications, such as vaso-occlusive crisis and acute chest syndrome, often increase in frequency when hydroxyurea treatment is interrupted. Obstetric complications, such as pre-eclampsia, fetal growth restriction, and preterm delivery, are more common in women with SCD. Recent meta-analysis–based studies support prophylactic transfusion. However, there have been no randomized trials assessing the benefits of prophylactic transfusion. Given the known risk of transfusion complications, including delayed hemolytic transfusion reaction and hyperhemolysis, transfusion is not systematically performed in pregnant women with SCD. We describe here a case-by-case approach to the management of pregnancy in women with SCD based on the medical and transfusion history of each patient.
Learning Objectives
Understand maternal and fetal complications during pregnancy in SCD patients
Evaluate the transfusion risk in pregnant women with SCD
Learn how to manage treatment and transfusion in pregnant patients with SCD
Introduction
During pregnancy, sickle cell disease (SCD)–related complications, vaso-occlusive crises (VOCs), acute chest syndrome (ACS), and infections increase in frequency. The rates of obstetric complications, pre-eclampsia, prematurity, intrauterine growth restriction, and mortality are significantly higher in women with SCD than in other pregnant women.1-4 Transfusion (TF)/exchange TF and hydroxyurea (HU) are the 2 most commonly prescribed preventatives and treatments for vaso-occlusive processes in patients with SCD.2,5 HU is not recommended during pregnancy and may be used only in special cases.6,7 There are not enough randomized trials assessing the benefits of prophylactic exchange TF in pregnancy to recommend its systematic use.5,8-10 The risks of a delayed hemolytic TF reaction (DHTR) and alloimmunization must be considered because DHTR is associated with significant morbidity; TFs cannot, therefore, be systematically recommended.11-13
The American Society of Hematology (ASH) Expert Panel concluded that the number of robust randomized trials is insufficient to recommend a systematic prophylactic TF strategy for pregnant women with SCD. Women with a history of severe complications or comorbid conditions related to SCD (nephropathy) or a history of complicated pregnancies could undergo prophylactic TF at regular intervals early in pregnancy. However, no threshold percentage of sickle cell Hb (HbS) has been established as a target for TF.
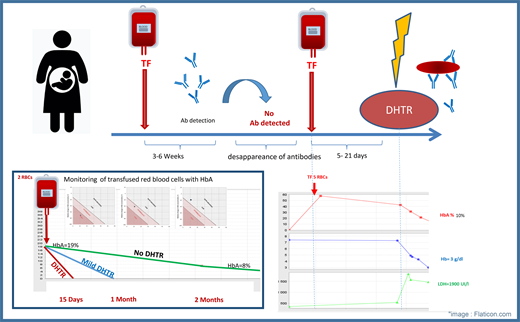
DHTR is a severe TF complication diagnosed by the rapid disappearance of transfused red blood cells (RBCs). The diagnosis is made a few days after a TF with a drop in Hb and an increase in hemolysis markers, often accompanied by a vaso-occlusive complication. The diagnosis can also be made by monitoring HbA, which represents the TF and disappears in 2 forms: “mild DHTR” and “DHTR or hyperhemolysis,” according to the Mekontso nomogram (Visual Summary).14
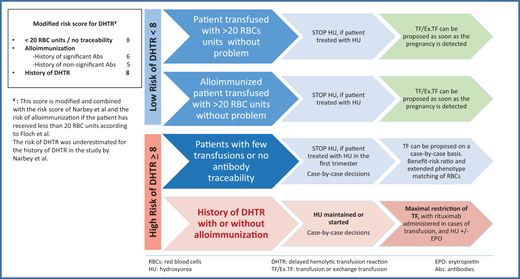
Patients at risk of DHTR are identified using the DHTR risk score developed by Narbey et al.15 This score is based on the number of RBCs received in the patient's lifetime, the presence of alloimmunization, and the patient's history of DHTR. Based on this risk, patients can be identified as “high responders” (patients with alloimmunization after a few TFs) and “low responders” (patients with no immunological reaction to TFs).14,15 Patients with a history of DHTR have a risk score for DHTR greater than 8 and are considered high responders with a greater risk of DHTR recurrence, which is fatal in 6% of cases (Figure 1).13 TFs should be limited as much as possible. Patients with a low DHTR risk score (<8) can undergo TF with no major risk, and patients who have received too few TFs or have undergone TF without antibody tracking are considered to be at risk because it cannot be determined whether they are high or low responders. Such patients are 8 times more likely to develop DHTR.14,16
How to manage TF decisions in pregnant SCD patients. abs, antibodies.
Using clinical cases, we attempt to refine the decision- making process based on TF risk analysis and the algorithms published in the ASH guidelines (Figure 1).
CLINICAL CASE 1
A 26-year-old woman with homozygous SCD was followed for pregnancy. Her basal hemoglobin (Hb) level was 8 g/dL, with approximately 3 VOCs per year and a history of 2 intensive care unit (ICU) admissions for ACS requiring TF with RBCs. HU treatment was initiated after her first hospitalization for ACS. She had received 45 RBC units during her lifetime, without alloimmunization. The patient had no chronic lesions other than minimal microalbuminuria treated with an angiotensin converting enzyme inhibitor (this treatment was stopped during pregnancy). The patient's obstetric history included only 1 early spontaneous miscarriage. HU treatment was stopped when the pregnancy was detected, and the patient was placed on a 6-weekly erythrocytapheresis program. No episodes of VOC or DHTR occurred during the pregnancy. The hematologist and obstetrician visited the patient alternately, at 2-week intervals. Proteinuria did not increase (proteinuria/creatininuria <50 mg/mmol), and blood pressure remained normal. Fetal growth was monitored monthly, with biometrics between the 10th and 20th percentiles. It was decided to induce labor after cervical preparation with prostaglandins at 38 weeks and 1 day. Epidural anesthesia was induced before artificial rupture of the membranes and oxytocin infusion. The patient spontaneously delivered a boy weighing 2705 g. Postpartum blood loss was estimated at 300 mL. The patient decided not to breastfeed in order to resume treatment with HU. No VOCs occurred during the postpartum period, Hb levels remained under control at 7.4 g/dL, and the patient was discharged home after 1 week.
Questions raised by this case: why was the decision to transfuse taken so early? The patient was considered a low responder with a very low TF risk, (DHTR risk score <8). She had already received a large number of RBC units without becoming alloimmunized or suffering any other adverse event. It was therefore considered reasonable to initiate an effective treatment, such as erythrocytapheresis or partial exchange (depending on the patient's venous capital) as soon as possible after stopping HU treatment. The patient's microalbuminuria constituted an additional risk factor for obstetric complications, further justifying the decision to transfuse.2
CLINICAL CASE 2
Mrs. B, a 28-year-old woman with homozygous SCD, was followed for pregnancy. Her baseline Hb level was 8 g/dL. She had 1 to 2 VOCs per year and had never been hospitalized for ACS. She had received only 6 RBC units during her lifetime and was alloimmunized, with anti-Jkb antibody detected at the last TF. The patient had no cardiac or neurological history but had been treated for proliferative retinopathy 1 year before the pregnancy. The patient reported a pregnancy termination for medical reason. The pregnancy that followed was spontaneous. HU treatment was stopped as soon as the pregnancy was discovered. The patient was hospitalized for 1 day for a VOC at 12 weeks' gestation. A second hospitalization for a VOC occurred at 14 weeks' gestation, by which time the patient's Hb concentration had fallen to 5.1 g/dL. The patient received a TF of 2 RBC units in the emergency department in accordance with the standard phenotyping protocol for RBCs. After TF the patient had an HbA level of 36%. One week later, she was readmitted to the hospital for a third VOC with dark urine and high lactate dehydrogenase levels. The patient's HbA level decreased to 15%, and her Hb concentration was 6 g/dL; a diagnosis of DHTR was retained (Figure 2). The patient required no specific treatment for DHTR other than erythropoietin (EPO) and iron. Twenty days after the TF, the patient's HbA level had fallen to 0%, and a nonspecific antibody was detected.
At 19 weeks' gestation, the patient was hospitalized for a fourth VOC and admitted to the ICU for ACS without associated pulmonary embolism. HU treatment was initiated at 20 weeks' gestation. The pregnancy was also characterized by vascular growth retardation (third percentile) at 25 weeks' gestation, followed by pre-eclampsia diagnosed at 30 weeks and 1 day. The patient did not receive antenatal corticosteroids because of the risk of VOC and ACS. It was decided to perform a cesarean delivery at 34 weeks and 5 days due to abnormal fetal heart rate. The cesarean was performed under spinal anesthesia, and a boy weighing 1735 g was delivered and immediately transferred to the neonatal ICU. The patient had another VOC on postpartum day 2 but did not require TF. She chose not to breastfeed in order to continue treatment with HU.
Questions raised by this case: why was the decision to transfuse made so late? The patient was considered a high responder with a high risk of DHTR (few TFs and prior alloimmunization). It was therefore decided not to transfuse early. TF was performed when the patient was admitted to the emergency department for a VOC. The interruption of HU treatment led to a recurrence of VOC and hospitalization. We were forced to manage the recent DHTR in this patient without the possibility of TF. HU with or without EPO is a real alternative in such patients. For patients with a history of DHTR, discussions can be held in advance to determine the true feasibility of TF in an emergency situation (the availability of frozen or liquid-phase units or the unavailability of compatible RBC units) and the timing of immunotherapy, such as rituximab before TF (off-label).16-19
Background and current knowledge about pregnancy in SCD patients
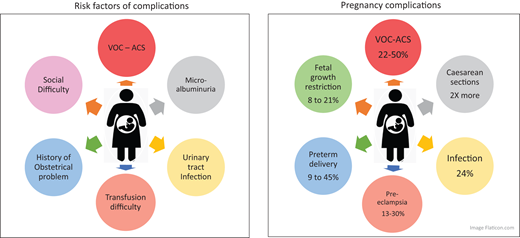
Improvements in the effectiveness of care for women with SCD have made it possible for many of them to become mothers, whereas in previous decades pregnancy was not recommended or was associated with very high-risk mortality rates.4 However, the risk of maternal death remains 50 times higher in these women than in the general population (Figure 3).2,20 Obstetric risks include preterm delivery, which has been reported in 9% to 45% of pregnancies in women with SCD, depending on the series considered.1,2,4,8,21 Several social and obstetric factors may account for the high risk of preterm delivery, including infections, particularly those affecting the urinary tract. It is important to ensure that patients receive appropriate doses of antibiotics relative to their glomerular filtration rate. Razazi et al. showed that plasma antibiotic levels were very low in patients with glomerular hyperfiltration and that young SCD patients often had high glomerular filtration rates.22
Pre-eclampsia is much more common in SCD patients than in the general population, occurring in 13% to 30% of pregnancies in women with SCD, vs only 2% to 5% of pregnancies in the general population.3,23,24 Preexisting maternal endothelial dysfunction and early placentation abnormalities account for this high incidence.
The incidence of fetal growth retardation ranges from 8% to 21%, depending on the study, and increases the likelihood of induced preterm birth and the risk of fetal or neonatal death.1,2 This growth retardation may be due to chronic fetal hypoxia and rheological abnormalities of maternal red blood cells in the placenta, as placental lesions are often found on pathology examination. Cordier et al recently showed that the placental villi of mothers with SCD were thinner than those of controls and that the rate of fibrinoid necrosis was higher in these mothers, who also had overabundant syncytial ganglia.18 Mifsud reported patchy placental infarction and fetal growth restriction relative to controls.25 The risk of fetal death is higher in this population than in the general population (1-4%). The causes of this higher risk remain unclear, but the risk appears to be highest during the third trimester.23 Women with SCD undergo physiological changes during pregnancy, with increases in both anemia rates and cardiac output. Special attention must be paid to cardiac ultrasound findings for these patients.2,26
More than 50% of pregnancies in women with SCD end in cesarean delivery.1,2,15 This high rate of cesarean delivery is due to a combination of several hematologic and obstetric risk factors, including a higher incidence of fetal growth restriction and pre-eclampsia and induced labor. The incidence of ACS increases in the postpartum period, and clinicians must be particularly attentive to the possibility of this complication.1,27
How should treatment be managed?
A large proportion of women with SCD are on HU treatment due to acute complications, such as VOC or ACS. Given the small number of publications on HU exposure during pregnancy, evidence on its safety is limited, and current recommendations state that HU treatment should be stopped 3 to 6 months before conception. HU is known to be teratogenic in animals, especially during the first trimester of pregnancy, but the doses used in animal studies are very different from those used in human treatment.6 Kroner et al performed a retrospective multicenter study of 1788 pregnancies, 241 of which were exposed to HU.7 Hydroxyurea increased the rate of spontaneous abortion and fetal death in utero when used just before and during early pregnancy, whereas the use of HU in the second and third trimesters appeared to improve pregnancy outcomes.7 However, TFs and their effect were not considered in this study. The ESCORT-HU study, a European prospective multicenter study of a cohort of 1960 patients, found no evidence of teratogenicity in the 110 pregnancies most exposed in the first trimester to HU. Additionally, the rate of miscarriage was similar to that in the general population, and obstetrical complications were similar to published incidence of complications in SCD women. However, these studies do not provide sufficient evidence to recommend the systematic use of HU during pregnancy.6
Transfusion and its risks
Most SCD patients have undergone TF/exchange TF procedures during their lifetime, regardless of the region or country in which they live. Several groups have reported the use of TF or exchange TF during pregnancy but not in randomized trials.7,15 A systematic review and meta-analysis suggested that prophylactic TF strategies may reduce maternal and neonatal morbidity and mortality.7 In addition, several groups have shown that TFs can effectively reduce the incidence of vaso-occlusive complications, such as VOC and ACS during pregnancy.5,8,10,23 When clinicians are considering the possibility of TF, they must take into account the risk of DHTR and alloimmunization associated with TF.13 The detection of placental abnormalities may lead to a decision to use TF in the early stages of pregnancy, during placental development, to improve blood circulation.23,27 In most of the studies reporting no obstetric benefit of TF, exchanges were not initiated during the first trimester of pregnancy.5,15 Knowledge of a patient's TF history is a key element to take into account when determining the risk/benefit ratio and deciding whether or not to proceed with TF. The indications for TF have changed significantly with improvements in our understanding of TF complications, including DHTR in particular. The immunohematological follow-up of patients remains an important issue due to the evanescence and secondary disappearance of antibodies detected at a given time.8,10,21 RBCs must be compatible for Rh and Kell phenotypes, taking into account the patient's history of TF-related antibodies. TF protocols are continually updated according to the patient's immunohematological status.14-16 In a series of 99 cases of DHTR, 31% of affected patients were pregnant women.3 In this study performed between 2000 and 2013, patients were transfused systematically from the 22nd week of pregnancy, regardless of the patient's TF history or number of previous TFs.15 Narbey et al attempted to identify risk factors for DHTR and found that patients who had previously received fewer than 12 RBC units were at risk because their low- or high-responder status could not be determined.9 Other risk factors identified were a history of DHTR or alloimmunization.9 Bauer et al reported an excess risk of alloimmunization in pregnant women.11 Based on the recent study by Floch et al showing that the majority of alloimmunizations of interest occur before the TF of 20 RBC units and the latest guidelines for the prevention of alloimmunization, we recommend the approach described in Figure 1.28
TFs: who should receive them, when and how?
When assessing the risk of alloimmunization and hemolysis, patients can be classified into 4 groups (Figure 1:
- 1)
Patients who have received more than 20 RBC units without adverse effects: These patients can be transfused according to the usual indications and recommendations. Patients treated with HU can be transfused as soon as HU is stopped, and patients without HU treatment can be transfused at the first sign of a clinical or biological problem or according to their history.11
- 2)
Alloimmunized patients with no history of DHTR who have received more than 20 RBC units with no adverse event can undergo TF at the time of pregnancy detection if they were on HU treatment. TF can also be initiated for patients not on HU treatment at the time of pregnancy diagnosis or at the first sign of a clinical or biological problem.15,16
- 3)
Patients with a history of DHTR: These patients are at high risk of DHTR if they undergo TF. If they are already on HU treatment, it is probably advisable to continue treatment, as vaso-occlusive complications may be more frequent if this treatment has already been prescribed. For patients not already on HU, this treatment should be initiated at the first clinical and laboratory signs of complications. EPO may also be used to treat anemia. The indications for TF in these patients are very limited. If an acute complication renders TF unavoidable, patients should receive prior immunotherapy (anti-CD20 antibody “off-label”), and TF-sparing measures (HU and high-dose EPO) can be implemented. The recommended TF protocol includes prophylactic expanded phenotype (FY, JK, MNS) matching for RBCs, regardless of the patient's alloimmunization status, and taking known antibodies into account. Outside the context of life-threatening emergencies, decisions about TF should be made in collaboration with experts and the TF center.10-13,22
- 4)
Patients who have received very few TFs (<20 RBC units) or who have been transfused without antibody traceability: In these patients, the risk/benefit ratio of TF must be carefully evaluated due to the absence of information about responder status and the likelihood of TF reactions. In such cases, we recommend prophylactic extended phenotype (FY, JK, MNS) matching for RBCs, with weekly monitoring of HbA levels to monitor efficacy and the use of Mekontso nomograms to detect mild DHTR.29 In case of complications, it should be borne in mind that DHTR remains a rare event and that TFs can be helpful. Finally, very complex situations may arise in patients with a rare blood phenotype who are also alloimmunized. In such cases the TF strategy must be discussed by a multidisciplinary team. Decisions depend on the resources available in the national rare blood bank, but alternatives to TF may be considered (HU, EPO). In all cases, TF performance should be monitored for up to 1 month by determining the percentage of HbA and through TF screening tests.14,29
Other actions: The National Institutes of Health recommends aspirin treatment, at a dose of 160 mg per night, in pregnant women with SCD.2,30 Ribeil et al showed, in a retrospective study, that home oxygen therapy at night may be safe and may reduce the need for TF.12 The results of a randomized trial of oxygen therapy are currently being analyzed. Teratogenic treatments, such as iron chelators and enzyme conversion inhibitors, are generally discontinued during pregnancy in accordance with recommendations.
Conclusion
Pregnancy is a high-risk period for both mother and fetus, but TFs can provide protection if the risk of DHTR is low. The alternative to TF in patients with a history of DHTR is HU. In other cases, the ASH Expert Panel concluded that each case should be discussed individually to ensure the most appropriate decision is made. Collaboration between the hematologist, obstetrician, TF center, and anesthesiologist is critical in the management of pregnant women with SCD.
Conflict-of-interest disclosure
Anoosha Habibi: consultancy: GBT, Vertex, Novartis, addmedica.
Alexandra Benachi: no competing financial interests to declare.
Edouard Lecarpentier: no competing financial interests to declare.
Off-label drug use
Anoosha Habibi: Rituximab, erythropoietin, hydroxycarbamide.
Alexandra Benachi: Rituximab, erythropoietin, hydroxycarbamide.
Edouard Lecarpentier: Rituximab, erythropoietin, hydroxycarbamide.