Abstract
B-cell maturation antigen (BCMA)–directed therapies, including antibody-drug conjugates, bispecific antibodies (BsAbs), and chimeric antigen receptor T cells (CARTs), have shown remarkable efficacy in patients with late-line myeloma with prior exposure to immunomodulatory agents, proteasome inhibitors, and anti-CD38 antibodies. However, optimal sequencing of these agents remains to be determined, and management of these patients once they relapse has become a new unmet need. Fortunately, there are multiple options with demonstrated activity after anti-BCMA therapy, including a different BCMA-directed therapy, non-BCMA-directed CARTs and BsAbs, novel non–T-cell–engaging drugs, and standard triplet/quadruplet regimens or salvage stem cell transplant. Factors to consider when choosing a next therapy after anti-BCMA therapy include patient characteristics and preferences, prior therapies and toxicities, disease biology, timing from last anti-BCMA therapy, and, in the future, BCMA expression and immune profiling. While current data are limited to retrospective studies and small prospective cohorts, the serial use of T-cell–engaging therapies looks particularly promising, especially as BCMA-directed therapies move up earlier in the myeloma treatment course and additional CARTs and BsAbs against alternative targets (eg, G protein–coupled receptor, family C, group 5, member D and Fc receptor-homolog 5) become available. Going forward, ongoing prospective studies, large real-world data sets, and better tools to interrogate antigen expression and immune cell fitness hopefully will provide further insight into how to best individualize therapy for this difficult-to-treat population.
Learning Objectives
Recognize currently available B-cell maturation antigen–targeted therapies for relapsed/refractory myeloma
Identify the potential treatment options available after progression on these therapies
Understand the expected safety and efficacy profiles of these treatment options
CLINICAL CASE
A 60-year-old man is diagnosed in 2015 with IgA κ multiple myeloma, International Staging System (ISS) stage 2 with deletion 13q and gain 1q by fluorescence in situ hybridization. He achieves a very good partial response (VGPR) after bortezomib, lenalidomide, and dexamethasone induction, followed by autologous stem cell transplant and lenalidomide maintenance. His disease progresses in 2019, and he subsequently progresses on daratumumab, pomalidomide, and dexamethasone; cyclophosphamide, bortezomib, and dexamethasone; and carfilzomib, pomalidomide, and dexamethasone. Most recently, he has received idecabtagene vicleucel (ide-cel), achieving complete response, but relapses 10 months later with new bone lesions and anemia. He is now 68 with normal renal function and Eastern Cooperative Oncology Group (ECOG) performance status of 1.
B-cell maturation antigen–directed therapies in late-line, relapsed/refractory myeloma
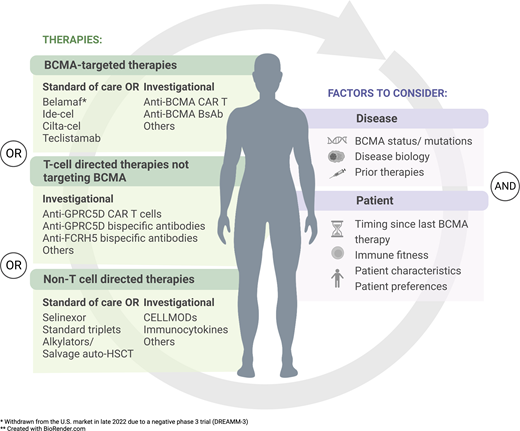
B-cell maturation antigen (BCMA), a cell surface receptor expressed on plasma cells, is now well established as a target for myeloma therapy.1 Several BCMA-targeted therapies have activity in relapsed/refractory myeloma, including antibody-drug conjugates (ADCs), bispecific antibodies or T-cell engagers (BsAbs), and chimeric antigen receptor T cells (CARTs). As of mid-2023, 4 of these had received regulatory approval—belantamab mafodotin (belamaf, an ADC), ide-cel (CART), ciltacabtagene autoleucel (cilta-cel, CART), and teclistamab (BsAb)—all for patients with myeloma who had at least 4 prior lines of therapy (3 prior lines in Europe), including a proteasome inhibitor, an immunomodulatory agent (IMID), and an anti-CD38 antibody (ie, “triple-class exposed”). Registration efforts are under way for several additional agents as well (Table 1). Of note, belantamab mafodotin was withdrawn from the US market in late 2022 due to a negative phase 3 trial (DREAMM-3)2 but remains available commercially outside the United States, and in the United States via an expanded access program, with other phase 3 trials ongoing.
Approved and selected investigational BCMA-targeted therapies for use in late-line MM*
| Agent (reference) . | Construct . | Trial (NCT#, status) . | Phase . | Design . | n . | % ORR (% ≥CR); median DOR in months (95% CI); median PFS in months (95% CI) at reported median follow-up . | Selected safety event % (G3 + 4%, if any) . |
|---|---|---|---|---|---|---|---|
| Belantamab mafodotin (belamaf)3† | Antibody-drug conjugate | DREAMM-2 (NCT03525678, active, not recruiting) | 2 | Open-label, 2-arm, randomized to receive 2.5 mg/kg or 3.4 mg/kg RP2D | 196 | 2.5 mg/kg: 31% (3% ≥ CR), 13.7 (9.9-NE); 2.9 (2.1-3.7) at 13 months 3.4 mg/kg: 34% (3% ≥ CR); NR; 4.9 (2.3-6.2) | 2.5 mg/kg: keratopathy 70% (27%), thrombocytopenia 35% (20%), anemia 24% (20%) 3.4 mg/kg: keratopathy 75% (21%), thrombocytopenia 54% (30%), anemia 37% (25%) |
| Idecabtagene vicleucel (ide-cel)5 | Autologous CART | KarMMa-1 (NCT03361748, active, not recruiting) | 1/2 | Open-label, single-arm, dose escalation and dose expansion | 128 | ORR 73% (33% ≥ CR); 10.7 (9.0-11.3); 8.8 (5.6-11.6) | CRS 84% (5%), neurotoxicity 18% (3%), neutropenia 91% (89%), anemia 91% (60%), thrombocytopenia 63% (52%), hypogammaglobulinemia (21%) |
| Ciltacabtagene autoleucel (cilta-cel)7 | Autologous CART | CARTITUDE-1 (NCT03548207, completed) | 1/2 | Open-label, single-arm, dose escalation and dose expansion | 97 | 97% (sCR 82.5%); 33.9 (25.5-NE); 34.9 (25.2-NE) | CRS 95% (4%), neurotoxicity 21% (9%), neutropenia 91% (89%), anemia 93% (95%), thrombocytopenia 81% (68%) |
| Teclistamab6‡ | BsAb (humanized IgG4) | MajesTec-1 (NCT04557098, recruiting) | 1/2 | Open-label, nonrandomized, IV or SC teclistamab in RRMM, dose expansion and dose escalation | 165 | 63% (43% ≥ CR); 24 (24-NE); 12.5 (8.8-17.2) at 22 months | CRS 72.1% (0.6%), neurotoxicity 14.5% (0.6%), neutropenia 70.9% (64.2%), anemia 52.1% (37%), pneumonia 18.2% (12.7%), COVID-19 17.6% (12.7%), hypogammaglobulinemia 74.5% (0%) |
| Elranatamab38‡ | BsAb (humanized IgG2a) | Magnetissm-3 (NCT04649359, active, not recruiting) | 2 | Open-label, multicenter, nonrandomized, single-agent SC | 123 | 61% (28% ≥ CR); NE (12-NE); NE (10.4-NE) at 10.4 months | CRS 57.7% (0%), neurotoxicity 3.4% (0%), peripheral neuropathy 17.1% (0.8%), infections 66.7% (35%) |
| Linvoseltamab (REGN5458)39‡ | BsAb (Veloci-Bi antibody) | LINKER-MM1 (NCT03761108, active, not recruiting) | 1/2 | Open-label, multicenter, nonrandomized, single-agent IV | 87 | 64% (24% ≥ CR); NE; NE at 3.2 months | CRS 37% (1%), ICANS 5.6% (1.2%), anemia 28% (24%), neutropenia 20% (17%), thrombocytopenia 15% (10%), infections 54% (29%) |
| Alnuctamab (CC-93269)40‡ | BsAb (2 + 1 humanized IgG1) | (NCT03486067, recruiting) | 1 | Open-label, multicenter, nonrandomized, single-agent IV or SC | 70 (IV), 68 (SC) | IV: 39%; 33.6 (10.6-NE); 3.1 (1.9-5.5) at 8 months SC: 53% (16%,7%); NE; NR at 4.1 months | CRS 53% (0%), peripheral neuropathy 6% (0%), ICANS 3% (0%), anemia 38% (25%), neutropenia 37% (32%), infections 34% (9%) |
| ABBV-383B41‡ | BsAb (2 + 1 humanized IgG4) | (NCT05286229, active, not recruiting) | 1b | Open-label, multicenter, nonrandomized, single-agent IV | 55 (40 mg), 61 (60 mg) | 40 mg: 58% (13% ≥ CR); NE (4.3); 13.7 (3.1-NE) at 3.5 months 60 mg: 61% (34% ≥ CR); NE (10.4); 11.2 (4.8-NE) at 12.7 months | CRS 60% (1%), ICANS 4.9% (1.6%), anemia 37% (16%), neutropenia 34% (26%), thrombocytopenia 29% (11%), infections NR (22%) |
| Agent (reference) . | Construct . | Trial (NCT#, status) . | Phase . | Design . | n . | % ORR (% ≥CR); median DOR in months (95% CI); median PFS in months (95% CI) at reported median follow-up . | Selected safety event % (G3 + 4%, if any) . |
|---|---|---|---|---|---|---|---|
| Belantamab mafodotin (belamaf)3† | Antibody-drug conjugate | DREAMM-2 (NCT03525678, active, not recruiting) | 2 | Open-label, 2-arm, randomized to receive 2.5 mg/kg or 3.4 mg/kg RP2D | 196 | 2.5 mg/kg: 31% (3% ≥ CR), 13.7 (9.9-NE); 2.9 (2.1-3.7) at 13 months 3.4 mg/kg: 34% (3% ≥ CR); NR; 4.9 (2.3-6.2) | 2.5 mg/kg: keratopathy 70% (27%), thrombocytopenia 35% (20%), anemia 24% (20%) 3.4 mg/kg: keratopathy 75% (21%), thrombocytopenia 54% (30%), anemia 37% (25%) |
| Idecabtagene vicleucel (ide-cel)5 | Autologous CART | KarMMa-1 (NCT03361748, active, not recruiting) | 1/2 | Open-label, single-arm, dose escalation and dose expansion | 128 | ORR 73% (33% ≥ CR); 10.7 (9.0-11.3); 8.8 (5.6-11.6) | CRS 84% (5%), neurotoxicity 18% (3%), neutropenia 91% (89%), anemia 91% (60%), thrombocytopenia 63% (52%), hypogammaglobulinemia (21%) |
| Ciltacabtagene autoleucel (cilta-cel)7 | Autologous CART | CARTITUDE-1 (NCT03548207, completed) | 1/2 | Open-label, single-arm, dose escalation and dose expansion | 97 | 97% (sCR 82.5%); 33.9 (25.5-NE); 34.9 (25.2-NE) | CRS 95% (4%), neurotoxicity 21% (9%), neutropenia 91% (89%), anemia 93% (95%), thrombocytopenia 81% (68%) |
| Teclistamab6‡ | BsAb (humanized IgG4) | MajesTec-1 (NCT04557098, recruiting) | 1/2 | Open-label, nonrandomized, IV or SC teclistamab in RRMM, dose expansion and dose escalation | 165 | 63% (43% ≥ CR); 24 (24-NE); 12.5 (8.8-17.2) at 22 months | CRS 72.1% (0.6%), neurotoxicity 14.5% (0.6%), neutropenia 70.9% (64.2%), anemia 52.1% (37%), pneumonia 18.2% (12.7%), COVID-19 17.6% (12.7%), hypogammaglobulinemia 74.5% (0%) |
| Elranatamab38‡ | BsAb (humanized IgG2a) | Magnetissm-3 (NCT04649359, active, not recruiting) | 2 | Open-label, multicenter, nonrandomized, single-agent SC | 123 | 61% (28% ≥ CR); NE (12-NE); NE (10.4-NE) at 10.4 months | CRS 57.7% (0%), neurotoxicity 3.4% (0%), peripheral neuropathy 17.1% (0.8%), infections 66.7% (35%) |
| Linvoseltamab (REGN5458)39‡ | BsAb (Veloci-Bi antibody) | LINKER-MM1 (NCT03761108, active, not recruiting) | 1/2 | Open-label, multicenter, nonrandomized, single-agent IV | 87 | 64% (24% ≥ CR); NE; NE at 3.2 months | CRS 37% (1%), ICANS 5.6% (1.2%), anemia 28% (24%), neutropenia 20% (17%), thrombocytopenia 15% (10%), infections 54% (29%) |
| Alnuctamab (CC-93269)40‡ | BsAb (2 + 1 humanized IgG1) | (NCT03486067, recruiting) | 1 | Open-label, multicenter, nonrandomized, single-agent IV or SC | 70 (IV), 68 (SC) | IV: 39%; 33.6 (10.6-NE); 3.1 (1.9-5.5) at 8 months SC: 53% (16%,7%); NE; NR at 4.1 months | CRS 53% (0%), peripheral neuropathy 6% (0%), ICANS 3% (0%), anemia 38% (25%), neutropenia 37% (32%), infections 34% (9%) |
| ABBV-383B41‡ | BsAb (2 + 1 humanized IgG4) | (NCT05286229, active, not recruiting) | 1b | Open-label, multicenter, nonrandomized, single-agent IV | 55 (40 mg), 61 (60 mg) | 40 mg: 58% (13% ≥ CR); NE (4.3); 13.7 (3.1-NE) at 3.5 months 60 mg: 61% (34% ≥ CR); NE (10.4); 11.2 (4.8-NE) at 12.7 months | CRS 60% (1%), ICANS 4.9% (1.6%), anemia 37% (16%), neutropenia 34% (26%), thrombocytopenia 29% (11%), infections NR (22%) |
sCR, stringent complete response; NE, not estimable/reached; NR, not reported; RRMM, relapsed and refractory multiple myeloma.
Nonexhaustive list of selected trials (search May 18, 2023)—for a comprehensive list, please visit clinicaltrials.gov.
Withdrawn from the US market in late 2022 due to a negative phase 3 trial (DREAMM-3).
Updated data as presented during ASH 2022 or ASCO 2023 meetings.
A summary of the data supporting registration of these therapies is in Table 1; more detailed discussion of these trials is beyond the scope of this review. In general, overall response rates (ORRs) in triple class-exposed, BCMA therapy-naive patients are roughly 30% for belamaf, 60% for teclistamab or other BCMA-targeted BsAbs, 73% for ide-cel, and 97% for cilta-cel. Responses can be quite durable, with median duration of response (DOR) in these trials reported as 11.0, 24.0, 10.0, and 33.9 months for belamaf, teclistamab, ide-cel, and cilta-cel, respectively.3-7 Unfortunately, however, there does not appear to be a plateau on progression-free survival (PFS) curves with these agents, and most patients ultimately relapse. Thus, additional therapeutic options following a BCMA-targeted therapy remain necessary.
Serial use of BCMA-targeted therapies
With so many BCMA-targeted therapies available, one obvious question is whether these can be used sequentially. We first reported in 2019 on 2 patients who responded serially to BCMA-targeted therapies (ADC→CART or CART→ADC),8 and safety and efficacy of sequential BCMA-directed therapies have since been confirmed in several retrospective and prospective studies (Table 2). As a caveat, most of these are small case series or clinical trial cohorts, and in general, they demonstrate that while responses can be recaptured by switching to a different BCMA-targeted agent, ORR and DOR appear lower compared with using the same agent in a BCMA therapy-naive population.
Activity of BCMA-targeting agents following prior anti-BCMA exposure*
| Agent . | Population/design . | n . | % ORR (≥CR); median DOR in months (95% CI); median PFS in months (95% CI) at reported median follow-up (if available) . | |||||
|---|---|---|---|---|---|---|---|---|
| Prior BCMA CART . | Prior BCMA BsAb . | Prior BCMA ADC . | Prior BCMA CART . | Prior BCMA BsAb . | Prior BCMA ADC . | Any prior anti-BCMA . | ||
| Belamaf13,18,19 | Commercial belamaf after prior anti-BCMA CART; 3 retrospective single-in stitution analysis—pooled results | 22 | None | None | 18% (NR); NR; NR | — | — | — |
| Ide-cel5 | KarMMa-1; prospective phase 2 trial. Analysis of patients retreated with ide-cel upon progression. | 28 | — | — | 21% (0% ≥ CR); NR; 1.0 (1.0-2.1) | — | — | — |
| Ide-cel 12† | Commercial ide-cel recipients; retrospective multi-institution analysis | 5 | 7 | 36 | 100% (60% ≥ CR) NR; NE | 85.7% (42.9% ≥ CR); NR; 2.83 | 67.6% (21.6% ≥ CR); NR; 3.19 | 74% (29% ≥ CR); NR; 3.2 |
| Cilta-cel14 | Cilta-cel recipients (cohort C CARTITUDE-2); prospective phase 2 trial | None | 7 | 13 | — | 57.1% (14.3% ≥ CR); 8.2 (4.4-NE); 5.3 (0.6-NE) at 10.9 months | 61.5% (38.5% ≥ CR); 11.5 (7.9-NE); 9.5 (0.99-NE) at 11.8 months | 60% (30% ≥ CR) 11.5 (7.9-NE); 9.1 (1.5-NE) at 11.3 months |
| CT103A11 | Recipients of CT103A BCMA CART (n = 103 total); subgroup analysis, prospective phase 1 trial | 12 | None | None | 75% (41.7% ≥ CR); 6.3 (2.9-NE); NR at 12.2 months | — | — | — |
| Teclistamab15† | Teclistamab recipients (MajesTec-1 cohort C), prospective phase 1/2 trial | 15 | None | 29 | 53.3% (26.7% ≥ CR); NR; NR at 12.5 months | — | 55.2% (24.1% ≥ CR); NR; NR at 12.5 months | 52.5% (27.5% ≥ CR); NE (10.5-NE); NR at 12.5 months |
| Elranatamab16† | Elranatamab recipients (4 MagnetisMM prospective studies); pooled sub-group analysis | 36 | None | 59 | 52.8% (19.6% ≥ CR); NE (9.8-NE); 10.0 (1.9-NE) at 11.3 months | — | 42.4% (18.7% ≥ CR); 13.9 (6.8-NE); 3.9 (1.9-6.6) at 11.3 months | 46% (18.4% ≥ CR); 17.1 (9.8-NE); 5.5 (2.2-10.0) at 11.3 months |
| Agent . | Population/design . | n . | % ORR (≥CR); median DOR in months (95% CI); median PFS in months (95% CI) at reported median follow-up (if available) . | |||||
|---|---|---|---|---|---|---|---|---|
| Prior BCMA CART . | Prior BCMA BsAb . | Prior BCMA ADC . | Prior BCMA CART . | Prior BCMA BsAb . | Prior BCMA ADC . | Any prior anti-BCMA . | ||
| Belamaf13,18,19 | Commercial belamaf after prior anti-BCMA CART; 3 retrospective single-in stitution analysis—pooled results | 22 | None | None | 18% (NR); NR; NR | — | — | — |
| Ide-cel5 | KarMMa-1; prospective phase 2 trial. Analysis of patients retreated with ide-cel upon progression. | 28 | — | — | 21% (0% ≥ CR); NR; 1.0 (1.0-2.1) | — | — | — |
| Ide-cel 12† | Commercial ide-cel recipients; retrospective multi-institution analysis | 5 | 7 | 36 | 100% (60% ≥ CR) NR; NE | 85.7% (42.9% ≥ CR); NR; 2.83 | 67.6% (21.6% ≥ CR); NR; 3.19 | 74% (29% ≥ CR); NR; 3.2 |
| Cilta-cel14 | Cilta-cel recipients (cohort C CARTITUDE-2); prospective phase 2 trial | None | 7 | 13 | — | 57.1% (14.3% ≥ CR); 8.2 (4.4-NE); 5.3 (0.6-NE) at 10.9 months | 61.5% (38.5% ≥ CR); 11.5 (7.9-NE); 9.5 (0.99-NE) at 11.8 months | 60% (30% ≥ CR) 11.5 (7.9-NE); 9.1 (1.5-NE) at 11.3 months |
| CT103A11 | Recipients of CT103A BCMA CART (n = 103 total); subgroup analysis, prospective phase 1 trial | 12 | None | None | 75% (41.7% ≥ CR); 6.3 (2.9-NE); NR at 12.2 months | — | — | — |
| Teclistamab15† | Teclistamab recipients (MajesTec-1 cohort C), prospective phase 1/2 trial | 15 | None | 29 | 53.3% (26.7% ≥ CR); NR; NR at 12.5 months | — | 55.2% (24.1% ≥ CR); NR; NR at 12.5 months | 52.5% (27.5% ≥ CR); NE (10.5-NE); NR at 12.5 months |
| Elranatamab16† | Elranatamab recipients (4 MagnetisMM prospective studies); pooled sub-group analysis | 36 | None | 59 | 52.8% (19.6% ≥ CR); NE (9.8-NE); 10.0 (1.9-NE) at 11.3 months | — | 42.4% (18.7% ≥ CR); 13.9 (6.8-NE); 3.9 (1.9-6.6) at 11.3 months | 46% (18.4% ≥ CR); 17.1 (9.8-NE); 5.5 (2.2-10.0) at 11.3 months |
Nonexhaustive list of selected studies.
Updated data as presented during ASH 2022, ASCO 2022, or ASCO 2023 meetings.
BCMA CART following prior BCMA CART: Although experience is limited, retreating with the same BCMA CART product has had disappointing outcomes (Table 2),5,9,10 possibly due to CAR-specific immune responses. However, subsequent treatment with a different BCMA CART product has demonstrated more promise, with ORR of 75% to 100% in small numbers of patients.11-13
BCMA CART following prior BCMA ADC or BsAb: In cohort C of the CARTITUDE-2 phase 2 study, cilta-cel was infused in 20 relapsed/refractory patients (median 8 prior lines) with prior exposure to a BCMA-targeted therapy (13 ADC [belamaf] and 7 BsAb [various]). The ORR was 60% (30% complete response [CR]) and was similar between the ADC-exposed and BsAb- exposed groups. Median DOR was 11.5 and 8.2 months, and median PFS 9.5 and 5.3 months, respectively, for these 2 groups.14 In a real-world analysis of outcomes following ide-cel infusion, 44 patients had prior belamaf (n = 37) or a BCMA-targeted BsAb (n = 7). Median prior lines of therapy was 9, with 62% penta-drug-refractory. ORRs were 68% for ADC exposed and 86% for BsAb exposed, with CR rates of 22% and 43%, respectively. However, median PFS was only 3.2 and 2.8 months, respectively, compared with a median 9.0 months for the BCMA treatment-naive population (n = 144).12 The toxicities of BCMA CARTs appear similar when given after a prior BCMA-directed therapy, although high-grade thrombocytopenia and infections may be more common.12,14 Overall, infusion of BCMA CART after prior a BCMA- targeted ADC or BsAb leads to responses in most patients, but the depth and duration of these responses appear inferior to that seen in BCMA treatment-naive patients.
BCMA BsAb following prior BCMA CART, ADC, or BsAb: In cohort C of the MajesTEC-1 study, patients with prior exposure to a BCMA-targeted ADC (n = 25), CART (n = 11), or both (n = 4) received teclistamab at a dose of 1.5 mg/kg weekly until progression. ORR was 53% and similar in both groups, with 28% CRs and median DOR not reached. Three of 4 patients with both prior ADC and CART responded. PFS and overall survival (OS) were not reported. Rates of cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and infections appeared similar to that seen in BCMA treatment-naive patients.15 In a pooled analysis of patients (n = 86, median 7 prior lines) receiving elranatamab following a prior BCMA- directed ADC or CART, ORR was 45%, with CR in 17%. Reponses were more frequent in CART-exposed patients compared with ADC-exposed patients (53% vs 41%). Median DOR was not reached, with a median PFS of 4.8 months and 60% alive at 10 months.16 These studies demonstrate the feasibility of using a BCMA-targeted BsAb after a prior BCMA-targeted ADC or CART (or both). No data are yet available for treating serially with different BCMA-targeted BsAbs. While it is suboptimal to compare across studies, current data suggest that the BCMA-targeted BsAbs may have less of a drop-off in response depth and duration between BCMA therapy-exposed and BCMA therapy-naive populations compared with that seen with BCMA CARTs (Table 2). This suggests that using a BCMA CART first followed by a BCMA BsAb later may be the better sequence compared with the other way around, although prospective trials and/or large real-world data sets are required to confirm this hypothesis.
BCMA ADC following prior BCMA CART or BsAb: There are limited data on the use of belamaf following prior BCMA-directed therapy. Gazeau et al17 described a patient progressing after a second infusion of ide-cel who achieved a VGPR after starting belamaf, with ongoing response at 5 months. Retrospective single-institution studies have reported responses in 0% to 29% of patients receiving belamaf after prior BCMA CART (Table 2).13,18,19
Non-BCMA-targeted, T-cell–engaging therapies
Talquetamab is a BsAb targeting G protein–coupled receptor, family C, group 5, member D (GPRC5D), a receptor expressed highly on myeloma cells, with lower levels of expression on normal plasma cells and keratinized tissues (eg, skin, nailbeds, tongue papillae). In an updated analysis of the MonumenTAL-1 study, ORRs at the recommended subcutaneous (SC) phase 2 doses of 405 µg weekly or 800 µg every other week were 74% and 72%, respectively, including roughly one-third with CR. Median DOR was 9.5 months and not reached, respectively.20 In a cohort of patients who received talquetamab after prior BCMA T-cell–engaging therapy, ORR was 65% (75% for prior CART [n = 36] and 44% for prior BsAb [n = 18]), with 35% CR and a median DOR of 11.9 months.20 Forimtamig is another GPRC5D- directed BsAb, with 2 GPRC5D-binding domains. In a preliminary report of a phase 1 study in patients with relapsed/refractory MM, ORR was 71% and 64% for intravenous (IV) (n = 49) and SC (n = 55) dosing, respectively, and was 52% (11/21) in patients previously exposed to a BCMA-targeted therapy.21 Common toxicities of GPRC5D-targeted BsAbs include CRS, skin and nail changes, dry mouth, and dysgeusia.
GPRC5D-targeted CART products have also shown efficacy in patients with relapsed/refractory MM, including those with prior BCMA-directed therapies (Table 3). In a phase 1 study, 18 patients received an infusion of MCARH109 at escalating doses. ORR was 71% (35% CR), with a median DOR of 7.8 months, and was 70% in the 10 patients with prior BCMA-directed therapies (8 with prior CART). Typical CART-related (eg, CRS, ICANS, cytopenias) and GPRC5D-related (eg, skin and nail changes, dysgeusia) toxicities were seen, although 2 patients developed grade 3 cerebellar toxicity at the highest dose level (450 × 10e6 CART cells).22 Several additional GPRC5D-targeted CART products have reported preliminary data, with similar efficacy in both BCMA treatment-naive and treatment-exposed patients, and no further cerebellar toxicity was reported.23-25 Of note, loss of GPRC5D expression has been described in several patients progressing after GPRC5D CARTs or BsAbs, suggesting this may emerge as a mechanism of resistance to this approach.22
Safety and efficacy of selected late line T-cell–directed therapies not targeting BCMA*
| Name . | Target . | Construct . | Trial (NCT#, status) . | Design . | n . | % ORR (sCR, CR); median DOR in months (95% CI); median PFS in months (95% CI) at reported median follow-up . | Select safety event % (G3+4%, if any) . | ||
|---|---|---|---|---|---|---|---|---|---|
| All . | BCMA-exposed . | All . | BCMA-exposed . | ||||||
| MCARH10922 | GPRC5D | Autologous CART | (NCT04555551, active, not recruiting) | Phase 1, open-label, single-center (MSKCC), dose escalation | 17 | 10 | 71% (35% ≥ CR); 7.8 (5.7-NE); NR at 10.1 months | 70% (40% ≥ CR); NR; NR at 10.1 months | CRS 88% (6%), ICANS 6% (6%), nail changes 65%, rash 18%, infections 18% (12%), cerebellar toxicity (12%) |
| BMS-986393 (CC 95266)25 | GPRC5D | Autologous CART | (NCT04674813, recruiting) | Phase 1, open-label, multienter, dose escalation | 21 | 7 | 86%; NE; NE at 4 months | 66.7%; NE; NE at 4 months | CRS 65%, ICANS 12%, neutropenia 41% (NR), thrombocytopenia 35% (NR), AEs of skin 18%, nails 12%, dysgeusia/dysphagia 12% |
| OriCAR-01723 | GPRC5D | Autologous CART (bi-epitope nanobody based) | POLARIS (NCT05016778, active, not recruiting) | Phase 1, open-label, single-center (First Affiliated Hospital of Zhejiang University) | 10 | 5 | 100% (60% ≥ CR); NE; NE at 7.8 months | 100% (40% ≥ CR); NE; NE at 7.8 months | CRS 100%, ICANS 0%, anemia 80% (70%), neutropenia 100% (100%), nail disorders 30% |
| Anti-GPRC5D CAR T24 | GPRC5D | Autologous CART | (Chinese Clinical Trial Register: ChiCTR2100048888) | Phase 2, open-label, single-center (Hospital of Xuzhou Medical University) | 33 | 9 | 91% (63% ≥ CR); NE; NE at 5.2 months | 100% (40% ≥ CR); NE; NE at 5.2 months | CRS 76%, ICANS 6% (3%), anemia 100% (52%), neutropenia 100% (100%), thrombocytopenia 100% (45%), nail changes 27% |
| Talquetamab20 † | GPRC5D | BsAb (humanized IgG4 Fc) | MonumenTal-1 (NCT03399799, recruiting; NCT04634552) | Phase 1/2, multienter, open-label, dose escalation and dose expansion | 143 (0.4 mg/kg SC weekly), 145 (0.8 mg/kg SC q2 wk) | 51 (36 CART, 18 BsAb) | 0.4 mg/kg: 74.1% (33.6% ≥ CR) 9.5 (6.7-13.3); 35% at 12 months 0.8 mg/kg: 71.7% (38.7% ≥ CR); NE (13.0-NE); 54% at 12 months | 64.7% (35.3 ≥ CR %); 11.9 (4.8-NE); 38% at 12 months (ORR 75% in patients with prior CART and 44% with prior BsAb) | 0.4 mg/kg: CRS 79% (2.1%), dysgeusia 63%, anemia 44.8% (31.5%), skin-related AEs 63%, nail disorders 51.7%, infections 57.3% (16.8%) 0.8 mg/kg: CRS 80% (0.7%), dysgeusia 46.2%, anemia 39.3% (24.8%), skin-related AEs 67.6% (0.7%), nail disorders 43.4%, infections 50.3% (11.7%) |
| Forimtamig (RG6234)21† | GPRC5D | BsAb (2:1 humanized antibody) | (NCT04557150, recruiting) | Phase 1, multienter, dose escalation and dose expansion | 51 (IV), 57 (SC) | 11 (IV), 12 (SC) (21 evaluable) | IV: 71.4% (34.7% ≥ CR); 10.8 (0-17.6); NR at 11.6 months SC: 63.6% (25.5% ≥ CR); 12.5 (1.2-12.5); NR at 8 months | IV: 50%; NR; NR SC: 54.5%; NR; NR | IV: CRS IV 82.4% (2.0%), ICANS 9.8% (2.0%), skin toxicity 78.4% (11.8%), hair and nail toxicity 23.5%, infections 56.9% (19.6%) SC: CRS 78.9% (1.8%), ICANS 12.3% (3.6%), skin 86% (22.8%), hair and nail toxicity 28.1%, infections 37.0% (24.1%) |
| Cevostamab26† | FCRH5 | BsAb (Humanized IgG1 Fc) | GO39775 (NCT03275103, recruiting) | Phase 1, multienter, fixed-duration of 17 cycles; 90 and 160 mg dose expansion cohorts | 161 (86 90 mg and 44 160 mg target dose level evaluable patients)† | 54 (27 CART, 13 BsAb, 27 ADC)—some patients had multiple BCMA therapies | 90 mg: 36.1% (9.6% ≥ CR); 11.5 (6.0-18.4; NR at 14.3 months 160 mg: 56.7% (8.4% ≥ CR); NR; NR at 6.5 months | All: 36.4% CART: 44.4% BsAbs: 33.3% ADCs: 50.0% | CRS 80.7% (1.2%), ICANS 14.3% (0.6%), infections 42.5% (18.8%), neurological/psychiatric 40.6% (3.8%), anemia 31.9% (21.9%) |
| Name . | Target . | Construct . | Trial (NCT#, status) . | Design . | n . | % ORR (sCR, CR); median DOR in months (95% CI); median PFS in months (95% CI) at reported median follow-up . | Select safety event % (G3+4%, if any) . | ||
|---|---|---|---|---|---|---|---|---|---|
| All . | BCMA-exposed . | All . | BCMA-exposed . | ||||||
| MCARH10922 | GPRC5D | Autologous CART | (NCT04555551, active, not recruiting) | Phase 1, open-label, single-center (MSKCC), dose escalation | 17 | 10 | 71% (35% ≥ CR); 7.8 (5.7-NE); NR at 10.1 months | 70% (40% ≥ CR); NR; NR at 10.1 months | CRS 88% (6%), ICANS 6% (6%), nail changes 65%, rash 18%, infections 18% (12%), cerebellar toxicity (12%) |
| BMS-986393 (CC 95266)25 | GPRC5D | Autologous CART | (NCT04674813, recruiting) | Phase 1, open-label, multienter, dose escalation | 21 | 7 | 86%; NE; NE at 4 months | 66.7%; NE; NE at 4 months | CRS 65%, ICANS 12%, neutropenia 41% (NR), thrombocytopenia 35% (NR), AEs of skin 18%, nails 12%, dysgeusia/dysphagia 12% |
| OriCAR-01723 | GPRC5D | Autologous CART (bi-epitope nanobody based) | POLARIS (NCT05016778, active, not recruiting) | Phase 1, open-label, single-center (First Affiliated Hospital of Zhejiang University) | 10 | 5 | 100% (60% ≥ CR); NE; NE at 7.8 months | 100% (40% ≥ CR); NE; NE at 7.8 months | CRS 100%, ICANS 0%, anemia 80% (70%), neutropenia 100% (100%), nail disorders 30% |
| Anti-GPRC5D CAR T24 | GPRC5D | Autologous CART | (Chinese Clinical Trial Register: ChiCTR2100048888) | Phase 2, open-label, single-center (Hospital of Xuzhou Medical University) | 33 | 9 | 91% (63% ≥ CR); NE; NE at 5.2 months | 100% (40% ≥ CR); NE; NE at 5.2 months | CRS 76%, ICANS 6% (3%), anemia 100% (52%), neutropenia 100% (100%), thrombocytopenia 100% (45%), nail changes 27% |
| Talquetamab20 † | GPRC5D | BsAb (humanized IgG4 Fc) | MonumenTal-1 (NCT03399799, recruiting; NCT04634552) | Phase 1/2, multienter, open-label, dose escalation and dose expansion | 143 (0.4 mg/kg SC weekly), 145 (0.8 mg/kg SC q2 wk) | 51 (36 CART, 18 BsAb) | 0.4 mg/kg: 74.1% (33.6% ≥ CR) 9.5 (6.7-13.3); 35% at 12 months 0.8 mg/kg: 71.7% (38.7% ≥ CR); NE (13.0-NE); 54% at 12 months | 64.7% (35.3 ≥ CR %); 11.9 (4.8-NE); 38% at 12 months (ORR 75% in patients with prior CART and 44% with prior BsAb) | 0.4 mg/kg: CRS 79% (2.1%), dysgeusia 63%, anemia 44.8% (31.5%), skin-related AEs 63%, nail disorders 51.7%, infections 57.3% (16.8%) 0.8 mg/kg: CRS 80% (0.7%), dysgeusia 46.2%, anemia 39.3% (24.8%), skin-related AEs 67.6% (0.7%), nail disorders 43.4%, infections 50.3% (11.7%) |
| Forimtamig (RG6234)21† | GPRC5D | BsAb (2:1 humanized antibody) | (NCT04557150, recruiting) | Phase 1, multienter, dose escalation and dose expansion | 51 (IV), 57 (SC) | 11 (IV), 12 (SC) (21 evaluable) | IV: 71.4% (34.7% ≥ CR); 10.8 (0-17.6); NR at 11.6 months SC: 63.6% (25.5% ≥ CR); 12.5 (1.2-12.5); NR at 8 months | IV: 50%; NR; NR SC: 54.5%; NR; NR | IV: CRS IV 82.4% (2.0%), ICANS 9.8% (2.0%), skin toxicity 78.4% (11.8%), hair and nail toxicity 23.5%, infections 56.9% (19.6%) SC: CRS 78.9% (1.8%), ICANS 12.3% (3.6%), skin 86% (22.8%), hair and nail toxicity 28.1%, infections 37.0% (24.1%) |
| Cevostamab26† | FCRH5 | BsAb (Humanized IgG1 Fc) | GO39775 (NCT03275103, recruiting) | Phase 1, multienter, fixed-duration of 17 cycles; 90 and 160 mg dose expansion cohorts | 161 (86 90 mg and 44 160 mg target dose level evaluable patients)† | 54 (27 CART, 13 BsAb, 27 ADC)—some patients had multiple BCMA therapies | 90 mg: 36.1% (9.6% ≥ CR); 11.5 (6.0-18.4; NR at 14.3 months 160 mg: 56.7% (8.4% ≥ CR); NR; NR at 6.5 months | All: 36.4% CART: 44.4% BsAbs: 33.3% ADCs: 50.0% | CRS 80.7% (1.2%), ICANS 14.3% (0.6%), infections 42.5% (18.8%), neurological/psychiatric 40.6% (3.8%), anemia 31.9% (21.9%) |
AE, adverse event; RP2D, recommended phase 2 dose.
Nonexhaustive list of select ongoing trials (search May 18, 2023)—for a comprehensive list, please visit clinicaltrials.gov.
Updated data presented during ASH 2021, ASH 2022, or ASCO 2023 meetings.
Another emerging target for myeloma therapy is Fc receptor- homolog 5 (FcRH5), a cell surface receptor highly expressed on myeloma cells, as well as on normal plasma and a subset of B cells. Cevostamab, a T-cell–engaging BsAb targeting FcRH5, is being evaluated in an ongoing phase 1 study exploring IV dosing every 3 weeks for a fixed duration (51 weeks). Preliminary analysis of 2 expansion cohorts showed ORRs of 37% (22/60) and 55% (24/44) at target doses of 90 mg and 160 mg, respectively, with an estimated median DOR of 11.5 months and not reported, respectively. CRS and ICANS were seen in 80% and 13% of patients, respectively. At target doses ≥90 mg, ORRs in patients with prior exposure to BCMA-directed CARTs, BsAbs, and ADCs were 44% (4/9), 33% (3/9), and 50% (7/14), respectively, demonstrating activity of cevostamab post-BCMA therapy.26 A phase 1/2 study of cevostamab specifically in patients with prior BCMA-directed therapy is ongoing (CAMMA-2, NCT05535244). Overall, T-cell–engaging therapies against GPRC5D and FcRH5 are showing promising efficacy, and these should be considered as next lines of therapy following a BCMA-directed agent as they become available. In fact, in a single-institution, retrospective analysis of various treatments given to patients progressing after BCMA CART therapy, the best OS was seen in patients who received a different T-cell–engaging therapy (ie, BsAb or CART, most targeting GPRC5D) following prior BCMA CART, with a median OS not reached at 21 months.18 This study, however, is limited by small size and potential selection bias, and prospective studies are needed.
Non–T-cell–engaging therapies
Data are limited regarding use of standard doublet/triplet/ quadruplet regimens incorporating IMIDs, proteasome inhibitors, alkylators, and/or monoclonal antibodies post-BCMA therapy. However, these patients are typically triple class-exposed/refractory, and we know from older studies (eg, MAMMOTH) that expected ORRs in this population with these regimens are roughly 30% to 40%, with median PFS 3 to 4 months.27 Similar efficacy numbers were reported in 2 retrospective single-institution experiences with these approaches for relapse following BCMA CART therapy.13,18 The use of cytotoxic chemotherapy (eg, dexamethasone, cyclophosphamide, etoposide, and cisplatinum) ± stem cell support, or salvage autologous stem cell transplant (SCT), was associated with responses in roughly 45% to 55% of patients in these studies and remains an option for patients with additional stem cells cryopreserved, especially in the setting of rapidly progressive disease or cytopenias.
Several additional novel agents have reported activity following BCMA-directed therapy (Table 4). A selinexor-based triplet or quadruplet combination induced responses in 7 of 11 patients (64%) with prior BCMA-directed therapy (8 prior ADC or mAb, 2 CART, 1 BsAb) in the STOMP trial, with 5 having responses lasting >6 months.28 Iberdomide, a novel, oral cereblon E3 ligase modulator (CELMoD), was studied in combination with dexamethasone in 38 patients with prior BCMA-directed therapies. ORR was 37% and was similar regardless of type of prior anti-BCMA therapy, with a median DOR of 7.5 months and a median PFS of 2.4 months.29 Mezigdomide, another potent oral CELMoD, also had significant activity in combination with dexamethasone in 30 BCMA treatment-exposed patients (22 ADC, 3 CART, 8 BsAb), with an ORR of 50%, a median DOR of 6.9 months, and a PFS of 5.4 months.30 Finally, in a phase 1/2 study of modakafusp alfa, an immunocytokine consisting of an anti-CD38 antibody fused to 2 attenuated interferon alpha molecules, the ORR for the 1.5 mg/kg IV every 4-week dose was 43% and was 27% for the 15 patients with a prior BCMA-targeted therapy, with DOR and PFS not yet reported.31 As these latter agents continue to move forward in development, they may provide additional non–T-cell–engaging, noncytotoxic options for patients following anti-BCMA exposure.
Select non–T-cell–engaging therapies with evidence of efficacy following relapse after BCMA-directed therapies
| Agent . | Population/design . | n . | % ORR (sCR, CR); median DOR in months (95% CI); median PFS in months (95% CI) at reported median follow-up . | |||
|---|---|---|---|---|---|---|
| Prior BCMA CART . | Prior BCMA BsAb . | Prior BCMA ADC (or mAB) . | BCMA-exposed . | All patients . | ||
| Selinexor (+ various)28 | BCMA-exposed subgroup in the STOMP trial (NCT02343042) —multicenter, open-label, phase 1b/2 study of selinexor in combination with backbone agents | 2 | 1 | 8 | 63.6% (0% ≥ CR); NE (10.6-NE); NE (6-NE) at 14.3 months, with various regimens including XPd, XVd, XKd, XPVd, and XPEd | Various |
| Iberdomide (+ dex)29,42 | BCMA-exposed cohort in CC-220-MM-001 trial (NCT02773030), multicenter, open-label, phase 1b/2 study | 17 | 9 | 13 | 37% (5.3% ≥ CR); 7.5 (3.2-NE); 2.4 (2.1-4.2) at 8.1 months | At RP2D (dose expansion cohort, n = 107): 26% (1% ≥ CR); 4 (2.4-10.5); 3.0 (2.8-3.7) |
| Mezigdomide (+ dex)30 | BCMA-exposed subgroup in CC-92480-MM-001 (NCT03374085), multicenter, open-label phase 1b/2 study | 3 | 8 | 22 | 50% (3.3% ≥ CR); 6.9 (4-NE), 5.4 (2.1-9.4) at 5.8 months | At RP2D (n = 101): 39.6% (5% ≥ CR); 8.3 (5.4-NE); 4.6 (3.2-6.3) at 5.8 months |
| Modakafusp (TAK-573)31 * | BCMA-exposed subgroup in multicenter, open-label phase 1/2 study (NCT03215030) | 15 at RP2D (8 prior CART, prior ADC, and BsAb not specified) | 27% (7% ≥ CR); NR; NR at 5.3 months | At RP2D (n = 30): 43% (10% ≥ CR); 12.5 (1-21); 5.7 (1.2-14) | ||
| Agent . | Population/design . | n . | % ORR (sCR, CR); median DOR in months (95% CI); median PFS in months (95% CI) at reported median follow-up . | |||
|---|---|---|---|---|---|---|
| Prior BCMA CART . | Prior BCMA BsAb . | Prior BCMA ADC (or mAB) . | BCMA-exposed . | All patients . | ||
| Selinexor (+ various)28 | BCMA-exposed subgroup in the STOMP trial (NCT02343042) —multicenter, open-label, phase 1b/2 study of selinexor in combination with backbone agents | 2 | 1 | 8 | 63.6% (0% ≥ CR); NE (10.6-NE); NE (6-NE) at 14.3 months, with various regimens including XPd, XVd, XKd, XPVd, and XPEd | Various |
| Iberdomide (+ dex)29,42 | BCMA-exposed cohort in CC-220-MM-001 trial (NCT02773030), multicenter, open-label, phase 1b/2 study | 17 | 9 | 13 | 37% (5.3% ≥ CR); 7.5 (3.2-NE); 2.4 (2.1-4.2) at 8.1 months | At RP2D (dose expansion cohort, n = 107): 26% (1% ≥ CR); 4 (2.4-10.5); 3.0 (2.8-3.7) |
| Mezigdomide (+ dex)30 | BCMA-exposed subgroup in CC-92480-MM-001 (NCT03374085), multicenter, open-label phase 1b/2 study | 3 | 8 | 22 | 50% (3.3% ≥ CR); 6.9 (4-NE), 5.4 (2.1-9.4) at 5.8 months | At RP2D (n = 101): 39.6% (5% ≥ CR); 8.3 (5.4-NE); 4.6 (3.2-6.3) at 5.8 months |
| Modakafusp (TAK-573)31 * | BCMA-exposed subgroup in multicenter, open-label phase 1/2 study (NCT03215030) | 15 at RP2D (8 prior CART, prior ADC, and BsAb not specified) | 27% (7% ≥ CR); NR; NR at 5.3 months | At RP2D (n = 30): 43% (10% ≥ CR); 12.5 (1-21); 5.7 (1.2-14) | ||
Dex, dexamethasone; XKd, selinexor, carfilzomib, and dexamethasone; XPd, selinexor, pomalidomide, and dexamethasone; XPd-40, selinexor 40 mg, bortezomib, and dexamethasone; XPd-60, selinexor 60 mg, bortezomib, and dexamethasone; XPEd, selinexor, pomalidomide, elotuzumab, and dexamethasone; XPVd, selinexor, pomalidomide, bortezomib, and dexamethasone; XVd, selinexor, bortezomib, and dexamethasone.
Updated data presented during ASH 2022 meeting.
Factors to consider when choosing treatment after prior anti-BCMA therapy
BCMA expression: BCMA expression on myeloma cells is dynamic and can decrease after BCMA-targeted CART cells, but in most cases, BCMA is still present at time of relapse.5,9,15 However, rare cases of biallelic genomic loss of BCMA (typically due to 16p deletion causing loss of the TNFRSF17 (BCMA) gene locus, in combination with a BCMA mutation) have been described,32,33 and the frequency of mutations or complete antigen loss may increase with the BsAb therapies, which provide more prolonged selective pressure due to their long-term administration. BCMA extracellular domain mutations have been identified that confer resistance to multiple BCMA-targeting BsAbs.34 Unfortunately, while several research tools exist to assess for the presence of BCMA, including serum soluble BCMA assays and immunohistochemistry and flow cytometry assays for myeloma cell BCMA expression, none of these are widely available yet in clinical practice. Hence, currently assessment for BCMA is not required prior to pursuing a second or third BCMA-targeted therapy. However, it is likely that assessing for BCMA protein expression combined with sequencing for BCMA mutations will become a useful tool to help guide therapeutic choice after prior anti-BCMA therapy.
Timing since last anti-BCMA therapy: In cohort C of the CARTITUDE-2 study, where cilta-cel was given after prior BCMA- directed ADC or BsAb, responding patients had a shorter median duration of prior anti-BCMA therapy (29.5 vs 63.5 days) and a longer median time from prior anti-BCMA therapy to CART infusion (235 vs 117.5 days) than nonresponders.14 A near-identical finding was observed with the use of ide-cel after prior BCMA- directed therapy.12 While these findings need to be confirmed in larger studies, they suggest that the optimal patient to consider for another anti-BCMA therapy may be one whose prior anti-BCMA exposure was relatively short and occurred remotely (eg, >6 months earlier). For a patient progressing after more recent BCMA-targeted therapy, switching to an alternative target first and then coming back to a different BCMA-directed modality later may potentially be more effective. Of note, response to prior anti-BCMA therapy was not predictive of response or PFS following subsequent cilta-cel or ide-cel therapy.12,14
Patient characteristics/disease biology: When choosing a therapy for relapsed/refractory myeloma, patient- and disease-specific features should always be taken into consideration, and this applies after anti-BCMA therapy as well. Comorbidities, performance status, renal function, presence of cytopenias, prior therapies and toxicities, distance from treatment center, and/or willingness to be hospitalized are examples of patient-specific factors that may impact choice of a T-cell–directed therapy (eg, CART or BsAb) vs reexploring a standard triplet or quadruplet regimen that could be given in the community. Disease-specific features may include cytogenetics, extramedullary disease (EMD), and/or rapid progression. Thus, for a t(11;14) patient, one might consider a venetoclax-based combination,35 and for patients with EMD and/or rapid disease progression, cytotoxic chemotherapy may be required to regain disease control and serve as a bridge to salvage SCT or a clinical trial.
Immune fitness: In additional to antigen loss, several potential immune-mediated mechanisms of resistance to BCMA-targeted BsAbs and/or CARTs have been identified, including a baseline decrease in T-cell receptor diversity, induction of T-cell exhaustion, and emergence of suppressive cell populations (eg, regulatory T cells, myeloid-derived suppressor cells).36,37 As with BCMA expression/mutation testing, we currently lack easy tools to assess this in clinical practice, but in the future, our workup may include assessment of T-cell fitness to help guide whether another T-cell–directed therapy vs non–T-cell–directed therapy has the highest likelihood of response after anti-BCMA treatment.
CLINICAL CASE (continued)
The patient was offered a clinical trial of cevostamab but declined as he wished to avoid hospitalization and receive treatment closer to home. He started isatuximab, carfilzomib, and dexamethasone, with a partial response lasting 5 months, before his myeloma progressed. He has since started teclistamab, with ongoing CR at 6 months.
Conclusions
Management of relapse after a BCMA-directed therapy has become a new unmet need in myeloma. Fortunately, patients can respond to additional BCMA- and non-BCMA-targeted T-cell–engaging therapies, as well as both older and newer myeloma therapies not directly dependent on T-cell engagement. Determining the optimal sequence of these therapies remains challenging, although based on the limited available data, we favor sequential T-cell–engaging strategies targeting different antigens, if possible. As usual with relapsed/refractory myeloma, however, treatment needs to be individualized for each patient, and ultimately ongoing trials, real-world data sets, and better biomarkers of response/resistance will help guide our decision-making.
Conflict-of-interest disclosure
Beatrice Razzo: no competing financial interests to declare.
Alfred L. Garfall has served as a consultant for BMS, Janssen, Novartis, GSK, and Legend; has received research funding form Novartis, Janssen, Tmunity, and CRISPR therapeutics; and has had intellectual property licensed by his institution to Novartis.
Adam D. Cohen has served as a consultant/advisory board member for GSK, Genentech/Roche, BMS/Celgene, Janssen, Abbvie, Pfizer, and Ichnos; has received research funding from Novartis, Janssen, GSK, Genentech/Roche, and BMS/Celgene; and has had intellectual property licensed from his institution to Novartis.
Off-label drug use
Beatrice Razzo: No off-label recommendations to disclose.
Alfred L. Garfall: No off-label recommendations to disclose.
Adam D. Cohen: No off-label recommendations to disclose.