Learning Objectives
Recognize common barriers limiting access to cellular therapies for patients with large B-cell lymphoma
Explore strategies for improving access to cellular therapy among patients treated in community settings
CLINICAL CASE
A 56-year-old Hispanic male without health insurance coverage was diagnosed with stage IV nonbulky diffuse large B-cell lymphoma, not otherwise specified (MYC, BCL2, BCL6 nonrearranged; Ki-67 70%). He received county health coverage and initial treatment at a local county hospital. Treatment with R-CHOP initially achieved complete response (CR), but unfortunately the lymphoma relapsed with bulky adenopathy. His cancer care system does not perform cellular therapies, but he is motivated to get the best available treatment.
Prior to 2022, the standard of care (SOC) approach for patients with relapsed or refractory large B-cell lymphoma (LBCL) consisted of salvage immunochemotherapy to achieve response followed by consolidation with high-dose therapy and autologous hematopoietic stem cell transplantation (auto-HSCT). However, only approximately 30% to 40% of patients with relapsed/refractory LBCL respond and proceed to auto-HSCT.1 Among patients receiving curative intent auto-HSCT, approximately 50% to 60% ultimately relapse.2 The prognosis for patients with primary refractory LBCL or LBCL that relapses in ≤12 months historically has been particularly poor.1
Utilization of CD19-directed chimeric antigen receptor T-cell (CAR T) therapy transformed the treatment landscape for patients with early-relapsed LBCL. CAR T-cell therapy first demonstrated significant efficacy in the third-line setting with the results of JULIET, TRANSCEND, and ZUMA-1 studies establishing these products as SOC options.3 In 2022, 2 multicenter, randomized phase 3 trials, ZUMA-7 and TRANSFORM, demonstrated superior efficacy of axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel) compared with SOC in fit patients relapsing within 12 months of fronte therapy.4,5 A recent update showed that axi-cel significantly improved overall survival compared to SOC.5 The BELINDA trial, however, failed to demonstrate improved efficacy with tisagenlecleucel (tisa-cel) compared with SOC in the second-line setting.
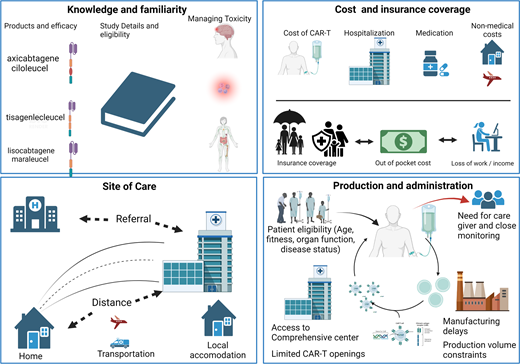
HSCT and CAR T therapy are technologically sophisticated, resource-intense, and costly procedures requiring specialized care at specially certified centers; they also require complex interactions between patients, caregivers, providers, and health care systems. Several sociodemographic and geographical factors can lead to disparities in access to these therapies, including insurance coverage, affordability, distance to centers, other geographic considerations, and referral patterns. In addition to barriers patients face when seeking HSCT therapy, access to CAR T therapy is limited by additional barriers, including manufacturing delays, limited availability, and provider familiarity. These barriers are particularly relevant for patients receiving treatment in community settings (Table 1).
Clinical outcomes and time to receipt of CART product in key CAR-T cell therapy clinical trials
| . | Axi-cel . | SOC . | Tisa-cel . | SOC . | Liso-cel . | SOC . |
|---|---|---|---|---|---|---|
| Second-line therapy . | ZUMA-7 . | Belinda . | Transform . | |||
| Received bridging chemotherapy (%) | 0 | — | 83 | — | 63 | — |
| Median time to CAR T-cell infusion (days) | 29 (27-34)* | — | 52 (31-135)† | — | NR | — |
| Received intended ASCT (%) | — | 36 | — | 32.5 | — | 45.6 |
| ORR | 83 | 50 | 46 | 43 | 86 | 48 |
| CR rate | 65 | 32 | 28 | 28 | 66 | 39 |
| EFS, median (months) | 8.3 | 2 | 3 | 3 | 10.1 | 2.3 |
| EFS HR (95% CI) | 0.4 (0.31-0.51) | 1.07 (0.82-1.4) | 0.35 (0.23-0.53) | |||
| PFS, median (months) | 14.7 | 3.7 | NR | NR | 14.8 | 5.7 |
| OS, median (months) | NE | 25.7 | 16.9 | 15.3 | NE | 16.4 |
| Third-line therapy (single-arm trials) . | ZUMA-1 . | JULIET . | TRANSCEND . | |||
| Follow-up, median (months) | 63.1 | — | 40.3 | 23.0 | — | |
| Received bridging chemotherapy (%) | 0 | — | 90 | — | 59 | — |
| Received intended CAR T cell (%) | 91 | — | — | — | ||
| Median time to CAR T-cell infusion (days) | 29 (27-34)* | — | 54 | — | 37 (27-224)† | — |
| ORR | 83% | 53% | — | 73% | — | |
| CR rate | 58% | — | 39% | — | 53% | — |
| Median DOR | 11.1 | — | 3 | — | 23.1 | — |
| Median duration of PR (months) | 1.9 | — | — | — | — | |
| Duration of CR (months) | 62.2 | — | 0.35 (0.23-0.53) | — | 26.1 | — |
| EFS, median (months) | 5.7 | — | NR | — | ||
| PFS, median (months) | 5.9 | — | — | 6.8 | — | |
| OS, median (months) | NR | 11.1 | 27.3 | — | ||
| . | Axi-cel . | SOC . | Tisa-cel . | SOC . | Liso-cel . | SOC . |
|---|---|---|---|---|---|---|
| Second-line therapy . | ZUMA-7 . | Belinda . | Transform . | |||
| Received bridging chemotherapy (%) | 0 | — | 83 | — | 63 | — |
| Median time to CAR T-cell infusion (days) | 29 (27-34)* | — | 52 (31-135)† | — | NR | — |
| Received intended ASCT (%) | — | 36 | — | 32.5 | — | 45.6 |
| ORR | 83 | 50 | 46 | 43 | 86 | 48 |
| CR rate | 65 | 32 | 28 | 28 | 66 | 39 |
| EFS, median (months) | 8.3 | 2 | 3 | 3 | 10.1 | 2.3 |
| EFS HR (95% CI) | 0.4 (0.31-0.51) | 1.07 (0.82-1.4) | 0.35 (0.23-0.53) | |||
| PFS, median (months) | 14.7 | 3.7 | NR | NR | 14.8 | 5.7 |
| OS, median (months) | NE | 25.7 | 16.9 | 15.3 | NE | 16.4 |
| Third-line therapy (single-arm trials) . | ZUMA-1 . | JULIET . | TRANSCEND . | |||
| Follow-up, median (months) | 63.1 | — | 40.3 | 23.0 | — | |
| Received bridging chemotherapy (%) | 0 | — | 90 | — | 59 | — |
| Received intended CAR T cell (%) | 91 | — | — | — | ||
| Median time to CAR T-cell infusion (days) | 29 (27-34)* | — | 54 | — | 37 (27-224)† | — |
| ORR | 83% | 53% | — | 73% | — | |
| CR rate | 58% | — | 39% | — | 53% | — |
| Median DOR | 11.1 | — | 3 | — | 23.1 | — |
| Median duration of PR (months) | 1.9 | — | — | — | — | |
| Duration of CR (months) | 62.2 | — | 0.35 (0.23-0.53) | — | 26.1 | — |
| EFS, median (months) | 5.7 | — | NR | — | ||
| PFS, median (months) | 5.9 | — | — | 6.8 | — | |
| OS, median (months) | NR | 11.1 | 27.3 | — | ||
interquartile range.
range.
ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; CR, complete response; DOR, duration of remission; EFS, event-free survival; HR, high risk; NE, neutrophil elastase; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; SOC, standard of care.
Manufacturing delays and limited availability
There are various steps involved in using autologous CAR T-cell products, including leukapheresis, manufacturing, quality checks, transportation to and from the manufacturer, and ultimately, CAR T administration. The median time from leukapheresis to product delivery or infusion was 17 days for axi-cel,5 36 days for liso-cel,4 and 54 days for tisa-cel.6 Given the potential for rapid progression of lymphoma, especially with refractory disease, early referral of patients to a center that offers cellular therapies is essential to maximize eligibility for this potentially lifesaving therapy. Long delays in time to CAR T infusion can result in increased tumor bulk, worsened organ function, and potentially loss of eligibility for CAR T due to decline in clinical status. These delays are particularly relevant for patients receiving care in the community who require referrals to certified centers. Several real-world studies at academic centers report CAR T administration rates after leukapheresis ranging from 90% to 93%.7 However, community-based practices have reported lower CAR T completion rates with 1 large community- based practice noting that 47% of patients referred for CAR T evaluation ultimately received CAR T therapy, with a median time from referral to CAR T infusion of 143 days.8 The primary reasons for inability to receive CAR T were disease-related issues such as disease progression and decline in clinical status. Unlike auto-HSCT, patients receiving CAR T therapy do not require demonstration of chemosensitive lymphoma at the time of infusion, though their disease should not be rapidly progressing to allow time for leukapheresis, manufacturing, and infusion of the CAR T cells. Early recognition of patients eligible for cellular therapies is important to decrease delays.
Additionally, use of effective bridging therapy is particularly important to improve disease control while undergoing evaluation for CAR T therapy. Numerous patients considered for CAR T-cell therapy have lymphoma with some degree of chemotherapy resistance. Novel targeted agents such as antibody drug conjugates and bispecific antibodies have shown promising efficacy for disease control and can improve eligibility for CAR T-cell therapy. Notably, these therapies also are expensive, and providers may encounter difficulties in obtaining access depending on insurance coverage and approval. Thus, early initiation of this process is crucial.
Cost and insurance coverage
Current list pricing for the 5 FDA-approved CAR T-cell therapies ranges between $373000 and $475000 per one-time infusion. However, the average total cost of care for patients was >$700000, and 12% of patients had costs exceeding $1 million.9 The high cost relates to combined costs of the CAR T-cell product, patient preparation (eg, leukapheresis and lymphodepletion), product infusion, pre- and post-infusion patient management, and monitoring for side effects. Despite the high cost, CAR T-cell therapy products are significantly efficacious and cost-effective in the second-line (axi-cel and liso-cel) or third-line setting (axi -cel, liso-cel and tisacel).10,11
Insurance coverage is critical, and prior studies have shown that patients who were uninsured or Medicare insured were less likely to receive CAR T compared with commercially insured patients.12 Despite the proven efficacy and cost- effectiveness of CAR T-cell therapy, based on current Medicare reimbursement structure, hospitals can lose up to $304000 on each inpatient administration of CAR T for Medicare beneficiaries, which disincentivizes appropriate use of these potentially curative therapies.13
In the era prior to CAR T, patients with LBCL who were Asian/Pacific Islander, American Indian/Alaska Native, Black/ African American, from rural neighborhoods, Medicaid insured, and/or uninsured had worse survival.14-16 Patients from disadvantaged socioeconomic status groups and racial/ethnic minority groups have been historically underinsured and are thus at increased risk of a major barrier to accessing CAR T-cell therapy due to insurance. Although data addressing the relationships between insurance coverage and access to salvage therapy and CAR T-cell therapy are lacking, data regarding stem cell transplantation suggest that expansion of coverage for uninsured or underinsured since the Affordable Care Act was enacted in 2014 led to increased access, with approximately 40% of HSCT procedures performed in the United States now reimbursed by governmental payers.17 Prior to expansion, this therapy that was routinely only offered to relatively young, otherwise healthy patients who likely had commercial, employer-based insurance coverage.17 Addressing inequities in access to CAR T therapy will likely require similar policy changes. Expanding access to insurance and decreasing the cost of specialized care can be accomplished through collaborative efforts across stakeholders, including health care system, payers/insurers, and pharmaceutical manufacturers.
Site of care and geographic limitations to access
Unlike cytotoxic chemotherapy regimens that are readily available at most centers that care for cancer patients, access to cellular therapies is currently limited to specific certified centers meeting the requirements set up by manufacturers and regulatory agencies. CAR T-cell administration is generally limited to well-resourced academic medical centers with HSCT experience and Foundation for the Accreditation of Cellular Therapy (FACT) accreditation.12,18 At the time of this article, there are approximately 307 FACT-accredited centers across the United States.19 As such, there is a geographic limitation to where CAR T therapy is available. Additionally, because of the unique toxicities of CAR T-cell therapies, patients are required to be monitored closely for the first week after cell administration and then required to stay within 2 hours of the facility for up to 30 days with a dedicated caregiver.12 One study found that one-third of patients lived >120 minutes of driving from where CAR T-cell therapy was administered.12 Geographically restricted access may disproportionately impact patients in rural locations and patients from socioeconomically disadvantaged backgrounds (such as those living below the federal poverty line). Distance can increase other out-of- pocket expenses not covered by insurance, including the costs of travel and lodging. Patients and caregivers may also experience financial consequences from short-term loss of income due to required relocation. A study using quantitative and qualitative methods identified that the financial impact on patients receiving CAR T-cell therapy extends beyond the cost of treatment. Expanding access to care through site-of-care planning could help address regional, rural-urban, and sociodemographic equity in the geographic allocation of CAR T-cell therapy.20
Conclusions
Auto-HSCT remains an important option for patients experiencing late relapse of LBCL who remain chemosensitive. CAR T-cell therapies revolutionized the treatment of LBCL in the second line and beyond. The wide adoption of these therapies presents challenges, particularly for patients who receive care in community centers and remote areas. Improving provider awareness of CAR T-cell products, uses, and toxicities is essential to improving familiarity with these products and expediting referrals to centers that offer these therapies. Understanding the impacts of insurance coverage and the full cost of CAR T for patients is important to expand access. This also must consider costs of travel, housing, medications, and other unseen costs like lost wages. We recommend early involvement of social workers and case management to aid patients in mitigating challenges. Engagement of disease-specific patient advocacy groups such as the Leukemia and Lymphoma Society (www.lls.org) and the Lymphoma Research Foundation (www.lymphoma.org) can offer additional guidance and support services to patients with lymphoma and their caregivers. These services may include financial support for socioeconomically disadvantaged patients. In the future, decreasing manufacturing time for CAR T products and expanding manufacturing capacity will be essential to increasing the ability to treat more patients with cellular therapies. We recommend timely considerations of effective bridging therapies (such as novel targeted therapies) where clinically indicated to optimize disease control and avoid clinical decline that could make patients ineligible while awaiting production of CAR T cells. Together these efforts can aid in wider application of CAR T therapy where clinically appropriate and improve outcomes for patients with LBCL.
Conflict-of-interest disclosure
Chijioke Nze: no competing financial interests to declare.
Christopher R. Flowers: consultant: AbbVie, AstraZeneca, Bayer, BeiGene, Bio Ascend, Bristol Myers Squibb, Celgene, Denovo Biopharma, Foresight Diagnostics, Genentech/Roche, Genmab, Gilead, Karyopharm, N-Power, Pharmacyclics/Janssen, Seagen, Spectrum; stock/stock options private company: Foresight Diagnostics, N-Power; researcher: 4D, AbbVie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, Celgene, Cellectis, EMD Serono, Genentech/Roche, Gilead, Guardant, Iovance, Janssen, Kite, MorphoSys, Nektar, Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, Ziopharm.
Off-label drug use
Chijioke Nze: nothing to disclose.
Christopher R. Flowers: nothing to disclose.