Learning Objectives
Understand the void filled by gene therapy in the treatment paradigm for transfusion-dependent β-thalassemia
Evaluate the appropriateness of gene therapy for individual patients with transfusion-dependent β-thalassemia
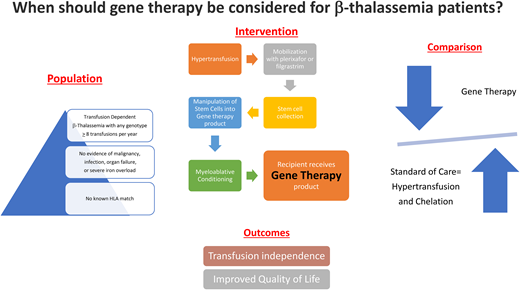
When should gene therapy be considered for transfusion-dependent β-thalassemia patients?
Case: The parent of a 12-year-old boy of Greek ancestry who has transfusion-dependent β-thalassemia are asking about curative options. He does not have any matched related donors, and a search through the international bone marrow donor registry has not identified a match either. He is transfused every 3 weeks to maintain a pretransfusion hemoglobin level of 9.5 to 10.5 g/dL and is well chelated, with a most recent liver magnetic resonance imaging showing a liver iron concentration of 3.4 mg/g dry weight and a cardiac T2* of 42 ms. The parents are concerned about school absences and their son's desire to participate in competitive sports as he gets older.
Transfusion-dependent β-thalassemia is a lifelong condition with a high physical and emotional burden of disease with tethering to the medical establishment, a propensity for many systemic complications, and impairment of quality of life. The recent approval of lentiviral gene addition therapy and the likely approval of CRISPR/CAS9-based gene-editing therapy dramatically increase “curative” options for this disease.1 However, as a treatment with significant potential for toxicity and side effects, patient selection will be key to ensuring excellent long-term outcomes. Using a population, intervention, comparison, outcome (PICO)-based analysis, we suggest an algorithm to assist in this. We have deliberately not included the economics of gene therapy to be able to focus on the clinical decision-making only.
Patient population: Individuals with transfusion- dependent (conventionally accepted as ≥8/y) β-thalassemia.
Intervention: Autologous hematopoietic stem cell transplant with lentiviral gene addition (the only currently approach approved by the US Food and Drug Administration uses beti-cel [a product of CD34+ cells transduced with the BB305 lentiviral vector encoding the β-globin (βA-T87Q) gene])2
Comparison: Continued standard-of-care management with regular transfusions and chelation or allogeneic hematopoietic stem cell transplantation (HSCT).
Outcome: Achievement of transfusion independence with improvement in quality of life.
Intervention: Gene therapy
Gene therapy for β-thalassemia involves harvesting stem cells by pheresis after plerixafor/granulocyte colony stimulating factor stimulation, modification of these cells (transduction of a lentiviral vector containing a copy of the β-globin gene, or genome editing to knock down BCL11A to allow for reactivated γ-globin expression so as to restore fetal hemoglobin production), and reinfusion after myeloablative conditioning.2,3 Once engraftment has occurred (taking somewhat longer than an allogeneic transplant), endogenous production of red cells containing HbA or HbF ameliorates the anemia, leading to transfusion independence. Data from clinical trials have confirmed durable engraftment with stable hemoglobin levels in both adults and children with transfusion-dependent thalassemia (91% of patients achieving transfusion independence in the Northstar trials using the lentiviral vector and 95% in the CLIMB trials using CRISPR/CAS9 editing).1,4 Early gene addition trials had better outcomes in those with non-β0/β0 genotypes, but more recent data suggest that age, genotype, and splenectomy status do not play a role. Complications are mostly related to the transplant procedure itself, including infections during the engraftment period, some delayed platelet recovery, and veno-occlusive disease (now not seen following introduction of standard prophylaxis with defibrotide), with no deaths, minimal graft-vs-host-disease, and no major concerns for marrow dysplasia.5 (Caveat: the bone marrow in individuals who have successfully undergone gene therapy still shows evidence of ineffective erythropoiesis.6 ) Infertility and risk for clonal disease may be comparable to allogeneic HSCT. Successful treatment resulted in improvements in health-related quality of life, and normalization of day-to-day activity.2 Table 1 provides a summary of trials and the key outcomes described in each.
Gene therapy trials for β-thalassemia
| Trial name . | Study design . | Inclusion criteria . | Exclusion criteria . | Intervention . | Comparison . | Outcome . |
|---|---|---|---|---|---|---|
| NorthStar Hgb 2042,6 | Phase 1/2 N = 19 Multisite | TDT age 12-35 years Any genotype | Severe iron overload Prior transplant | Beti-cel | Standard of care: Transfusion dependence and chelation | Transfusion independence for 24 months in 61% |
| Hgb2052,6 | Phase 1/2 Single site (Paris) N = 4 | TDT Age 5-35 years Any genotype | Malignancy Organ damage Infection Neutropenia | Beti-cel | Standard of care: Transfusion dependence and chelation | Transfusion independence for 24 months in 75% |
| Northstar 2 Hgb 2072,3 | Phase 3 N = 23 Multisite | TDT Age <50 years Non-β0/β0 genotype | β0/β0 genotype Known HLA match | Beti-cel | Standard of care: Transfusion dependence and chelation | Transfusion independence in 91% |
| Northstar 3 Hgb 2122,3 | Phase 2 Multisite N = 23 | TDT β0/β0, β0/β+ IVS-I-110, and β+ IVS-I-110/β+ IVS-I-110 genotypes Age <50 years | Presence of a mutation characterized as other than β0 (eg, β+, βE, βC) on at least 1 β-globin gene (HBB) allele | Beti-cel | Standard of care: Transfusion dependence and chelation | Transfusion independence in 86% |
| LTF-3032,8 | Multiphase Multisite N = 41 | TDT Previously treated with beti-cel | None | Follow-up after beti-cel 13 years | Standard of care: Transfusion dependence and chelation | Transfusion and chelation independence in 68% in phase 1/2 and 89% in phase 3 |
| β-Thalassemia Major With Autologous CD34+ Hematopoietic Progenitor Cells Transduced With TNS9.3.55 a Lentiviral Vector Encoding the Normal Human β-Globin Gene1,9 | Phase 1 Multisite N = 4 | TDT of any genotype | Infection Diabetes Myelodysplasia | Autologous CD34+ HSPCs transduced with TNS9.3.55, a lentiviral vector encoding the normal human β-globin gene Reduced-intensity busulfan | Standard of care: Transfusion dependence and chelation | Transfusion requirements reduced by 50% |
| GLOBE1,10 | Phase 1/2 N = 7 | TDT of any genotype Age >3 years | If age <18 years with an HLA match | GLOBE lentiviral vector–transduced CD34+ cells | Standard of care: Transfusion dependence and chelation | Three adults with reduced transfusion requirements Four pediatric patients with transfusion independence |
| CLIMB-THAL 1111,4 | Phase 1/2 | TDT of any genotype Age 3-64 years | Infection Malignancy | Autologous CRISPR-Cas9–edited CD34+ HSPCs | Standard of care: Transfusion dependence and chelation | 42/44 = 95% transfusion independence 2/44 = 5% reduced transfusion requirements |
| Trial name . | Study design . | Inclusion criteria . | Exclusion criteria . | Intervention . | Comparison . | Outcome . |
|---|---|---|---|---|---|---|
| NorthStar Hgb 2042,6 | Phase 1/2 N = 19 Multisite | TDT age 12-35 years Any genotype | Severe iron overload Prior transplant | Beti-cel | Standard of care: Transfusion dependence and chelation | Transfusion independence for 24 months in 61% |
| Hgb2052,6 | Phase 1/2 Single site (Paris) N = 4 | TDT Age 5-35 years Any genotype | Malignancy Organ damage Infection Neutropenia | Beti-cel | Standard of care: Transfusion dependence and chelation | Transfusion independence for 24 months in 75% |
| Northstar 2 Hgb 2072,3 | Phase 3 N = 23 Multisite | TDT Age <50 years Non-β0/β0 genotype | β0/β0 genotype Known HLA match | Beti-cel | Standard of care: Transfusion dependence and chelation | Transfusion independence in 91% |
| Northstar 3 Hgb 2122,3 | Phase 2 Multisite N = 23 | TDT β0/β0, β0/β+ IVS-I-110, and β+ IVS-I-110/β+ IVS-I-110 genotypes Age <50 years | Presence of a mutation characterized as other than β0 (eg, β+, βE, βC) on at least 1 β-globin gene (HBB) allele | Beti-cel | Standard of care: Transfusion dependence and chelation | Transfusion independence in 86% |
| LTF-3032,8 | Multiphase Multisite N = 41 | TDT Previously treated with beti-cel | None | Follow-up after beti-cel 13 years | Standard of care: Transfusion dependence and chelation | Transfusion and chelation independence in 68% in phase 1/2 and 89% in phase 3 |
| β-Thalassemia Major With Autologous CD34+ Hematopoietic Progenitor Cells Transduced With TNS9.3.55 a Lentiviral Vector Encoding the Normal Human β-Globin Gene1,9 | Phase 1 Multisite N = 4 | TDT of any genotype | Infection Diabetes Myelodysplasia | Autologous CD34+ HSPCs transduced with TNS9.3.55, a lentiviral vector encoding the normal human β-globin gene Reduced-intensity busulfan | Standard of care: Transfusion dependence and chelation | Transfusion requirements reduced by 50% |
| GLOBE1,10 | Phase 1/2 N = 7 | TDT of any genotype Age >3 years | If age <18 years with an HLA match | GLOBE lentiviral vector–transduced CD34+ cells | Standard of care: Transfusion dependence and chelation | Three adults with reduced transfusion requirements Four pediatric patients with transfusion independence |
| CLIMB-THAL 1111,4 | Phase 1/2 | TDT of any genotype Age 3-64 years | Infection Malignancy | Autologous CRISPR-Cas9–edited CD34+ HSPCs | Standard of care: Transfusion dependence and chelation | 42/44 = 95% transfusion independence 2/44 = 5% reduced transfusion requirements |
HLA, human leukocyte antigen; HSPC, hematopoietic stem cells; TDT, transfusion-dependent thalassemia.
Comparison: Standard transfusion and chelation
Management of transfusion-dependent thalassemia is extremely patient time-intensive and burdensome, necessitating frequent all-day visits to the hospital for transfusions and the potential for long-term complications, including transfusion-associated infections, alloimmunization, with systemic iron overload and organ dysfunction despite chelation therapy. Chelation therapy requires excellent compliance to prevent iron-related organ damage and has its own toxicities. This has a tremendous physical, mental, emotional, and economic burden on patients and families, with symptoms from chronic anemia, organomegaly (extramedullary hematopoiesis), endocrinopathy, bone disease and vasculopathy (pulmonary hypertension, silent cerebral infarction), anxiety and depression, and loss of school or work days. Median age at death in the United States is ~37 years, half that of the average American, mostly related to iron overload–induced heart failure.2 Until recently, allogeneic HSCT from a matched related donor (MRD) (more recently matched unrelated donor as well) has been the best “curative” therapy, achieving transfusion independence in over 90% of individuals when performed at a younger age.7 However, the nonavailability of a matched donor limits this as a viable option in most patients.
Patients should have a detailed discussion on the process and timeline, efficacy and safety profile, short- and long-term complications, and alternatives. It would be appropriate to offer gene therapy to motivated patients after further discussion of the following:
Metanalysis of registry data showed the best results in children under age 7 years (with limited fertility preservation options) who had MRD, with less than optimal results in patients over the age of 15 years, especially without matched donors.7 No such age-related trend was seen in the gene therapy clinical trials. While most trials limited participation to patients between 4 and 35 years, age has not been defined in the recent Food and Drug Administration approval. It would be appropriate to offer gene therapy to younger patients, since they would be most likely to have good outcomes, and derive the longest benefit. It may be reasonable to offer this to even older individuals, particularly those who have been well transfused and chelated and do not have the preexisting morbidities described, and would therefore be less likely to have adverse outcomes.2
Individuals with available MRDs were excluded from the trials.1,2 However, with demonstrated equivalent efficacy in achieving transfusion independence and the low risk of graft-vs-host-disease, gene therapy may now be a consideration even in when a MRD is available so long as there is a continued demonstrable durable response without increased risk of development of clonal disease (which may be related to an inherent risk in thalassemic stem cells).
Individuals with iron overload–induced hepatic fibrosis or cirrhosis, as well as those with renal dysfunction, would be high-risk candidates to undergo HSCT and likely should be excluded.
Fertility preservation is an important consideration and should feature in all discussions. Being able to receive gene therapy with equivalent success at an older age has the benefit of being able to wait until postpubertal fertility preservation is possible or family planning is completed.
Continuing transfusion and chelation therapy may be considered by patients/families who feel the risks of stem cell transplantation are too great, and the discussion should include (1) recent data7 showing improving outcomes with allogeneic HSCT and comparable results with gene therapy, (2) the potential for development complications related to iron overload or vascular disease, (3) potential reduced life expectancy, and (4) the ongoing burden of being tightly tethered to the medical system.
Outside of the clinical trial setting, the challenge is to offer the option of gene therapy to patients who will not only be most likely to benefit but also be least likely to have adverse events. Given that there may still be some uncertainty around durability and potentially some unknown risk for clonal disease, we believe the certainty of benefit in the form of transfusion independence and reduced risk of future complications is high, compared with continuing transfusions and chelation with the attendant risks of iron overload, endocrinopathies, bone disease, and vasculopathy.2 Gene therapy has benefits over allogeneic HSCT, but uncertainties around long-term safety and durability limit this comparison.
Most pressing need: Transfusion-dependent thalassemia has a high burden of disease, potential for many systemic life-limiting morbidities, and limited conventional “curative” options. A durable, well-tolerated treatment approach with acceptable toxicity which achieves transfusion independence would provide a viable “curative” option for more patients.
Conflict-of-interest disclosure
Cheryl Mensah reports consultancy fees from Vertex.
Sujit Sheth reports consultancy fees from Agios Pharmaceuticals, bluebird bio, Bristol Myers Squibb, Forma, and Chiesi and serves on a clinical trial steering committee for CRISPR/Vertex CTX001 for thalassemia.
Off-label drug use
Cheryl Mensah: There is no off-label drug use mentioned.
Sujit Sheth: There is no off-label drug use mentioned.