Abstract
Iron deficiency is a very common and treatable disorder. Of all the tests available to diagnose iron deficiency, the serum ferritin is the most able to discriminate iron deficiency from other disorders. However, the reference range for ferritin in many laboratories will lead to underdiagnosis of iron deficiency in women. Studies have shown that 30%-50% of healthy women will have no marrow iron stores, so basing ferritin cutoffs on the lowest 2.5% of sampled ferritins is not appropriate. In addition, several lines of evidence suggest the body physiologic ferritin “cutoff” is 50 ng/mL. Work is needed to establish more realistic ferritin ranges to avoid underdiagnosing a readily treatable disorder.
Learning Objectives
Understand why serum ferritin is the best test to diagnose iron deficiency
Learn why iron deficiency in women is vastly underdiagnosed
CLINICAL CASE
A 24-year-old runner self-refers to hematology because of fatigue and decreased exercise performance. Over the past year, her running pace has slowed and she tires more readily with exertion. Now when she rock climbs, she also notes more muscle fatigue and has noticed diminished concentration at work. She is concerned her symptoms may be related to iron deficiency after reading an article in a running magazine. Upon taking a menstrual history, she states she had menarche at age 11 and has regular menses every 28 days, which last around 5 days. On her heaviest days she has to change her tampon every 2 hours and wakes up at night to change her pad. She experiences flooding around once per cycle. Initially, she presents to an urgent care clinic for further evaluation but was told not to worry as her hemoglobin was normal. Given her progressive symptoms, she is seen at another urgent care clinic, where a serum ferritin was obtained and returns at 10 ng/mL. Given the lower limit of normal of ferritin at this clinic was 8 ng/mL, she was reassured that her iron stores were normal.
Introduction
Iron deficiency is one of the leading contributors to the global burden of disease, disproportionately impacting women of reproductive age and people living in low- and middle-income countries.1 It has become apparent that the prevalence of iron deficiency is much higher than what has been appreciated in the past, in part due to a lack of standardized guidelines to diagnose iron deficiency, as well as provider misconceptions on how to accurately interpret iron studies. As a result, iron deficiency is often overlooked because blood counts or ferritin fall within “normal range.” This article reviews testing for iron deficiency, the wide array of symptoms associated with iron deficit even in the absence of anemia, and the need for more appropriate and standardized laboratory reference ranges for serum ferritin.
What is ferritin?
Ferritin is the cellular storage molecule for iron.2 Ferritin consists of 24 subunits that form a hollow sphere with 6 openings that allow up to 5000 atoms of iron to be stored inside, permitting the cell to store a large amount of iron without concern of free iron catalyzing toxic reactions. The organs that contain the highest concentration of ferritin are the liver and reticuloendothelial system in the spleen and bone marrow.
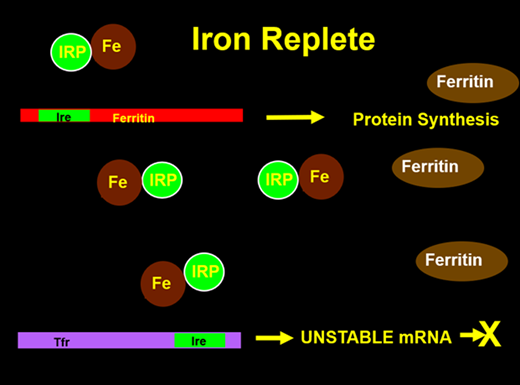
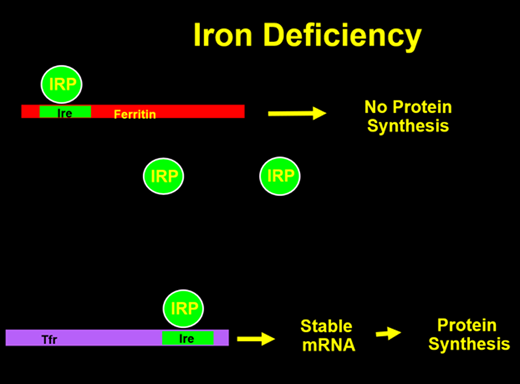
The cellular production of ferritin is tightly regulated by total iron stores within the body3 (Figures 1 and 2). The 5’ end of the ferritin messenger RNA (mRNA) contains a sequence known as the iron response element, upon which a protein called the iron regulatory protein (IRP) binds in the absence of cellular iron. When this binding occurs, translation of ferritin mRNA into protein cannot occur, thus making iron more readily available for cellular use. When iron is present, it can bind to the IRP, which then dissociates from the IRE, allowing ferritin protein synthesis to occur. The regulation of the transferrin receptor (TfR) is also controlled by a 3’ IRE in its mRNA. Conversely, when the IRP is bound, the mRNA is stabilized, allowing for increased protein synthesis leading to mobilization and use of iron. With abundant cellular iron, the IRP is released, leading to destabilization of the TfR mRNA and decreased synthesis. By this elegant mechanism, iron stores can control the production of key iron metabolism proteins.
With cellular iron, the IRP binds iron and releases it from the ferritin IRE to allow translation of mRNA to ferritin protein while destabilizing the transferrin receptor mRNA.
With cellular iron, the IRP binds iron and releases it from the ferritin IRE to allow translation of mRNA to ferritin protein while destabilizing the transferrin receptor mRNA.
With no cellular iron, the IRP binds the ferritin IRE to blocking translation of mRNA to ferritin protein while stabilizing the transferrin receptor mRNA to allow protein synthesis.
With no cellular iron, the IRP binds the ferritin IRE to blocking translation of mRNA to ferritin protein while stabilizing the transferrin receptor mRNA to allow protein synthesis.
Why do we use ferritin as a measure of iron stores?
Traditional tests to diagnose iron deficiency have key limitations that affect their clinical utility (Table 1). For example, serum iron levels are low both in inflammation and iron deficiency, and levels can vary based on dietary intake. Total iron binding capacity, which is typically elevated in iron deficiency, is a specific but not sensitive finding, as inflammation, age, and malnutrition can falsely lower levels. Transferrin saturation is low both in iron deficiency and in inflammation, limiting its utility in differentiating 2 of the most common forms of anemia. Reticulocyte hemoglobin content is low with any iron-deficient erythropoiesis and therefore cannot discriminate between anemia of inflammation and iron deficiency.4 Finally, a low mean corpuscular volume is a late finding of iron deficiency with several cofounders, such as liver disease, alcohol use, among others, that prohibit its specificity.
Tests for iron deficiency
| Test for iron deficiency . | Limitations . |
|---|---|
| Mean corpuscular volume | Decreases late in iron deficiency, confounded by liver disease, alcohol use, etc |
| Serum iron | Decreased in inflammation, daily variation, affected by diet |
| Total iron binding capacity | Not sensitive as in the setting of iron deficiency can be decreased by inflammation and malnourishment |
| Iron saturation | Low in inflammation |
| Free erythrocyte protoporphyrin | Affected by inflammation |
| Red cell distribution width | Not specific for iron deficiency |
| CHr (Ret-He) | Can also be abnormal in inflammation or thalassemia |
| Ferritin | Best performing test |
| Test for iron deficiency . | Limitations . |
|---|---|
| Mean corpuscular volume | Decreases late in iron deficiency, confounded by liver disease, alcohol use, etc |
| Serum iron | Decreased in inflammation, daily variation, affected by diet |
| Total iron binding capacity | Not sensitive as in the setting of iron deficiency can be decreased by inflammation and malnourishment |
| Iron saturation | Low in inflammation |
| Free erythrocyte protoporphyrin | Affected by inflammation |
| Red cell distribution width | Not specific for iron deficiency |
| CHr (Ret-He) | Can also be abnormal in inflammation or thalassemia |
| Ferritin | Best performing test |
For reasons that are poorly understood, small amounts of ferritin leak from the cytoplasm into the blood when synthesized to store cellular iron. Serum ferritin levels correlate with total body iron stores with 1 ng/mL of ferritin equating to 8-10 mg of storage iron.5 While it is true that, as an acute phase reactant, ferritin can be markedly elevated in inflammation, this is only the case with good iron stores. Recall that ferritin protein synthesis is dependent on the presence of cellular iron. With concurrent inflammation and iron deficiency, lack of iron prevents the translation of ferritin mRNA into protein, thus blunting any extreme rise in serum ferritin (over 100 ng/mL).
In a systematic review of the literature, Guyatt et al concluded that serum ferritin “was by far the most powerful test” for diagnosing iron deficiency.6 This test strongly outperformed other traditional tests of iron deficiency, including red cell protoporphyrin, mean corpuscular volume, transferrin saturation, and red cell distribution width. This review noted that a ferritin level of over 100 ng/mL ruled out absolute iron deficiency (absent iron stores), while a lower limit was dependent on the clinical scenario. A newer test of iron status, serum soluble transferrin receptor (sTR), has been proposed to differentiate anemia related to iron deficiency from chronic inflammation, in which sTR would be expected to be elevated in iron deficiency but not impacted by inflammation.7 However, an important caveat is that sTR can be elevated in any condition associated with increased erythropoietic activity/ineffective erythropoiesis, leading to potential misinterpretation and diagnosis of iron deficiency.8
Are low iron stores without anemia an issue?
While adverse effects of iron deficiency anemia have been recognized for over a century, it is increasingly appreciated that low iron stores with normal blood counts can also lead to clinical complications (Table 2). Three studies have shown that administering iron to women with normal blood counts and ferritin levels less than 50 ng/mL significantly improved symptoms of fatigue.9-11 A systematic review and meta-analysis also demonstrated the detrimental effects of low iron stores and exercise, with the supplementation of iron leading to improved aerobic capacity (VO2 max) and athletic performance.12 Supplementing iron in nonanemic iron-deficient adolescent girls also improved tests of verbal learning and memory.13 Finally, restless legs syndrome is another symptom resultant of low iron stores that resolves with iron supplementation.14
Symptoms of iron deficiency
| Alopecia |
| Decreased exercise performance |
| Fatigue |
| Impaired cognition |
| Impaired thyroid function |
| Increased bruising |
| Increased susceptibility to acute mountain sickness |
| Pica |
| Pruritus |
| Restless legs |
| Alopecia |
| Decreased exercise performance |
| Fatigue |
| Impaired cognition |
| Impaired thyroid function |
| Increased bruising |
| Increased susceptibility to acute mountain sickness |
| Pica |
| Pruritus |
| Restless legs |
A potential explanation of the adverse effects seen with low iron stores in the absence of anemia is that anemia reflects an end stage complication of progressive iron deficiency. As iron stores fall, iron is stripped out of muscles and other tissue to maintain adequate erythropoiesis. Interestingly, a study supporting this hypothesis found a direct correlation with muscle iron depletion as serum ferritin fell from 75 ng/mL to 36 ng/mL.15 Thus waiting for anemia to develop, instead of acknowledging the symptomatic implications of iron deficiency, will result in underdiagnosis of a very treatable and preventable disease.
How common are low iron stores in women?
Because of obligate menstrual losses, women are at higher risk of iron deficiency. Menstrual losses can average 35 mL of blood (16 mg of iron) per cycle, leading to higher dietary iron requirements, which are often not met by diet alone or hindered by inadequate gastrointestinal absorption. The recommended iron intake for women is 18 mg/day, but an Institute of Medicine Report shows that on average, the intake of iron ranges from only 12.6-13.5 mg/day.16
When iron losses chronically outpace iron intake, iron deficiency develops. In a 1966 study, investigators performed bone marrow studies on 114 healthy college women and found that 24% had no stainable iron in their marrow, and another 37% had depleted (1+) iron stores.17 These remarkable findings have been further validated by Puolakka et al, who found absent marrow iron in 50% of healthy women,18 and Hallberg et al, who showed absent iron stores in 34%.19 Thus, due to the inability to keep up with iron losses, a substantial number of women are iron deficient.
Should women have a different ferritin reference range than men?
Established reference ranges are historically derived from values observed in 95% of individuals within a sample population.20 Commonly cited flaws with this approach include the nonrepresentative sample populations, the assumption of a Gaussian distribution, and the fact that only 2.5% of values are pathologic. However, since ~30%-50% of women have absent iron stores in their marrow, deriving a cutoff at the lowest 2.5% will both dramatically underdiagnose iron deficiency and result in an inappropriately low lower limit of normal. There is no physiologic reason that ranges of normal serum ferritin should differ between men and women; rather, this reflects the fact many women have little to no total body iron stores. The use of population normal for ferritin's cutoff is reminiscent of decades ago when a “normal” cholesterol was said to be under 300 mg/mL. To reiterate, most laboratories use a descriptive statistical finding for their ferritin reference range instead of one that reflects the reality of the high prevalence of iron deficiency. Therefore, there is a critical need to develop a standardized lower limit of ferritin that more appropriately reflects physiology to assist in accurate diagnosis of low iron stores.
What should the ferritin lower limit be to diagnose iron deficiency?
Several studies have employed a physiologic approach to determine a more accurate ferritin lower limit. Since iron absorption in the gastrointestinal tract can increase severalfold in iron- deficient states, a study using absorption of a stable iron isotope showed this physiologic compensation does not return to baseline until the serum ferritin is over 50 ng/mL, perhaps reflecting a more precise threshold of iron deficiency.21 Another study using sensitive biomarkers of iron depletion—soluble transferrin receptor and hepcidin—confirm the use of 50 ng/mL as a physiologic cutoff.22 Importantly, this proposed threshold corresponds with the above-mentioned studies that show repletion of the serum ferritin to over 50 ng/mL reduces fatigue (Table 3).
Why the ferritin cutoff should be 50 ng/mL
| • Gastrointestinal absorption of iron returns to baseline at 50 ng/mL |
| • Clinical trials show this cutoff is associated with fatigue |
| • Biochemical markers of iron deficiency normalize at 50 ng/mL |
| • Gastrointestinal absorption of iron returns to baseline at 50 ng/mL |
| • Clinical trials show this cutoff is associated with fatigue |
| • Biochemical markers of iron deficiency normalize at 50 ng/mL |
What are the clinical implications?
Laboratory reference ranges are essential for the accurate interpretation of test results and clinical decision making; however, inappropriate ranges may inadvertently contribute to inequitable care, particularly among women. Clinical implications of using ferritin ranges that do not accurately reflect women's iron stores have led to systemic underdiagnosis and underrecognition of iron deficiency. Additionally, flawed reference ranges also limit eligibility for coverage of parenteral iron therapy. It is the unfortunate truth that many women are denied parenteral iron therapy until their untreated iron deficiency progresses to anemia, resulting in significant morbidity.
Those who disagree with raising the serum ferritin cutoff have voiced concern that this change will dramatically increase the number of women diagnosed with iron deficiency. Ironically, however, this highlights the crux of the issue. Given resounding evidence that many women are iron deficient and symptomatic, greater emphasis on developing ferritin ranges using physiologic data can lead to more accurate estimation of the global burden of disease.
Another key question that clinicians should consider is if hematologic reference ranges for hemoglobin/hematocrit are appropriate, acknowledging the high prevalence of women with iron deficiency. While men have higher upper ranges for their hemoglobin due to the effects of testosterone, there is no physiologic explanation for why women should have a lower hemoglobin compared to men. And, as such, there are several studies to support that, among iron-replete women, the hemoglobin lower limit of normal does not differ. As people age, the reference ranges for men and women draw closer together.23 A study conducted in 1936 showed that iron administration raised women's hemoglobin by 10%. Interestingly, the discussion included the prescient comment that “the accepted ‘normal’ for women's heamoglobin may not be a true normal, but should perhaps be regarded as mildly pathological.”24 A paper published in 1967 also showed that iron supplementation in a “normal control” group of women resulted in an increase in hemoglobin by -1 g/dL.25 Taken together, these findings highlight sex-based inequities that lead to normalization of disease states and the critical need to update hematologic ranges truly reflective of iron repletion.
What should be done?
There are several ways to improve recognition and treatment of this common clinical scenario (Table 4). First, we propose broader implementation of educational programs highlighting the prevalence and clinical implications of undiagnosed and untreated iron deficiency. Second, hematologists need to collaborate with their hospital laboratory leadership to develop new ferritin reference ranges that allow for accurate diagnosis of iron deficiency. Finally, standardized guidelines to diagnose and treat iron deficiency may allow for more accessible, consistent, and widespread implementation. Ultimately, hematologists are in a prime position to substantially decrease the global burden of disease by more aggressively screening and correcting iron deficits. This is another example of how hematologists are on the front lines of combating structural inequality in medicine.26
Action steps
| • Educate your colleagues about |
| ○ The symptoms that can be seen with iron deficiency without anemia |
| ○ The very high incidence in women |
| ○ The need for appropriate laboratory ranges for ferritins |
| • Work with your hospital laboratory to provide accurate ferritin reference ranges |
| • Work to establish accurate reference ranges for women's hemoglobin/hematocrit cutoffs |
| • Educate your colleagues about |
| ○ The symptoms that can be seen with iron deficiency without anemia |
| ○ The very high incidence in women |
| ○ The need for appropriate laboratory ranges for ferritins |
| • Work with your hospital laboratory to provide accurate ferritin reference ranges |
| • Work to establish accurate reference ranges for women's hemoglobin/hematocrit cutoffs |
CLINICAL CASE (continued)
The patient was appropriately diagnosed with iron deficiency and treated with oral iron supplementation. Within weeks, she noticed improvement in her exercise capacity and her ability to concentrate at work. In addition, her astute hematologist thoroughly asked about reversible causes of iron deficiency and diagnosed her with heavy menstrual bleeding as the likely cause of ongoing blood loss. She was referred to a gynecologist with prompt resolution of her heavy periods with the placement of a progesterone intrauterine device.
Conflict-of-interest disclosure
Kylee Martens: no competing financial interests to declare.
Thomas G. DeLoughery: no competing financial interests to declare.
Off-label drug use
Kylee Martens: nothing to disclose.
Thomas G. DeLoughery: nothing to disclose.