Abstract
Discussions regarding gonadal function and possible disease or treatment-related ovarian or testicular dysfunction, sexual dysfunction, and possible future infertility can be challenging in the sickle cell disease (SCD) pediatric care setting. A construct that stratifies topics into those that are time sensitive and those that require reproductive care expertise vs address gonadal health as a part of normal SCD care may be helpful. Pediatric health care discussions of gonadal function/dysfunction for patients with SCD can include (1) time-sensitive fertility consults preceding the start of gonadotoxic therapy and (2) targeted discussions at key time points during normally scheduled hematology clinic visits. The former conversations are best led by individuals with expertise in the risk for treatment-related infertility and fertility preservation. The latter discussions can be incorporated into targeted regularly scheduled visits with hematologists. These topics can be addressed as a part of planned education in pediatric care for adolescents and incorporated into transition plans as young adults transfer care to adult providers. Although the topics of puberty and gonadal health can be uncomfortable and many complex interdisciplinary and ethical issues arise in this process, these discussions can be aided by the collaterals and teaching handouts presented in this article.
Learning Objectives
Identify areas of normal pubertal development and gonadal health that SCD or its treatments can affect, and discuss how hematologists can address these topics as a component of longitudinal health care in sickle cell clinic visits
Utilize reproductive health specialists, such as fertility teams, to assist in counseling before the initiation of gonadotoxic therapies, both to establish the level of risk for infertility/gonadal dysfunction and to provide information about fertility preservation options
Ensure that all sickle cell patients coming of age and assuming responsibility for making decisions about their health care are offered a comprehensive discussion about reproductive health and a fertility status assessment and other referrals as needed
Introduction
Discussions regarding gonadal function and disease or treatment-related ovarian or testicular dysfunction, sexual dysfunction, and possible future infertility are challenging in the setting of the pediatric care of sickle cell disease (SCD). Discussions in the pediatric health care setting can include: (1) time-sensitive fertility consults preceding the initiation of gonadotoxic therapy and (2) targeted discussions at key time points during regular hematology visits. The former conversations are best led by experts in treatment-related infertility and fertility preservation. Such experts are often available to pediatric hematology/oncology programs. The latter discussions can be incorporated into targeted regularly scheduled hematology visits. These topics can be addressed as part of planned education in pediatric care for adolescents and incorporated into plans as young adults transition their care to adult hematologists. Although discussions of puberty and gonadal health can be uncomfortable and bring complex interdisciplinary and ethical issues, we hope to mitigate this process via the collaterals and teaching handouts presented in this manuscript.
Discussions regarding gonadal health should be tailored to the patient's age and developmental stage, as well as treatment scenario, and may include the following topics: pubertal development, risk of failure to produce adequate sex hormones (estrogen and testosterone), risk of future infertility, and risk of sexual dysfunction. In addition, these are opportune times to discuss the use of contraception, safe sex practices, and pregnancy risks for patients with SCD. These topics must be reviewed repeatedly as patients mature, each time adapting to the patient's developmental stage. These discussions can be awkward and challenging for patients, families, and pediatric providers. The following recommendations may help in guiding these discussions.
The first recommendation is to use a patient communication strategy such as PLISSET (a construct that aids reproductive health conversations and includes the concepts of permission, limited information, specific situation, and extensive therapy).1,2 The PLISSET methodology provides a structured outline that can aid practitioners in their approach to sensitive conversations. PLISSET begins with asking the patient/family if they give permission to engage in a discussion of how SCD, current treatments, or proposed treatments can have an impact on reproductive health. Allowing this buy-in process is empowering and may improve receptivity to information. The other components of PLISSET recommend that limited and specific information be communicated and professional support offered for distressed patients.
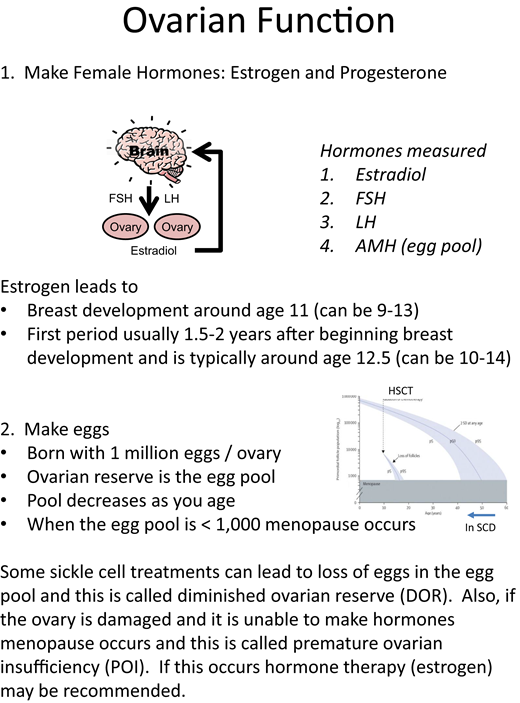
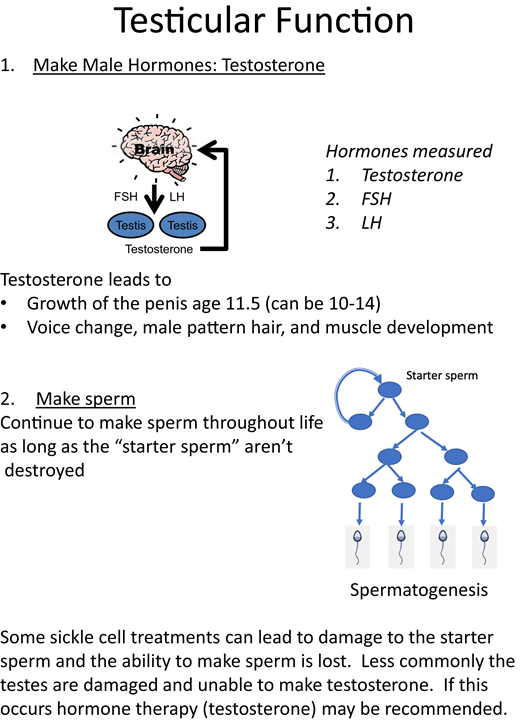
The second recommendation is to set a foundation of understanding about the function of the ovaries/testes (Figures 1 and 2). It is helpful to define the 2 roles of the gonads as hormone production and gamete (oocyte or sperm) production. The normal timing of puberty, normal variations in the timing of the onset of puberty, and delayed puberty in patients with chronic disease should be discussed. Patients with SCD may experience a constitutional delay of growth and puberty related to the malnutrition and hypermetabolism associated with anemia.3 In patients considering any type of hematopoietic stem cell transplantation (HSCT), discussions should include information about how HSCT can interrupt the progression through the stages of puberty (Figure 3) and result in premature ovarian insufficiency (POI) in females and infertility in both sexes.4-6 Be sure to define terms, like “puberty” and “infertility,” that the patient may not fully understand. Puberty consists of the development of secondary sex characteristics and in patients with SCD may be associated with increased pain frequency in females and males and priapism in males.7-10 Infertility is defined as being unable to conceive after 1 year of unprotected sex or 6 months in the context of fertility-risking conditions.11 These concepts lay the foundation for ongoing discussions about reproductive health. However, it is important to emphasize that having SCD does not automatically cause infertility and that unprotected intercourse may lead to pregnancy and/or sexually transmitted infections.
Concepts to include in discussions about ovarian function. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Concepts to include in discussions about ovarian function. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Concepts to include in discussions about testicular function. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Concepts to include in discussions about testicular function. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
The third recommendation is to provide information about how alterations in gonadal function can be treated. Estrogen or testosterone deficiency can be treated with hormone replacement, often chronically. In considering estrogen for replacement therapy and or contraception, thrombotic risk must be considered. The severity and reversibility of the lost ability to produce gametes is variable and dependent on the specific gonadotoxic exposure, such as hydroxyurea therapy vs HSCT.4-6,12-16
The last recommendation is to identify 3 or 4 key concepts the patient/family should retain from the discussion and utilize techniques such as the “teach-back” method to ensure the patient/family's understanding of these key concepts.17,18 Handouts given to the patient can help solidify understanding and retention.2
The following case examples illustrate how to have these conversations and potential psychosocial and ethical considerations.
CLINICAL CASE 1
The parents of a 2-year-old boy with hemoglobin SS are considering HSCT and request information on preimplantation genetic diagnosis to select an embryo that might become a donor for their child. They also want to discuss the risk of infertility after HSCT. Who should answer these questions? What are his options? How would the consultation differ if this were a 6-year-old girl?
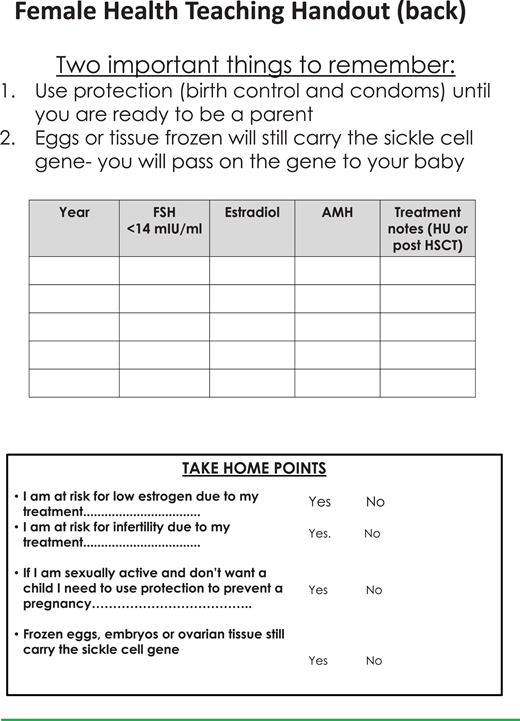
This scenario warrants consultation with a physician specializing in treatment-related infertility, assisted reproductive technology, and fertility preservation. Pediatric hematologists/oncologists often have access to these experts either as part of the pediatric system (fertility preservation team or practitioner) or in adult reproductive endocrine infertility or urology specialties. These fertility consultants can evaluate the risk for gonadal dysfunction in the child related to pretransplant therapies and the gonadotoxicity of the proposed HSCT- conditioning regimen. No HSCT has been proven to be free from gonadotoxicity; infertility risk should be discussed, and fertility preservation should be offered prior to all HSCT.4 Alterations in gonadal function have been preliminarily described in patients on hydroxyurea, including diminished ovarian reserve in some females5,6,12-14 and potentially reversible impaired spermatogenesis.15,19 Although the loss of spermatogonial stem cells in testicular tissue harvested for fertility preservation has been reported, it is unclear whether this is hydroxyurea related or disease related.20 Patients at a significant level of risk for treatment-related gonadal dysfunction/infertility should be offered fertility preservation. Standard of care options for fertility preservation include sperm banking in pubertal boys and oocyte cryopreservation in pubertal girls. In 2019, ovarian tissue cryopreservation, involving the laparoscopic removal of an ovary and the cryopreservation of cortical strips, was deemed no longer experimental and can now be offered to prepubertal and postpubertal females at high risk for future infertility.21 The only option for prepubertal boys is testicular tissue cryopreservation, which remains experimental, with no human births from this intervention.21 It is always important to discuss two key facts in pre-HSCT consults: first, any cryopreserved oocytes/sperm will carry the sickle cell gene, and second, until pregnancy is desired, contraception should be used.
Psychosocial and ethical considerations
When decisions are made for minors, the accepted approach to promoting autonomy is to utilize shared decision-making, including the maximum reasonable participation from the child and support for their right to an open future. This means preautonomous minors should have their future decision-making preserved, such as maintaining the option for fertility when possible.22-24 This is difficult when very young patients with SCD are recommended for HSCT, which does not allow for decisions to be put off for maturity.25 A child being transplanted prior to an age at which they can participate in shared decision-making should have their future autonomy preserved via cryopreservation. Although risks and uncertainties are involved with this choice, physicians must not assume these barriers are insurmountable and should educate families to the greatest extent possible.
CLINICAL CASE 2
A female patient underwent HSCT at age 5 and is now 13 with no signs of puberty. Her parents would like to discuss delayed puberty and ovarian failure but do not want her to know her risk of infertility. How do you navigate this conversation? Would you discuss the situation differently if the patient were male? How would you discuss gonadal health if the patient had not had HSCT but was on hydroxyurea or exclusively receiving chronic transfusions?
As a part of normal health maintenance, we recommend a discussion of puberty and the effects of SCD and its treatments on reproductive health beginning at age 13 or 14. For patients with SCD who have been transplanted, this can be overlooked since it may not be clear where long-term follow-up should occur. HSCT results in significant hormonal deficiency in females, with 68% to 100% presenting with amenorrhea or POI/ovarian failure.4 Females post HSCT are likely to require hormone therapy, but the need for hormone replacement is uncommon in adolescent males. Coordination between the hematology team, the HSCT team, and endocrine specialists is necessary to ensure there is timely surveillance for gonadal dysfunction and opportunities for counseling regarding gonadal health in these patients. For patients with SCD who have not had curative therapies, these conversations should occur in an SCD clinic. Discussion can begin by reviewing the 2 functions of the ovaries/testes, the normal timing of puberty, and possible delays in puberty in adolescent patients with SCD.26-30 The central hypogonadism associated with chronic transfusion and the hypothalamic/pituitary iron loading observed in patients with beta-thalassemia do not seem to occur in SCD.31 Primary hypogonadism secondary to iron overload is a theoretical concern but is not supported by the literature. Diminished ovarian reserve and impaired spermatogenesis have been reported in patients with SCD on hydroxyurea therapy; however, sex hormonal production does not appear to be affected.5,6 Figures 1 and 2 may be helpful to the provider when reviewing the normal roles of the ovary/testis and can be used in guiding the discussion with patients and their families. Figure 3 reviews Tanner staging, which only needs to be done for breast development in females and genitalia in males. Pubic hair Tanner staging can be misleading and may be related to adrenarche. Although adrenarche and gonadarche can occur at similar ages, the production of dehydroepiandrosterone sulfate by the adrenal glands can lead to pubic hair development while the hypothalamic gonadal axis is still inactive. If females have delayed puberty, puberty arrest, or POI, they should be referred to endocrinology or gynecology for evaluation and possible hormone replacement therapy with attention to minimizing thrombotic risk. The topic of gonadal health can be addressed annually and provides opportunities to discuss sexual health and contraception.
Psychosocial and ethical considerations
Navigating parental requests not to disclose the risk of infertility vary based on the age and maturity of the patient and the jurisdiction in which the physician practices.32 Parents from cultures where families, rather than individuals, are considered the central unit of decision-making are more likely to request that distressing information not be shared with the minor.33 Some parents may intuitively sense that their child is not ready to process this kind of medical information. Requests not to disclose information should be the starting point for conversations about how decisions are made in their culture, what the family and patient understand thus far, any distress the patient has previously exhibited, and, where applicable, any relevant state laws governing how teens should receive relevant medical care. Minor patients should always be given the opportunity to ask questions, and we would recommend that any questions they formulate should always be answered honestly. A simple standard is that a patient who can articulate a question is probably ready for the answer. However, it is ethically acceptable not to force this conversation to happen. Although our culture and sometimes our laws strongly support autonomy, this is not universal, and we should respect other cultural morals and minimize prejudice against divergent cultural practices, a concept known as cultural relativism.34,35 This is especially relevant in the treatment of SCD, given the diverse patient populations affected by it. That said, the approach should not differ based on sex, gender identity, or sexual orientation.
CLINICAL CASE 1 AND 2 (Continued)
The patients are now 18 years of age and are legally responsible for their own health care decisions. As they prepare to transition to adult health care providers, what information about reproductive health should their pediatric providers be sure the patients are aware of?
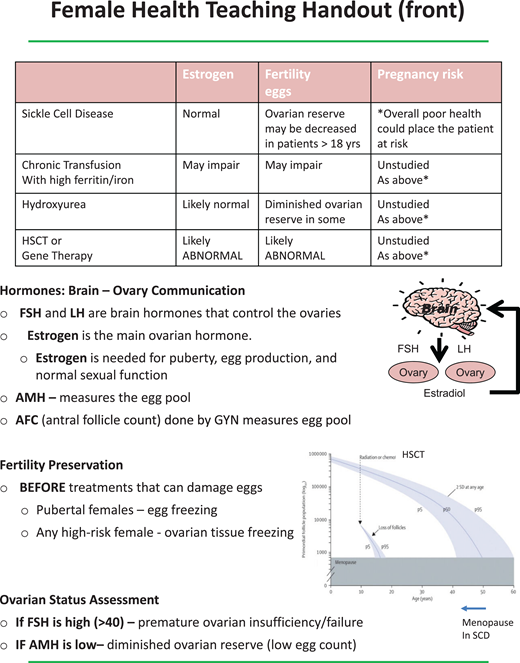
Following a model used in cancer survivorship, at age 18 patients become responsible for their own health care decisions and are offered a discussion of how their disease and its treatments can affect sex hormone production, fertility, and sexual function. The patient can choose whether to include a parent in the discussion.2 Teaching sheets to help guide these discussions are provided (Figures 4-7). For males, potential effects on hormone production, infertility, and sexual function are listed for SCD, as well as recurrent priapism, chronic transfusions with iron overload, hydroxyurea, and HSCT/gene therapy. For female patients the handout addresses the effects on hormone production, fertility, and pregnancy risk from SCD, chronic transfusions with iron overload, hydroxyurea, and HSCT/gene therapy. The tables in the handouts are not comprehensive but instead offer a platform for discussing the many factors that can have an impact on gonadal health in SCD. Fertility assessment can be offered, including semen analysis and measurement of follicle-stimulating hormone levels and early-morning testosterone levels for males and an evaluation of anti-Mullerian hormone (AMH), follicle-stimulating hormone, and estradiol, drawn on days 3 to 5 of the menstrual cycle, for females. If semen analysis on hydroxyurea is abnormal, a 2-cycle (180 day) trial off hydroxyurea may be considered and should be coordinated with the hematologist. If the semen parameters improve off hydroxyurea, sperm cryopreservation prior to restarting hydroxyurea may be advised. If AMH is low in females on hydroxyurea, referrals can be made to reproductive endocrinology for further evaluation, and recommendations can be made regarding future SCD treatments and fertility preservation options. It is imperative to review the need for contraception until pregnancy is desired even in patients with significant ovarian or testicular dysfunction. This information is helpful to review in the last visit before the transition to adult care.
Teaching sheets on female health to aid discussions with patients. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Teaching sheets on female health to aid discussions with patients. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Teaching sheets on female health to aid discussions with patients (part 2). FSH, follicle-stimulating hormone; HU, hydroxyurea.
Teaching sheets on female health to aid discussions with patients (part 2). FSH, follicle-stimulating hormone; HU, hydroxyurea.
Teaching sheets on male health to aid discussions with patients. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Teaching sheets on male health to aid discussions with patients. FSH, follicle-stimulating hormone; LH, luteinizing hormone.
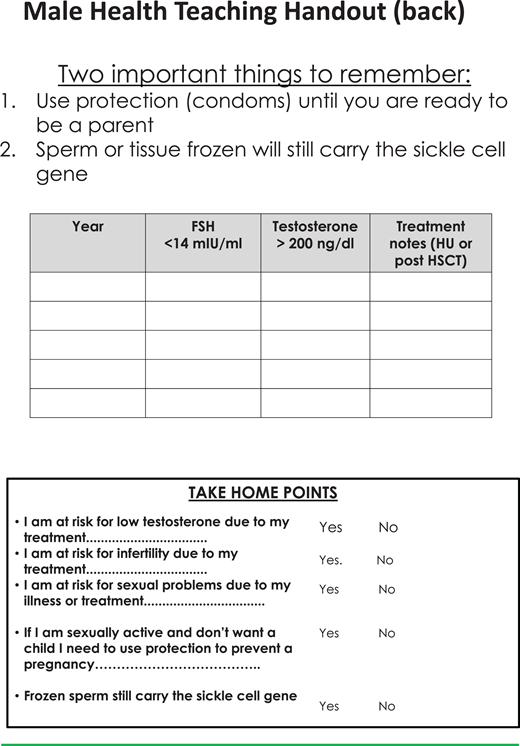
Teaching sheets on male health to aid discussions with patients (part 2). HU, hydroxyurea.
Teaching sheets on male health to aid discussions with patients (part 2). HU, hydroxyurea.
Psychosocial and ethical considerations
As patients attain majority and maturity, clinicians should be prepared to fully educate them about the sexual and reproductive effects their disease and treatments may have caused. Both patients and physicians may at times be hesitant to discuss infertility caused by SCD and its treatments. Patients may be reticent about focusing on undesirable aspects of their disease or may be uncomfortable talking about sexual anatomy, function, and reproduction. Patients and clinicians may assume that the costs of accessing assisted reproductive technology are insurmountable. Physicians may be uncomfortable talking about sex, especially with younger patients.24,36 Physicians also face discomfort discussing iatrogenic morbidity, areas with insufficient research and information, decisions already made by parents without the patient's awareness, and the inability to offer a perfect medical option.36-38 We are empathic to these concerns for both patients and clinicians, and we hope the materials provided here help reduce barriers to these conversations.
These recommended models of care utilize members of hematology and fertility teams, but not all programs have access to specialists. In lower-resource settings, any team member can provide this information to patients once adequately trained. The data around gonadotoxicity and the risk of infertility in some areas are evolving, and the lack of consensus in the risk level should also be disclosed. However, even when no fertility preservation intervention is available, patients/families need to be fully informed prior to the start of therapy to allow for informed participation in shared decision- making.
Conflict-of-interest disclosure
Lillian R. Meacham: no competing financial interests to declare.
Lydia H. Pecker: Consultant for Global Blood Therapeutics.
Beatrice Gee: no competing financial interests to declare.
Adrienne Mishkin: no competing financial interests to declare.
Off-label drug use
Lillian R. Meacham: nothing to disclose.
Lydia H. Pecker: nothing to disclose.
Beatrice Gee: nothing to disclose.
Adrienne Mishkin: nothing to disclose.