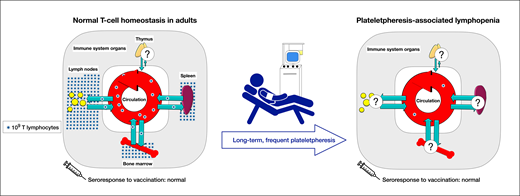
Abstract
In the United States, more than 2 000 000 apheresis platelet units are collected annually from volunteer donors. Platelet donors in the United States and elsewhere are permitted to donate up to 24 times per year. Recently, frequent apheresis platelet donation has been associated with severe T-cell lymphopenia. Several frequent platelet donors have been found to have peripheral blood CD4+ T-cell counts below 200 cells/µL, the threshold for AIDS in HIV-positive individuals. Independent risk factors for plateletpheresis-associated lymphopenia include lifetime donations, age, and donations on the Trima Accel instrument (Terumo BCT), which uses a leukoreduction system (LRS) chamber to trap white blood cells. Less often, severe lymphopenia can occur in donors collected on the Fenwal Amicus instrument (Fresenius Kabi), which has no LRS. For Trima Accel donors, lymphopenia can be partially mitigated by performing a plasma rinseback step at the end of collection. To date, there is no definitive evidence that plateletpheresis-associated lymphopenia is harmful. In a study of frequent platelet donors with lymphopenia who were administered COVID-19 messenger RNA vaccines, immune responses were normal. The homeostatic mechanisms responsible for maintaining a normal peripheral blood T-cell count remain obscure, as do the causal mechanisms underlying plateletpheresis-associated lymphopenia.
Learning Objectives
Examine risk factors for plateletpheresis-associated lymphopenia
Evaluate clinical consequences and mitigation approaches for plateletpheresis-associated lymphopenia
CLINICAL CASE
On routine screening, a 58-year-old man in his usual state of good health was discovered to be lymphopenic (lymphocyte count 512 cells/µL; reference range, 720-4100 cells/µL.) The patient's hemoglobin level, white blood cell count, and platelet count were within normal limits. Follow-up studies were remarkable for a CD4+ T-cell count of 106 cells/µL (441-2156 cells/µL) and a CD8+ T-cell count of 92 cells/µL (125-1312 cells/µL). The patient's IgG level was normal, and he tested negative for HIV by immunoassay and nucleic acid testing. The patient was referred by his primary care physician to a hematologist, who performed a bone marrow biopsy. No abnormalities were found. The patient reported donating platelets every 2 weeks for the past 10 years.
Introduction
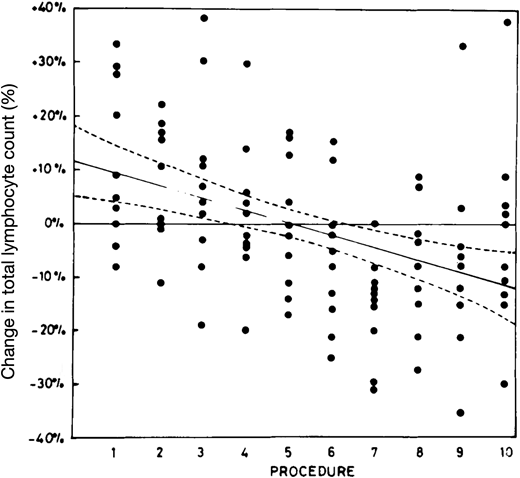
By the 1970s, apheresis technology had advanced to the point where it became feasible to collect high numbers of platelets from individual volunteer donors during a single donation. Concerns were soon raised about whether frequent apheresis platelet donations might render donors thrombocytopenic, potentially putting them at risk for bleeding. Investigators also noticed that frequent platelet donors' lymphocyte counts tended to decline, and worries were raised about possibly making donors immunodeficient. In 1981, Koepke et al1 reported a prospective study of lymphopenia in plateletpheresis donors. Ten healthy male volunteers, aged 21 to 33 years, donated platelets by apheresis 10 times over 12 weeks. None had donated whole blood or platelets previously. The volunteers' total lymphocyte counts decreased by approximately 20% during the study period (Figure 1). The investigators concluded that this was a statistically significant change but not one that was clinically meaningful.
Peripheral blood lymphocyte trends in naive volunteers undergoing frequent plateletpheresis. Ten healthy male volunteers donated apheresis platelets 10 times in 12 weeks on the Haemonetics Model 30 blood processor. Blood samples were obtained at baseline and at each of the plateletpheresis procedures. The plotted points show, for each volunteer, the percentage change in total peripheral blood lymphocytes relative to that volunteer's mean lymphocyte count for the entire study period. The solid regression line shows the average change for all participants; ±2 SD confidence intervals are indicated by the dashed lines.
Peripheral blood lymphocyte trends in naive volunteers undergoing frequent plateletpheresis. Ten healthy male volunteers donated apheresis platelets 10 times in 12 weeks on the Haemonetics Model 30 blood processor. Blood samples were obtained at baseline and at each of the plateletpheresis procedures. The plotted points show, for each volunteer, the percentage change in total peripheral blood lymphocytes relative to that volunteer's mean lymphocyte count for the entire study period. The solid regression line shows the average change for all participants; ±2 SD confidence intervals are indicated by the dashed lines.
Apheresis technology continued to evolve, and with the introduction of better automated instruments, the question of whether plateletpheresis could cause lymphopenia was set aside. In 2007, the US Food and Drug Administration dropped its requirement that donor consent forms include the potential risk of lymphocyte depletion.2 A 2008 editorial by Ronald Strauss3 asserted, “Donor lymphocytes collected or lost during plateletpheresis are so few that the risk of significant lymphocytopenia and/or immune dysfunction is nil, and no additional measures are needed.” That is where things remained until 2017, when a frequent platelet donor in Boston was incidentally discovered to have a CD4+ T-cell count below 200 cells/µL. The observation of severe T-cell lymphopenia in this donor led to subsequent investigations in frequent plateletpheresis donors, summarized below.
Risk factors for acquiring plateletpheresis-associated lymphopenia
In 2019, Gansner et al4 reported a single-center, cross-sectional study of 60 apheresis platelet donors. The donors were divided into 3 groups based on the number of platelet donations in the past 365 days: 1 or 2, 3 to 19, or 20 to 24. All donors had donated on the Trima Accel (Terumo BCT) instrument exclusively. CD4+ T-cell counts below 200 cells/µL were found, respectively, in 0 of 20, 2 of 20 (10%), and 6 of 20 (30%) donors in the 3 groups (P = .019). Similarly, CD8+ T-cell counts in the 3 groups were below the lower limit of normal in 0 of 20, 4 of 20, and 11 of 20, respectively (P < .001). There appeared to be a cumulative donation effect: a lifetime history of donating platelets 50 or more times was associated with a significant decrease in CD4+ and CD8+ T-cell counts (Figure 2). In multivariable analyses, both donor age and platelet donations were independently associated with reduced CD4+/CD8+ counts. All the donors in this study reported being in good health.4
Peripheral blood T-cell counts in frequent plateletpheresis donors. Cell counts are plotted for the 3 donor groups. (A) CD4+ T-cell counts. The horizontal dotted line indicates 200 cells/µL. (B) CD8+ T-cell counts. The horizontal dotted line indicates the lower limit of normal. Blue symbols indicate volunteers who donated 20 to 24 times in a 365-day period during the past 20 years. (C) CD4+ T-cell counts by total plateletpheresis sessions. (D) CD8+ T-cell counts by total sessions. The vertical dotted line indicates 50 donations.
Peripheral blood T-cell counts in frequent plateletpheresis donors. Cell counts are plotted for the 3 donor groups. (A) CD4+ T-cell counts. The horizontal dotted line indicates 200 cells/µL. (B) CD8+ T-cell counts. The horizontal dotted line indicates the lower limit of normal. Blue symbols indicate volunteers who donated 20 to 24 times in a 365-day period during the past 20 years. (C) CD4+ T-cell counts by total plateletpheresis sessions. (D) CD8+ T-cell counts by total sessions. The vertical dotted line indicates 50 donations.
The Trima Accel instrument's circuit incorporates a leukoreduction system (LRS) chamber, which is a small plastic cone that traps approximately 15% to 20% of circulating lymphocytes and monocytes. Approximately 1 to 2 × 109 of mononuclear cells are retained in the LRS following a platelet donation.4,5 Donor centers often provide postplateletpheresis LRS chambers to researchers, as they provide a convenient source of mononuclear cells.5 It was hypothesized that bulk removal of T cells by the LRS could contribute to the development of plateletpheresis-associated lymphopenia in donors collected on the Trima Accel. On that basis, a cross-sectional study was performed of frequent platelet donors collected on the Fenwal Amicus (Fresenius Kabi) apheresis instrument, which does not have an LRS. Among 30 frequent platelet donors collected on the Fenwal Amicus, none had a CD4+ T-cell count below 200 cells/µL. In contrast, a large single-center retrospective study conducted in Germany found that frequent donors collected mainly using the Amicus instrument had a median lymphocyte count of 1530 cells/µL, compared with 1960 cells/µL among new donors. The authors concluded that plateletpheresis-associated lymphopenia can occur without an LRS, only to a lesser degree than when an LRS is used.6 In a recent international study conducted by the Biomedical Excellence for Safer Transfusion Collaborative, CD4+ T-cell counts below 200 cells/µL were observed in 10% of frequent platelet donors collected on the Trima Accel and 4% of frequent platelet donors collected on the Fenwal Amicus (Richard M. Kaufman, unpublished data). Fenwal Amicus donors had lymphocyte counts and CD4+/CD8+ counts that were intermediate between those of age-matched whole-blood donor controls, who had higher counts, and frequent Trima Accel donors, who had lower counts. Donation on the Trima Accel vs the Fenwal Amicus was thus determined to be an independent risk factor for plateletpheresis-associated lymphopenia. However, severe lymphopenia can develop following frequent platelet donation on the Fenwal Amicus instrument, in the absence of an LRS.
Are platelet donors with severe T-cell lymphopenia immunodeficient?
An intrinsic problem with studying volunteer platelet donors is that they are, by definition, healthy. (To qualify for blood donation, donors must answer “yes” to the first question on the Donor History Questionnaire: “Are you feeling healthy and well today?”7 ) Rahmani et al8 attempted to contact individuals who used to donate platelets frequently but had stopped donating for at least 1 year for any reason. Of 15 former donors, 2 were found to have CD4+ T-cell counts below 200 cells/µL despite not donating for 1 to 2 years. Thus, plateletpheresis-associated lymphopenia can persist long after donors stop donating. But does severe T-cell lymphopenia in platelet donors reflect an acquired immunodeficiency state, or is it simply an abnormal laboratory result without meaningful clinical consequences?
In a nationwide cohort study, Zhao et al9 attempted to look for signs of clinical risk in frequent platelet donors using the Swedish Scandinavian donations and transfusions (SCANDAT3-S) database. SCANDAT3-S pulls together data from national registers in Sweden and Denmark on blood donors, blood products, transfusions, and transfusion recipients. The data span from 1968 through 2017 and encompass over 26 million person-years of donor follow-up.10 At the time the SCANDAT3-S study was performed, lymphopenia had been observed in platelet donors collected on the Trima Accel instrument but not yet among donors collected on the Fenwal Amicus.8 Zhao and colleagues9 also sought to avoid potential confounding by donor self-selection—the so-called healthy donor effect—whereby donors who feel unwell donate less often. Therefore, the investigators chose to compare apheresis platelet donors collected using an LRS chamber (COBE Spectra and Trima Accel) with platelet and plasma donors who donated the same number of times but without an LRS (Spectra Optia; all 3 instruments from Terumo BCT). A second maneuver to mitigate against the healthy donor effect involved using a time-dependent analysis. The investigators defined a 10-year exposure window that excluded donations in the most recent 1 year, to try to prevent imbalances in the numbers of donations analyzed due to donors becoming ill and donating less frequently as a result.
A total of 74 048 plateletpheresis and plasmapheresis donors were included in the SCANDAT3-S analysis. Among donors with the same number of donations, there were significantly more immunosuppression-related infections (Figure 3A) and common bacterial infections (Figure 3B) among donors collected using an LRS compared to those collected without an LRS. Notably, no immunosuppression-related infections were observed among the highest-risk group, LRS donors who had donated more than 50 times. In all, only 11 immunosuppression-related infections were found among LRS donors, mainly herpes zoster. No donor was found to have had a severe illness (eg, disseminated mycobacterial infection or aspergillus). While provocative, there were important limitations to the Zhao et al9 study. This was a retrospective, observational study with potential for residual confounding. There were relatively few frequent plateletpheresis donors: just 95 donors in the data set (1.4%) had donated 20 or more times in a single year. Finally, as noted above, the number of infection events was small.11
Risk of infections with LRS+ donations vs LRS− apheresis platelet donations, in relation to number of donations between 1 and 11 years in the past, modeled as a restricted cubic spline. (A) Immunosuppression-related infections. (B) Common bacterial infections. Confidence intervals are shown in gray. HR, hazard ratio.
Risk of infections with LRS+ donations vs LRS− apheresis platelet donations, in relation to number of donations between 1 and 11 years in the past, modeled as a restricted cubic spline. (A) Immunosuppression-related infections. (B) Common bacterial infections. Confidence intervals are shown in gray. HR, hazard ratio.
Laumaea et al12 evaluated the ability of frequent apheresis platelet donors to respond to COVID-19 vaccination. The investigators recruited a cohort of 43 COVID-19 infection-naive platelet donors who had donated more than 5 times per year on the Trima Accel instrument. The donors were administered 2 doses of a COVID-19 messenger RNA vaccine. Blood samples were collected at baseline and approximately 5 or 6 weeks after the first dose and second doses, respectively (Figure 4A). The donors were divided into 2 groups: those with CD4+ T-cell counts below 400 cells/µL (n = 27) and those with CD4+ T-cell counts at or above 400 cells/µL (n = 16). Consistent with earlier studies, donors in the CD4+-low group had a median of 166 lifetime donations vs a median of 24 lifetime donations in the CD4+-high group (P < .0001). Antibody to SARS-CoV-2 receptor binding domain was low at baseline (V0) in both groups and increased significantly in both groups following vaccination. The IgG response in the CD4+-high group was slightly higher than in the CD4+-low group, but the difference was not statistically significant (Figure 4B). The CD4+-low and CD4+-high groups were also similar in their IgM and IgA anti–receptor binding domain responses, in spike-specific antibody formation, in pseudovirus neutralization assays, and in an anti–SARS-CoV-2 antibody-dependent cellular cytoxicity assay. In aggregate, these data provide reassuring evidence of preserved immune function in frequent plateletpheresis donors.
Anti–receptor binding domain (RBD) antibody responses in plateletpheresis donors receiving COVID-19 messenger RNA (mRNA) vaccines. (A) Study schema. COVID-19 infection-naive CD4+-low (teal) and CD4+-high (orange) platelet donors were administered 2 mRNA vaccine doses per the schedule shown. (B) Anti-RBD IgG levels at baseline (V0) and after (V1, V2) each dose of vaccine. **P < .01; ****P < .0001.
Anti–receptor binding domain (RBD) antibody responses in plateletpheresis donors receiving COVID-19 messenger RNA (mRNA) vaccines. (A) Study schema. COVID-19 infection-naive CD4+-low (teal) and CD4+-high (orange) platelet donors were administered 2 mRNA vaccine doses per the schedule shown. (B) Anti-RBD IgG levels at baseline (V0) and after (V1, V2) each dose of vaccine. **P < .01; ****P < .0001.
Mitigation
T-cell lymphopenia tends to be more frequent and severe when donating on the Trima Accel instrument vs the Fenwal Amicus instrument, consistent with mononuclear cell capture by the LRS contributing to the development of lymphopenia. Although not routinely used by most blood donor centers, the Trima Accel provides a “plasma rinseback” option, which returns to the donor 22%13 to 74% (A. Razatos, personal communication, 2022) of white blood cells remaining in the disposable tubing of the apheresis circuit. The rinseback procedure was designed to minimize red blood cell loss in the disposable tubing and does not flush cells out of the Trima Accel's LRS chamber. In 2013, Canadian Blood Services (CBS) instituted rinseback for all platelet collections using the Trima Accel instrument. Multiple CBS blood centers contributed data to the Biomedical Excellence for Safer Transfusion Collaborative study of plateletpheresis-associated lymphopenia. The CBS blood centers were directly compared to other centers using the Trima Accel that do not perform rinseback. The results suggest that Trima Accel plasma rinseback partially mitigates against the development of plateletpheresis-associated lymphopenia. Among 40 frequent platelet donors collected on the Trima Accel using rinseback, the mean CD4+ T-cell count was significantly lower than that of matched whole-blood donor controls. However, 0 of 40 Trima Accel donors receiving rinseback had a CD4+ T-cell count below 200 cells/µL, compared with 13 of 91 Trima Accel donors (14%) collected without rinseback.
T-cell homeostasis and other open questions
In adult humans, approximately 1011 naive T cells circulate in the peripheral blood and through lymphoid organs.14 T-cell progenitors originate in the bone marrow, then migrate to the thymus, where they undergo maturation and selection. Some of these cells are eventually released from the thymus as naive T cells, ready to respond should they encounter specific antigen. In mice, the thymus continually produces large numbers of naive T cells throughout the life of the organism. In contrast, thymic export of naive T cells is sharply curtailed in childhood in humans. A small number of new thymic emigrants can be found in middle-aged adults, but the bulk of the naive T-cell population in adult humans is sustained by cell proliferation in other lymphoid organs.15-18 T cells circulating in the blood are easy to access and can provide critical prognostic information, as in patients infected with HIV. But only about 2% to 3% of the body's total T cells circulate in the peripheral blood at any given time.16,19 Most naive T cells reside in lymph nodes.18 Mouse experiments suggest that within lymph nodes, IL-7 and IL-15 provide key survival and proliferation signals to resident T cells.20 More recently, the cysteine-rich with EGF-like domains 1 (CRELD1) gene has been implicated as a regulator of T-cell homeostasis in humans.21
Overall, however, how the peripheral blood T-cell count is normally maintained remains poorly understood. In frequent platelet donors with low blood T cells, nothing is known at this point about T-cell numbers and homeostatic proliferative activity within their lymphoid tissues. The vaccine study12 summarized above provides reassuring evidence of preserved immune function in donors with plateletpheresis-associated lymphopenia. As illustrated by the Clinical Case, hematologists should be aware of this entity to avoid unnecessary medical workups in otherwise healthy individuals. Donor centers using the Trima Accel instrument are advised to perform plasma rinseback routinely to reduce the risk of donor lymphopenia. The mononuclear cell content of LRS chambers is largely preserved following plasma rinseback,13 so the LRS chambers remain useful for research. Finally, during the donation consent process, frequent platelet donors should be made aware of the possibility of developing plateletpheresis-associated lymphopenia.
Conflict-of-interest disclosure
Richard M. Kaufman: no competing financial interests to declare.
Off-label drug use
Richard M. Kaufman: Nothing to disclose.