Abstract
In the current treatment paradigm, the use of anti-CD38 monoclonal antibodies (mAbs) in frontline has notably increased, for both transplant-ineligible and transplant-eligible patients with newly diagnosed multiple myeloma (NDMM) patients. As a result, patients with multiple myeloma (MM) are frequently exposed to or develop resistance to anti-CD38 mAb therapy during the initial stages of treatment. Here, we review second-line (first relapse) and some third-line (second relapse) therapies for patients with MM with disease progression after exposure to anti-CD38 mAb-based therapy. We discuss therapies including B-cell maturation antigen (BCMA)–targeted and non-BCMA-targeted therapeutic options in the setting of prior anti-CD38 mAb exposure/refractoriness.
Learning Objectives
Discuss non–B-cell maturation antigen (BCMA)–directed multiple myeloma (MM) treatment options in first and second relapse after anti-CD38 monoclonal antibody (mAb) exposure
Review the use of BCMA-directed MM therapies in first and second relapse after anti-CD38 mAb exposure
Review management of MM in second relapse after anti-CD38 mAb exposure
CLINICAL CASE
A 63-year-old African American man was diagnosed with multiple myeloma (MM) 3 years ago. At the time of diagnosis, he had immunoglobulin G (IgG) κ, stage II disease per the Revised International Staging System. Additionally, bone marrow pathology revealed standard-risk features, including t(11;14) and monosomy 13. Positron emission tomography/computed tomography showed no fluorodeoxyglucose (FDG) avid osseous lesions. He was treated with daratumumab (Dara), lenalidomide (Len), bortezomib (Bortez), and dexamethasone (Dex) (DRVd) for 4 cycles. He then underwent stem cell harvest, followed by another 4 cycles of DRVd consolidation. Given his standard-risk disease and achievement of stringent complete remission after the first 4 cycles of induction therapy, he opted to forego autologous stem cell transplantation (ASCT). He received maintenance therapy with Dara-Len for 2 years and then Len single-agent maintenance onward. The following year, he developed relapsed disease with a new paraspinal L5 plasmacytoma and symptomatic bilateral rib lytic lesions. M spike was 1.8 g/dL, repeat bone marrow biopsy showed 40% plasma cell involvement, and fluorescence in situ hybridization analysis detected an additional finding of 3 copies of duplication 1q.
Use of anti-CD38 mAbs in frontline treatment of NDMM
The integration of anti-CD38 monoclonal antibodies (mAbs) in the frontline treatment of newly diagnosed multiple myeloma (NDMM) has become a standard-of-care (SoC) approach based on various clinical trials. The pivotal MAIA trial, which encompassed a cohort of patients with transplant-ineligible (TI) NDMM treated with Dara in the frontline setting laid the foundation for this approach. Data published in 2022 showed a 44% reduction in risk of death or progressive disease and sustained measurable residual disease (MRD) negativity (using next-generation sequencing at 10−5),1 with nearly 15% of patients with TI NDMM maintaining MRD negativity at or beyond 6 months and ∼11% at or beyond 12 months, which translated to favorable progression-free survival (PFS) outcomes.1 The tolerability and safety of Dara-Len-Dex in the MAIA regimen proved to be reassuringly comparable to those of Len-Bortez-Dex,2,3 leading to a surge in the utilization of anti-CD38 mAb therapy as a primary treatment option for patients with TI NDMM.
In the United States, there is an increasing trend in the utilization of quadruplet mAb-based therapy as frontline treatment for patients with transplant-eligible (TE) NDMM based on the phase 2 GRIFFIN trial. Results published in 2022 showed an impressive MRD-negative rate of 64% in the Dara cohort compared to 30% in the non-Dara arm (P < .0001), with 44% of patients sustaining MRD negativity at or beyond 12 months,4 as well as superior PFS and MRD negativity and deepening of complete remission rates over time in the Dara-containing arm. No additional safety concerns and no notable differences in ability to proceed with ASCT were observed in the Dara-quadruplet group compared to the triplet group.4 The PERSEUS phase 3 clinical trial evaluation of this quadruplet regimen in patients with NDMM with PFS as the primary end point is ongoing.5
In Europe, the phase 3 randomized CASSIOPEIA trial assessed Dara in combination with Bortez, thalidomide, and dex (D-VTd) vs VTd induction in patients with TE NDMM. The first randomization was 1:1 to quadruplet versus triplet therapy, and the second randomization allowed patients with partial or better response to receive maintenance with Dara vs observation only. The D-VTd group had clinically superior complete remission (CR) of 39% vs 26% in the VTd group, with MRD-negative rates of 64% and 44% (P < .0001), respectively.6 At a median follow-up of 35.4 months after the second randomization, median PFS for the Dara cohort was not reached, whereas it was 46.7 months for the VTd cohort.6
Another notable European quadruplet phase 3 trial (ALCYONE) evaluated Dara, Bortez, melphalan, and prednisone (D-VMP) vs VMP in TI NDMM, focusing on PFS. In the final analysis at a 40.1-month median follow-up, both PFS and overall survival (OS) were significantly better in the D-VMP cohort, with hazard ratios of 0.60 (P = .0003; 95% confidence interval [CI], 0.46-0.80) and 0.42 (P < .0001; 95% CI, 0.34-0.51), respectively.7 Additionally, the overall response rate (ORR), CR or better, and MRD negativity rates in the D-VMP-treated patients were all significantly superior to those in the VMP arm. The infectious adverse events (AEs) were notably more increased in the Dara cohort.7
Finally, the GMMG-HD7 trial, a phase 3 trial, successfully achieved its primary end point in just 18 weeks, with the isatuximab (Isa)–RVd quadruplet regime attaining >50% MRD negativity in the quadruplet group pre-ASCT. The post-ASCT results from this trial will possibly be practice informing, given the planned double-randomization study design before and after ASCT.8
What would best be used for second-line therapy for this patient?
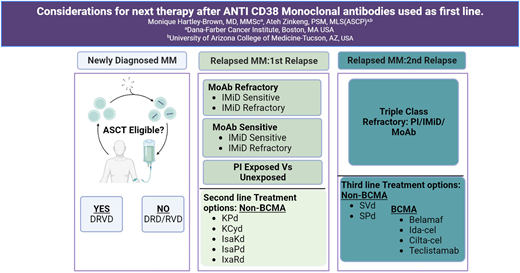
There are several factors to consider when choosing the next best regimen for a patient with MM with relapsed disease. These can be categorized as follows: (1) prior regimen received, (2) disease risk stratification, and (3) patient conditions (Table 1). In this clinical case, the patient experienced disease progression in less than 4 years with prior Dara exposure and prolonged Len therapy with progression on Len. It is important to factor in his prior exposure to Dara and prolonged Len exposure when determining the best next course of therapy.
Factors to consider when selecting the next line of therapy in patients with relapsed MM
| Prior MM directed therapies (induction/first line) . | MM disease risk stratification . | Patient conditions . |
|---|---|---|
| Triplet vs quadruplet therapy • Triplet o DRd vs RVd • Quadruplet ○ D-RVd* ○ Clinical trial | High risk vs standard risk • Cytogenetics ○ Hypoploidy ○ Hyperploidy • Fluorescence in situ hybridization ○ Del 17p, t(4;14), t(14; 16), t(14;20), gain 1q, loss 1p • Next-generation sequencing • Genomic expression profiling | Renal disease • Cast nephropathy • Nonmalignant etiology • Severity • Medication toxicity |
| Alkylator† • Bendamustine • Cyclophosphamide Anthracycline • Doxorubicin • Doxil | Time to relapse • Early (<12 mo vs <24 mo) • Late (>48 mo) • Slow (asymptomatic/biochemical) • Rapid (symptomatic) | Neuropathy • MM related • Nonmalignant etiology |
| Proteasome inhibitor • Bortezomib • Carfilzomib • Ixazomib | Extramedullary disease • Plasmacytomas ○ Visceral ○ Skin • Central nervous system disease | Bone lesions (symptomatic vs asymptomatic) |
| ASCT • Transplant ineligible • Transplant eligible | Peripheral blood plasma cells • <5% • >5% • >20% | Steroid (tolerant vs intolerant) |
| Institutional/resources • Advanced cancer care center • Community medical center | Non-MM-related health conditions • Cardiovascular • Renal disease |
| Prior MM directed therapies (induction/first line) . | MM disease risk stratification . | Patient conditions . |
|---|---|---|
| Triplet vs quadruplet therapy • Triplet o DRd vs RVd • Quadruplet ○ D-RVd* ○ Clinical trial | High risk vs standard risk • Cytogenetics ○ Hypoploidy ○ Hyperploidy • Fluorescence in situ hybridization ○ Del 17p, t(4;14), t(14; 16), t(14;20), gain 1q, loss 1p • Next-generation sequencing • Genomic expression profiling | Renal disease • Cast nephropathy • Nonmalignant etiology • Severity • Medication toxicity |
| Alkylator† • Bendamustine • Cyclophosphamide Anthracycline • Doxorubicin • Doxil | Time to relapse • Early (<12 mo vs <24 mo) • Late (>48 mo) • Slow (asymptomatic/biochemical) • Rapid (symptomatic) | Neuropathy • MM related • Nonmalignant etiology |
| Proteasome inhibitor • Bortezomib • Carfilzomib • Ixazomib | Extramedullary disease • Plasmacytomas ○ Visceral ○ Skin • Central nervous system disease | Bone lesions (symptomatic vs asymptomatic) |
| ASCT • Transplant ineligible • Transplant eligible | Peripheral blood plasma cells • <5% • >5% • >20% | Steroid (tolerant vs intolerant) |
| Institutional/resources • Advanced cancer care center • Community medical center | Non-MM-related health conditions • Cardiovascular • Renal disease |
Based on the GRIFFIN trial, a phase 2 trial; the phase 3 (PERSEUS) trial needed to support FDA approval of the quadruplet regimen is ongoing.
Some patient cases require induction triplet inclusive of traditional chemotherapeutic agent, for various reasons, such as renal insufficiency.
In cases where the patient does not exhibit refractoriness to Dara, the APOLLO and CANDOR trials provide appropriate second-line options. The APOLLO trial evaluated 304 patients randomized to receive Dara-pomalidomide-Dex (DPd; n = 151) vs Pd (n = 153) post–first relapse (1 to 3 prior lines of therapy). Most patients (79.6%) were Len refractory, with almost half (48%) being proteasome inhibitor (PI) refractory and 42.4% being both PI and Len refractory. After a median follow-up of 39.6 months, DPd had a median OS of 34.4 months (95% CI, 23.7-40.3) vs 23.7 months for Pd (95% CI, 19.6-29.4). Almost 10% of the DPd cohort was exposed to prior anti-CD38 mAb therapy.9 The CANDOR trial compared Dara-carfilzomib-Dex (DKd) vs Kd after 1 to 3 prior lines of therapy and primarily assessed PFS. Final analysis showed that at a median follow-up of 50 months, the DKd group demonstrated a median PFS of 28.4 months, whereas the Kd group had a PFS of 15.2 months, with MRD negativity rates of 28% in the DKd group and 9% in the Kd group.10 The use of carfilzomib instead of Bortez in the CANDOR trial is supported by several trials, including the pivotal phase 3 ENDEAVOR trial, which compared carfilzomib-Dex (Kd) to Bortez-Dex (Vd) in patients with relapsed or refractory MM (RRMM). The trial showed a median PFS of 18.7 months with Kd vs 9.4 months with Vd (hazard ratio = 0.53; 95% CI, 0.44-0.65; P < .0001). Updated interim analysis also showed improved OS for the carfilzomib group compared to the Bortez cohort.11
Isatuximab (Isa) is another MAb that targets CD38 plasma cell surface antigen but differs from Dara in epitope binding.12 This difference may translate to distinct treatment outcomes when either agent is used. An in vitro study suggested that Isa exhibits a higher potency than Dara in inhibiting CD38 enzymatic activity13 ; despite this, a phase 2 study evaluating the use of Isa in patients with Dara-refractory MM (within 6 months of Dara exposure) yielded disappointing results.14 In the same study, minimal responses were observed in patients with ≥6 months from the last Dara dose treated with Isa. This distinction highlights the importance of considering the timing of prior Dara exposure when treatment strategies involving another anti-CD38 mAb are planned.
Notably, isatuximab is approved by the Food and Drug Administration (FDA) in the United States for use in combination with carfilzomib and Dex (IsaKd) in patients with RRMM after 1 to 3 prior lines of therapy, based on the IKEMA study.15 The final analysis, after a median follow-up of 44 months, showed a median PFS of 35.7 months with IsaKd (95% CI, 25.8-44.0) vs 19.2 months for Kd (95% CI, 15.8-25.0), translating to a 42% risk reduction of death or MM disease progression for the Isa-containing regimen.16
For the patient in this clinical case, retaining Bortez as a component of his second-line therapy is a reasonable consideration. This decision is grounded in the facts that his prior exposure to Bortez was limited to just 8 cycles and his last encounter with this drug was over 12 months ago.
The BOSTON regimen, which combines selinexor (Seli) with Bortez and Dex (SVd), is a viable non–B-cell maturation antigen (BCMA) second-line treatment option for this patient. Seli is an oral selective inhibitor of nuclear export medication that binds and inhibits XP01, resulting in myeloma cell destruction. Seli has shown efficacy in Len-resistant and mAb-exposed patients with RRMM. The BOSTON trial had a median PFS for SVd of ∼14 months compared to ∼9.5 months for Vd (P = .0075).17,18 Another key trial (STORM) that investigated Seli in patients with RRMM showed favorable efficacy and tolerability in combination with pomalidomide and Dex (SPd).19-21 Experience with Seli use has shown the importance of anticipatory management of potential gastrointestinal and electrolyte adverse effects, often well mitigated with acceptable safety and tolerance, especially in the combination regimens with doses of Seli below 80 mg weekly. For many patients, especially in the community oncology setting, the BOSTON regimen may be an ideal option to salvage patients in second relapse and/or bridge patients to clinical trial opportunities or next therapeutic care such as immune cellular therapy or ASCT for those eligible. The triplet regimens from the OPTIMISMM (PVd) and CASTOR (DVd) trials are potential Bortez- based second-line therapies.
Two other potential second-line non-BCMA therapeutic options to consider are carfilzomib, pomalidomide, and Dex (KPd), and carfilzomib, cyclophosphamide (Cy), and Dex (KCyd), as supported by findings from the EMN011/HOVON114 and MCRN-003/MYX.1 phase 2 trials.22 The caveat is that those trials were smaller phase 2 institutional studies with no phase 3 data support, although the trials’ results are compelling.
It is worth noting that the TOURMALINE-MM123 and ELOQUENT24 studies, which evaluated ixazomib (Ixa) with Len-Dex (IxaRd) and elotuzumab (Elo) with Len-Dex (EloPd), respectively, in patients after 1-3 prior lines of therapy, would not be suitable next-line options in this case. This is because the patients enrolled in these studies were not exposed to Dara.
The case described here, use of ASCT in second-line treatment is possible, given the patient completed stem cell harvest and deferred frontline transplantation. Both the phase 3 IFM 2009 and DETERMINATION trials, which evaluated ASCT in frontline vs later in relapse, indicate that ASCT remains important for select MM patients.25,26 The IFM 2009 trial included 700 patients randomly assigned to ASCT early after 3 cycles of RVd with consolidation of 2 RVd cycles, compared to RVd for 8 cycles. Len maintenance was for 1 year. The primary end point was PFS, and at a median follow-up of 93 months, the median PFS was 47.3 months vs 35 months for the ASCT group vs the RVd-alone group, respectively (hazard ratio = 0.70; 95% CI, 0.95-0.83). The MRD negativity rates were superior in the ASCT group (29.8%) vs RVd alone (20%; P = .01). Of the patients who relapsed, 68.4% proceeded to second-line therapy (ASCT, n =217; RVd alone, n = 262); 22.6% received a second ASCT and 76.7% of the RVd-alone group received ASCT post–first relapse. The median PFS showed no statistical difference between the groups, and the median OS was 62.2% and 60.2% in the ASCT and RVd-alone groups, respectively.12,25
The DETERMINATION trial evaluated patients with NDMM who received RVd induction with ASCT up front (n = 365) vs no frontline ASCT (n = 357), with both groups receiving Len maintenance until disease relapse. PFS was the primary end point of the trial. The median follow-up was 76.0 months, with a median PFS of 67.5 months vs 46.2 months, respectively. The OS at 5 years was 80.7% and 79.2%, respectively.26
These studies suggested that whether ASCT is performed up front or delayed, the overall survival benefit does not seem to be significantly different. It is important to note that both DETERMINATION and IFM 2009 were preplanned for PFS as the primary end point. Additionally, only ~35% of the patients in the DETERMINATION trial received ASCT at the time of first relapse, which may have impacted the OS outcome.25,26 Consequently, the timing of ASCT frontline vs post first relapse remains controversial, with the data suggesting that ASCT is a reasonable option for second-line treatment if not performed previously.
CLINICAL CASE (continued)
The patient is fully cognizant of the reoccurring nature of his myeloma disease and is concerned about treatment options in the event of a second relapse.
What would best be used for third-line therapy for this patient?
Depending on the choice of second-line therapy, third-line treatment options may include options as discussed above (Table 2). The choice of third-line therapy may also depend on the nature of the relapse, whether it is rapidly progressive or indolent. Treatment options in this scenario can encompass combination chemotherapy such as Dex, Cy, etoposide, and cisplatin (DCEP) or Bortez, Dex, cisplatin, doxorubicin, Cy, and etoposide (VD-PACE), as well as BCMA-targeted therapies such as chimeric antigen receptor T-cell (CAR-T) therapies. Some examples of sequencing therapies from NDMM through second RRMM are listed below:
DRVd (TE) → ASCT → DR → first relapse → PVd, SVd, or clinical trial → second relapse → clinical trial, BCMA-targeted therapy (CAR-T or bispecific [eg, teclistamab]), or non-BCMA-targeted therapy (eg, talquetamab)
DRd (TI) → R → first relapse → DPd, DVd, KRd, or clinical trial → second relapse → clinical trial, SVd, PVd, BCMA- targeted therapy (CAR-T or bispecific [eg, teclistamab]), or non-BCMA-targeted therapy (eg, talquetamab)
RVd (TI or TE) → R → first relapse → ASCT (TE), PVd, DPd, SVd, DVd, or clinical trial → second relapse → clinical trial, BCMA-targeted therapy (CAR-T or bispecific [eg, teclistamab]), or non-BCMA-targeted therapy (eg, talquetamab).
Current US FDA-approved triplet regimens for management of RRMM in the second line
| Regimen (study) . | Survival outcomes, PFS (mo) and OS (mo) . | Median lines of therapy (range) . | Included anti-CD38 mAb–exposed/ refractory patients . | Included Len-refractory patients . |
|---|---|---|---|---|
| DPd (APOLLO) | Median OS 34.4 (95% CI, 23.7-40.3) DPd vs 23.7 (95% CI, 19.6-29.4) Pd | 2 (1-5) | No | Yes |
| KRd (ASPIRE) | Median PFS 26.1 (95% CI, 23.3-30.5) KRd vs 16.6 (95% CI, 15-20.6; P < .001) Rd Median OS 48.3 (95% CI, 42.4-52.8) KRd vs 40.4 (95% CI, 33.6-44.4; P = .0045) Rd | 2 (1-3) | No | No |
| SVd (BOSTON) | Median PFS 13.9 SVd vs 9.46 Vd (P = .0075) | 2 (1-3) | Yes | Yes |
| DKd (CANDOR) | Median PFS 28.4 DKd vs 15.2 Kd | 2 (1-5) | No | Yes |
| DVd (CASTOR) | Median PFS 16.7 DVd vs 7.1 Vd (P < .0001) | 2 (1-9) | No | Yes |
| EloRd (ELOQUENT-2) | Median PFS 19.4 EloRd vs 14.9 Rd (P = .014) Median OS 43.7 EloRd vs 39.6 Rd (P = 0.025) | 2 (1-4) | No | No |
| IsaKd (IKEMA) | Median PFS 35.7 (95% CI, 25.8-44.0) IsaKd vs 19.2 (95% CI, 15.8-25.0) Kd | 2 (1-4) | No | Yes |
| PVd (OPTIMISMM) | Median PFS 11.2 (95% CI, 9.66-13.73) PVd vs 7.1 (95% CI, 5.88-8.48; P < .0001) Vd | 2 (1-2) | No | Yes |
| DRd (POLLUX) | Median PFS 44.5 (95% CI, 34.1-NE) DRd vs 17.5 (95% CI, 13.9-20.8; P < .0001) Rd 65% vs 57% OS | 1 (1-11) | No | Yes |
| IxaRd (TOURMALINE-MM1) | Median PFS 19.4 IxaRd vs 14.9 Rd (P = 0.0014) Median OS 43.7 IxaRd vs 39.6 Rd (P = 0.025) | Range: 1-3 | No | No |
| Regimen (study) . | Survival outcomes, PFS (mo) and OS (mo) . | Median lines of therapy (range) . | Included anti-CD38 mAb–exposed/ refractory patients . | Included Len-refractory patients . |
|---|---|---|---|---|
| DPd (APOLLO) | Median OS 34.4 (95% CI, 23.7-40.3) DPd vs 23.7 (95% CI, 19.6-29.4) Pd | 2 (1-5) | No | Yes |
| KRd (ASPIRE) | Median PFS 26.1 (95% CI, 23.3-30.5) KRd vs 16.6 (95% CI, 15-20.6; P < .001) Rd Median OS 48.3 (95% CI, 42.4-52.8) KRd vs 40.4 (95% CI, 33.6-44.4; P = .0045) Rd | 2 (1-3) | No | No |
| SVd (BOSTON) | Median PFS 13.9 SVd vs 9.46 Vd (P = .0075) | 2 (1-3) | Yes | Yes |
| DKd (CANDOR) | Median PFS 28.4 DKd vs 15.2 Kd | 2 (1-5) | No | Yes |
| DVd (CASTOR) | Median PFS 16.7 DVd vs 7.1 Vd (P < .0001) | 2 (1-9) | No | Yes |
| EloRd (ELOQUENT-2) | Median PFS 19.4 EloRd vs 14.9 Rd (P = .014) Median OS 43.7 EloRd vs 39.6 Rd (P = 0.025) | 2 (1-4) | No | No |
| IsaKd (IKEMA) | Median PFS 35.7 (95% CI, 25.8-44.0) IsaKd vs 19.2 (95% CI, 15.8-25.0) Kd | 2 (1-4) | No | Yes |
| PVd (OPTIMISMM) | Median PFS 11.2 (95% CI, 9.66-13.73) PVd vs 7.1 (95% CI, 5.88-8.48; P < .0001) Vd | 2 (1-2) | No | Yes |
| DRd (POLLUX) | Median PFS 44.5 (95% CI, 34.1-NE) DRd vs 17.5 (95% CI, 13.9-20.8; P < .0001) Rd 65% vs 57% OS | 1 (1-11) | No | Yes |
| IxaRd (TOURMALINE-MM1) | Median PFS 19.4 IxaRd vs 14.9 Rd (P = 0.0014) Median OS 43.7 IxaRd vs 39.6 Rd (P = 0.025) | Range: 1-3 | No | No |
Doublet FDA-approved options are not included in this table. KCyD (MYX.1/MCRN-00) and KPd (EMN011/H0114) are not included although they are strongly supported by smaller phase 2 studies.
NE, not evaluable.
CLINICAL CASE (continued)
The patient is interested in newer therapies and asks about BCMA-targeted treatment options in first and second relapse. He is aware of the market withdrawal of belantamab mafodotin (belamaf) in 2022 and has many questions about this treatment.
What is belamaf and how does it work? Why was it withdrawn? What other BCMA-directed second-line therapeutic options exist for patients with MM after anti-CD38 resistance?
Belamaf is an antibody-drug conjugate that targets BCMA found on the surface of the malignant plasma cells in MM. Belamaf is conjugated to monomethyl auristatin-F (MMAF), a microtubule inhibitor that binds to tubulin and impedes microtubule assembly, resulting in cell cycle arrest and apoptosis.27 An additional effect of belamaf is the activation of MM cell death through the antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis.28,29 Belamaf gained conditional accelerated FDA approval in August 2020 based on the phase 2 DREAMM-2 trial.30 The safety and efficacy data were favorable, except for keratopathy, an unusual adverse effect seen only in antibody drug conjugate MM therapy.30,31 The FDA approval for belamaf was later withdrawn in November 2022, when the phase 3 DREAMM-3 trial failed to meet the conditional requirement, falling short of its primary PFS end point with a hazard ratio of 1.03 (95% CI, 0.72-1.47).32 Of note, belamaf remains approved for use in Europe.33
The ALGONQUIN, DREAMM-12, and DREAMM-15 trials are currently evaluating the management of keratopathy, focusing on both efficacy and mitigation of toxicity. Preliminary findings indicate that when belamaf was combined with pomalidomide and Dex at different doses, the ORR increased from 82% to 95%.25,26 The belamaf dose of 1.92 mg/kg every 4 weeks in the combination with pomalidomide and Dex has had significant efficacy with reduced keratopathy adverse effects. Further results from the ALGONQUIN, DREAMM-12, and DREAMM-15 trials are forthcoming.
Other BCMA-targeted therapies have shown significant clinical benefit in patients with MM with triple refractory disease (refractory to PIs, immunomodulatory drugs [IMiDs], and anti-CD38 mAbs). The current US FDA-approved BCMA-targeted therapies are the CAR-T therapies idecabtagene vicleucel (ide-cel)34 and ciltacabtagene autoleucel (cilta-cel),35 plus the bispecific antibody teclistamab.36 These newer therapies are currently approved for use in the late refractory and relapsed setting, beyond fourth-line MM therapy. Due to their promising results in treating advanced RRMM, ongoing clinical trials are now assessing the efficacy of these BCMA therapies in the first and second relapse as well (Table 3).
Clinical trials assessing the efficacy of BCMA-targeted therapies in earlier lines of therapy
| Trial . | No. of prior lines of therapy . | Median PFS, mo (median follow up) . | ORR, n (%); CR, n(%); MRD, n(%) . | Hazard ratio (95% CI) . |
|---|---|---|---|---|
| Cilta-cel trials | ||||
| CARTITUDE-239 | 1-3 | N/A | 18 (88.9); 18 (27.8); 9 (100) | N/A |
| CARTITUDE-437 | 1-3 | Not reached (15.9 mo) | 176 (85); 152 (73); 126 (61) | 0.26 (0.18-0.38) (P < .0001) |
| CARTITUDE-540 | 0 (NDMM, TI) | N/A (recruiting) | N/A | N/A |
| CARTITUDE-6 [NCT05257083] | 0 (NDMM, TE) | N/A (recruiting) | N/A | N/A |
| Ide-cel trials | ||||
| KarMMa-241 | 1-3 | N/A (recruiting) | 37 (83.8); 37 (45.9); 13 (85) | N/A |
| KarMMa-338 | 2-4 | 13.3 (18.6) | 181 (71); 98 (39); 50 (20) | 0.49 (0.38-0.65) (P < .001) |
| KarMMa-442 | 0 (NDMM, high-risk) | N/A | N/A | N/A |
| KarMMa-743 | 1-3 | N/A | N/A | N/A |
| Elranatamab trials | ||||
| MagnetisMM-144 | 2-15 | 55 (64); 35 (31); N/A | ||
| MagnetisMM-345 | 123 (61); 123 (35); 29 (89.7) | |||
| MagnetisMM- 446 | ≥3 | N/A | N/A | N/A |
| MagnetisMM-547 | >1 | N/A | N/A | N/A |
| Teclistamab trial | ||||
| MajesTEC-348 | 1-3 | N/A (recruiting) | N/A | N/A |
| Talquetamab trials | ||||
| TRIMM-249 | >3 | N/A | 50 (78); 50 (16); N/A | N/A |
| RedirecTT-150 | On/after lines of therapy | 20.9 (13.4) | 93 (86.6); 93 (22); N/A | N/A |
| Trial . | No. of prior lines of therapy . | Median PFS, mo (median follow up) . | ORR, n (%); CR, n(%); MRD, n(%) . | Hazard ratio (95% CI) . |
|---|---|---|---|---|
| Cilta-cel trials | ||||
| CARTITUDE-239 | 1-3 | N/A | 18 (88.9); 18 (27.8); 9 (100) | N/A |
| CARTITUDE-437 | 1-3 | Not reached (15.9 mo) | 176 (85); 152 (73); 126 (61) | 0.26 (0.18-0.38) (P < .0001) |
| CARTITUDE-540 | 0 (NDMM, TI) | N/A (recruiting) | N/A | N/A |
| CARTITUDE-6 [NCT05257083] | 0 (NDMM, TE) | N/A (recruiting) | N/A | N/A |
| Ide-cel trials | ||||
| KarMMa-241 | 1-3 | N/A (recruiting) | 37 (83.8); 37 (45.9); 13 (85) | N/A |
| KarMMa-338 | 2-4 | 13.3 (18.6) | 181 (71); 98 (39); 50 (20) | 0.49 (0.38-0.65) (P < .001) |
| KarMMa-442 | 0 (NDMM, high-risk) | N/A | N/A | N/A |
| KarMMa-743 | 1-3 | N/A | N/A | N/A |
| Elranatamab trials | ||||
| MagnetisMM-144 | 2-15 | 55 (64); 35 (31); N/A | ||
| MagnetisMM-345 | 123 (61); 123 (35); 29 (89.7) | |||
| MagnetisMM- 446 | ≥3 | N/A | N/A | N/A |
| MagnetisMM-547 | >1 | N/A | N/A | N/A |
| Teclistamab trial | ||||
| MajesTEC-348 | 1-3 | N/A (recruiting) | N/A | N/A |
| Talquetamab trials | ||||
| TRIMM-249 | >3 | N/A | 50 (78); 50 (16); N/A | N/A |
| RedirecTT-150 | On/after lines of therapy | 20.9 (13.4) | 93 (86.6); 93 (22); N/A | N/A |
N/A, not applicable.
The CARTITUDE-4 trial, a phase 3 randomized study, has shown impressive early results of chimeric antigen receptor T-cell therapy in patients with RRMM who had received 1 to 3 prior lines of therapy, including PIs and IMiDs, and with disease unresponsive to Len. The trial compared cilta-cel to the SoC regimens pomalidomide, Bortez, and Dex (PVd) and Dara, pomalidomide, and Dex (DPd). The primary end point of the study was evaluation of PFS in the intent-to-treat population. At a median follow-up of 16 months, the median PFS was not reached in the cilta-cel–treated patients, while it was 11.8 months in the SoC cohort. This translates to an impressive 74% reduction in the risk of disease progression or death in the cilta-cel cohort (hazard ratio, 0.26; 95% CI, 0.18-0.38; P < .0001). ORR was 85% vs 67% (P < .0001), and MRD negativity was 61% vs 16% (P < .0001) in favor of cilta-cel. These favorable results may lead to use of cilta-cel as second-line therapy after first relapse for patients with RRMM.37
Similarly, the international KARMMA-3 trial evaluated ide-cel vs SoC (5 regimens) in patients with RRMM after receiving 2 to 4 prior lines of therapy. The primary end point was PFS, which at a median follow-up of 18.6 months was 13.3 months for ide-cel vs 4.4 months in the SoC, translating to a risk reduction of disease progression or death of 61% for ide-cel (95% CI, 0.38-0.65; P < .001). The ORR was 71% vs 42% (P < .001), and complete remission rate was 39% vs 5%, favoring ide-cel. There were no unexpected adverse toxicities, and cytokine release syndrome (CRS) and neurotoxicity (NT) rates were similar to other ide-cel trial rates, with the majority being grade 1 (88% CRS; 15% NT) and few grade 3 or above (5% CRS; 3% NT).38
Bispecific antibodies are also being assessed in earlier lines of therapy in RRMM; some examples include the MajesTEC trial series evaluating teclistamab in combination with Len and Dara. Other BCMA-targeted bispecifics also being evaluated in combination with Len and Dara include elranatamab in the MagnetisMM trials (Table 3).
Discussion
The treatment landscape for patients with RRMM is rapidly changing, with use of anti-CD38 mAb–based therapy now routinely administered as SoC for both TE and TI patients with NDMM. This change has impacted the next line of therapy offered post–first relapse. In relapsed disease, it is important to consider the prior therapeutic regimen received; the depth and duration of prior treatment response; disease cytogenetic status (high-risk vs standard risk); use of frontline ASCT; refractoriness of disease to PIs, IMiDs, and/or anti-CD38 mAbs; and patient tolerability and fitness when choosing the next therapeutic regimen after anti-CD38 mAb exposure in the front line. Moving anti-CD38 mAbs to NDMM has resulted in newer therapies with novel mechanisms of action, such as BCMA-targeted therapies being moved earlier in the treatment paradigm. These include cellular therapies, such as ide-cel and cilta-cel CAR-T therapies and bispecific antibodies (teclistamab) that have been approved by the FDA within the last 2 years; current clinical trials are addressing evaluation of CAR-T therapies and bispecific antibodies in the early post–first relapse setting, potentially moving us closer to a cure for the disease. The toxicity profile of these therapies, namely, CRS, neurotoxicity, and cytopenias, along with its impact on the T-cell immune milieu of treated patients with MM, remains to be a concern, requiring close monitoring and specialized training.
Conflict-of-interest disclosure
Monique Hartley-Brown: Consultancy honorarium: AbbVie, Bristol Myers Squibb/Celgene, GSK, Janssen, Karyopharm, Sanofi.
Ateh Zinkeng: no conflicts to disclose.
Off-label drug use
Monique Hartley-Brown: nothing to disclose.
Ateh Zinkeng: nothing to disclose.