Learning Objectives
Recognize the different strategies to prevent and manage severe alloimmune platelet refractoriness
Understand when to suspect and how to diagnose alloimmune platelet refractoriness
CLINICAL CASE
A 35-year-old woman from Mexico with idiopathic aplastic anemia receives leukocyte-reduced apheresis platelets for a platelet count of 8 × 109/L. A 30-minute posttransfusion platelet count is 9 × 109/L. There is no change after a second unit of apheresis platelets. Recent abdominal imaging shows no splenomegaly. The patient has been pregnant 4 times and has received non-leukoreduced blood products in her home country. She demonstrates no signs or symptoms of bleeding. A human leukocyte antigen (HLA) antibody screen reveals a calculated panel-reactive antibody (cPRA) of 100%. The blood bank is notified and low-resolution HLA typing is requested.
Introduction
Platelet transfusion refractoriness can be a serious problem among patients with hematologic malignancy. Nonimmune causes such as fever, infection, bleeding, medications (eg, amphotericin and vancomycin), and splenic sequestration account for approximately 80% of cases, while immune-mediated causes comprise the remaining 20%.1 In the absence of nonimmune causes, immune-mediated refractoriness is suspected when a corrected count increment measured within 10 minutes to an hour is less than 5000 on 2 sequential occasions (Figure 1).
Diagnostic and management algorithm for severe alloimmune platelet refractoriness. Key factors involved in the identification, diagnosis, management, and prevention of alloimmune platelet refractoriness. The corrected count increment (CCI) may be calculated using the following formula: [Posttransfusion platelet count – Pretransfusion platelet count (/L)] × body surface area (m2)/platelets transfused (1011). The number of platelets transfused may be assumed to be 3 × 1011 when this information is unavailable. In clinical practice, refractoriness may be suspected when the posttransfusion platelet increment is less than 10 × 109/L. IVIG, intravenous immune globulin.
Diagnostic and management algorithm for severe alloimmune platelet refractoriness. Key factors involved in the identification, diagnosis, management, and prevention of alloimmune platelet refractoriness. The corrected count increment (CCI) may be calculated using the following formula: [Posttransfusion platelet count – Pretransfusion platelet count (/L)] × body surface area (m2)/platelets transfused (1011). The number of platelets transfused may be assumed to be 3 × 1011 when this information is unavailable. In clinical practice, refractoriness may be suspected when the posttransfusion platelet increment is less than 10 × 109/L. IVIG, intravenous immune globulin.
Risk factors for alloimmune platelet refractoriness
Alloimmunization develops after exposure to nonself HLAs or human platelet antigens (HPAs) through pregnancy, transfusion, or solid organ or mismatched hematopoietic stem cell transplantation. HLA antibodies are more common and clinically relevant. Since its widespread adoption, leukoreduction has decreased rates of alloimmunization resulting from transfusion by more than 50%.1 While alloimmune platelet refractoriness itself is relatively uncommon, patients with hematologic malignancy who receive frequent transfusions remain at risk and comprise up to 80% of patients requiring HLA-matched platelets.2
Current testing methods
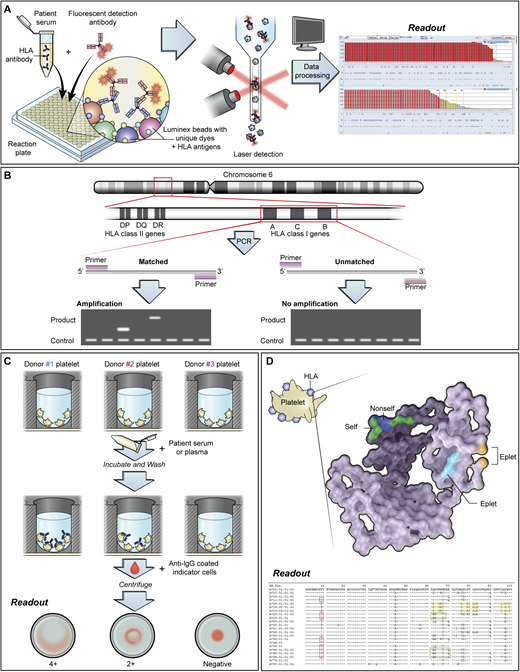
Most laboratories use solid-phase methods (Figure 2A) to detect anti-HLA and anti-HPA alloantibodies. Solid-phase testing is more sensitive and specific compared with traditional lymphocytotoxicity assays. Following antibody detection, a cPRA is generated. The cPRA represents the percentage of donors who possess HLA antigens to which the patient has made antibodies. Low-resolution HLA typing is performed for HLA class I antigens (Figure 2B). These tests can take hours to days depending on institutional resources. Platelet crossmatching (Figure 2C) may identify best-matched units in inventory while awaiting results of the more definitive tests. These and other HLA testing methods are reviewed elsewhere.3
Testing methods for platelet refractoriness due to HLA alloimmunization. (A) Solid-phase assays are increasingly used to test for anti-HLA, anti-HPA, and anticomplement antibodies. In testing for HLA antibodies, beads are coated with 1 or more HLA antigens. Patient serum is added to the beads, followed by fluorescently labeled anti–human globulin to detect bound anti-HLA antibodies. Each bead is impregnated with a different ratio of dye, which produces a unique fluorescent signature that can be identified by Luminex technology. The mean fluorescence intensity (MFI) of the antibody-bound beads is used to determine positivity. Antibody screening platforms use beads coated with more than 1 antigen, while antibody specificity testing involves the use of single antigen beads. Other techniques for HLA antibody testing include flow cytometry and enzyme-linked immunosorbent assay–based methods. Caveats to solid-phase testing include the lack of a standardized MFI cutoff for determining antibody positivity. Low-level “positive” alloantibodies may not be clinically associated with transfusion refractoriness. Despite efforts to couple solid-phase assays with functional testing, newer methods that detect C1q-binding anti-HLA antibodies have not consistently demonstrated utility in platelet refractoriness. Additionally, assays may vary in their ability to detect anti-HPA alloantibodies. (B) HLA antigens are encoded on the short arm of chromosome 6. Among the HLA class I antigens, HLA-A and HLA-B are primarily implicated in alloimmune platelet transfusion refractoriness. Low-resolution HLA typing is generally performed for platelet transfusions, although high-resolution typing is required for epitope matching. (C) Platelet crossmatching identifies donor platelets that are not reactive to patient plasma. Crossmatching may be performed using various methods, including the solid-phase red cell adherence assay (which is shown), wherein wells are coated with donor platelets available in inventory. The platelet-coated wells are then incubated with patient plasma or serum, washed, reacted with anti-IgG coated indicator red cells, centrifuged, and read visually. Compatible donor products are selected for transfusion. (D) HLA class I antigens comprise a light chain (β subunit) and a heavy chain (α subunit), which have a standard crystalline structure with regions of polymorphic amino acid sequences, termed epitopes, that are immunogenic and serve as the basis of alloimmunization. Short groups of these polymorphic amino acids on the molecular surface form eplets, which may be linear sequences or discontinuous residues that lie in close proximity in the native, 3-dimensional conformation of the antigen. Eplets may be shared across many HLA subtypes. Epitope matching uses the nonself-self paradigm, in which alloantibodies are hypothesized to be directed against unfamiliar “nonself” eplets rather than “self” eplets. Therefore, in cases where there are mismatched patient-donor HLA antigens, the degree of eplet mismatch (ie, the number of “nonself” eplets present in the donor HLA antigens) may be calculated using computer algorithms to predict the likelihood of compatibility. PCR, polymerase chain reaction.
Testing methods for platelet refractoriness due to HLA alloimmunization. (A) Solid-phase assays are increasingly used to test for anti-HLA, anti-HPA, and anticomplement antibodies. In testing for HLA antibodies, beads are coated with 1 or more HLA antigens. Patient serum is added to the beads, followed by fluorescently labeled anti–human globulin to detect bound anti-HLA antibodies. Each bead is impregnated with a different ratio of dye, which produces a unique fluorescent signature that can be identified by Luminex technology. The mean fluorescence intensity (MFI) of the antibody-bound beads is used to determine positivity. Antibody screening platforms use beads coated with more than 1 antigen, while antibody specificity testing involves the use of single antigen beads. Other techniques for HLA antibody testing include flow cytometry and enzyme-linked immunosorbent assay–based methods. Caveats to solid-phase testing include the lack of a standardized MFI cutoff for determining antibody positivity. Low-level “positive” alloantibodies may not be clinically associated with transfusion refractoriness. Despite efforts to couple solid-phase assays with functional testing, newer methods that detect C1q-binding anti-HLA antibodies have not consistently demonstrated utility in platelet refractoriness. Additionally, assays may vary in their ability to detect anti-HPA alloantibodies. (B) HLA antigens are encoded on the short arm of chromosome 6. Among the HLA class I antigens, HLA-A and HLA-B are primarily implicated in alloimmune platelet transfusion refractoriness. Low-resolution HLA typing is generally performed for platelet transfusions, although high-resolution typing is required for epitope matching. (C) Platelet crossmatching identifies donor platelets that are not reactive to patient plasma. Crossmatching may be performed using various methods, including the solid-phase red cell adherence assay (which is shown), wherein wells are coated with donor platelets available in inventory. The platelet-coated wells are then incubated with patient plasma or serum, washed, reacted with anti-IgG coated indicator red cells, centrifuged, and read visually. Compatible donor products are selected for transfusion. (D) HLA class I antigens comprise a light chain (β subunit) and a heavy chain (α subunit), which have a standard crystalline structure with regions of polymorphic amino acid sequences, termed epitopes, that are immunogenic and serve as the basis of alloimmunization. Short groups of these polymorphic amino acids on the molecular surface form eplets, which may be linear sequences or discontinuous residues that lie in close proximity in the native, 3-dimensional conformation of the antigen. Eplets may be shared across many HLA subtypes. Epitope matching uses the nonself-self paradigm, in which alloantibodies are hypothesized to be directed against unfamiliar “nonself” eplets rather than “self” eplets. Therefore, in cases where there are mismatched patient-donor HLA antigens, the degree of eplet mismatch (ie, the number of “nonself” eplets present in the donor HLA antigens) may be calculated using computer algorithms to predict the likelihood of compatibility. PCR, polymerase chain reaction.
HLA-matched platelets
Patients with alloimmune platelet refractoriness are frequently supported with platelets matched for HLA-A and B antigens. HLA-matched platelets are graded based on degree of match. Preference is given to grade A units. The highest grades include a perfect 4/4 antigen match (grade A) and grade B1U and B2U units, where the donor is homozygous at 1 or 2 HLA alleles, respectively, such that the recipient will not perceive donor antigens as mismatched. While HLA-C typing data are available in most instances, only rare cases of clinically relevant anti–HLA-C antibodies are reported so matching at HLA-C is not routinely performed. Even with matching for HLA-A and B antigens alone, the ability to provide well-matched units depends on the frequency of the patient's HLA type, the HLA makeup of the donor pool, and access to a large HLA-typed donor pool (≥3000 donors). For patients with rare HLA types requiring frequent transfusion, identifying and maintaining a steady supply of matched units is challenging.
CLINICAL CASE (Continued)
Low-resolution HLA typing reveals a combination of rare HLA-A and HLA-B antigens and no HLA antigen-matched donors are identified. No HPA antibodies are identified. There are no suitable donors for hematopoietic stem cell transplantation and the patient is initiated on immunosuppressive therapy. Over the next month, she receives intermittent HLA epitope-matched and antigen-negative platelets for persistent severe thrombocytopenia, with satisfactory response.
Epitope-matched, antigen-negative, and crossmatched units
When HLA antigen-matched units are unavailable, epitope-matched units and antigen-negative units are considered equivalently efficacious.4,5
Epitope matching is based on the theory that patients do not make antibodies against epitopes (polymorphic amino acid configurations shared among HLA antigens) present on nonself HLA antigens if they are also present on self HLA antigens. Computer algorithms, like HLAMatchmaker, predict matching at the epitope level regardless of antigen match status (Figure 2D). Using the patient's HLA type, the software estimates the likelihood of epitope mismatch with each available HLA-typed donor and may be used to rank donors by degree of mismatch (low to high). Originally used for renal transplantation, its use in alloimmune platelet transfusion refractoriness has been reported effective by several centers. Recently, a randomized, double-blind, noninferiority, crossover trial compared epitope-matched platelets identified by HLAMatchmaker to standard HLA-matched platelets in 37 patients with aplastic anemia and alloimmune platelet refractoriness.5 One-hour posttransfusion increments were not significantly different between epitope-matched and antigen- matched units. Epitope matching correlated with transfusion success (mean epitope mismatch 3.2 for units associated with adequate 1-hour posttransfusion increment vs 5.5 for units with inadequate increment). Like HLA antigen matching, epitope matching requires a large HLA-typed donor pool and is resource intensive.
Alternatively, blood centers may select platelets that lack the HLA antigens against which the patient's alloantibodies are directed (antigen-negative or antigen-exclusion units).4 Among 16 patients with HLA antibodies who received a total of 556 transfusions, using platelets lacking “prohibited” HLA antigens yielded higher 1-hour posttransfusion corrected count increment compared with randomly selected units.6
For patients with low to moderate levels of alloimmunization (cPRA <70%), crossmatched platelets are effective, do not require specialized HLA testing, and can be readily accessible.7 Crossmatch-compatible units are unlikely to be identified when cPRA is >90%, as in this case. An additional drawback is the need for repeated testing as each crossmatch involves testing patient plasma against a panel of approximately 10 apheresis units. Some institutions offer platelets pooled from multiple donors as an interim strategy while awaiting HLA-matched units, although their efficacy over random donor apheresis platelets is questionable.8,9
Another potential approach is to identify donors with a reproducibly low density of specific antigens, which may minimize antibody-mediated platelet phagocytosis and potentially expand the donor pool for a subset of patients with alloantibodies to these subtypes.10 Up to a third of donors have platelets with minimal antigen expression of HLA-B8, B12 (B44 and B45), or B35 and antibody-mediated macrophage clearance of these platelets is not observed in vitro. These data support findings that units mismatched only at HLA-B12 produced satisfactory increments in >50% of transfusions provided to refractory, alloimmunized patients, including those with anti–HLA-B12 antibodies.11
In cases where platelet refractoriness persists despite appropriate product support, concomitant nonimmune causes and HPA alloimmunization should be considered. Beyond this, there is sparse evidence to guide management. When suitable platelet products are not available, possible practical strategies include using ABO-matched2 units and raising the hematocrit transfusion threshold to 26% to potentially mitigate bleeding.12 Although attempted previously, slow platelet infusions and agents such as antifibrinolytics,13 intravenous immune globulin, and anticomplement therapies are of unclear benefit. Future studies may evaluate the utility of HLA class I depletion,14 HLA universal platelets,15 and HLA desensitization therapies.16 In instances of unrelenting refractoriness with poor response to appropriately selected units, it is also critical to reevaluate the need for prophylactic platelet transfusions considering the burden on the donor pool and health care resource utilization associated with these cases.
Transfusion refractoriness may improve over time as alloantibody levels wane. In the landmark Trial to Reduce Alloimmunization to Platelets, 56% of patients who developed alloantibodies during the study lost antibody expression after a median of 14 weeks.17 As such, we typically repeat HLA antibody testing every month to ascertain an ongoing need for HLA-matched units. Repeat testing is also warranted if there is a waning response to appropriately selected units, suggesting an evolution in antibody profile.
Preventative strategies
Platelet transfusions should be prescribed only when needed to prevent primary and secondary alloimmunization. International consensus panels recommend prophylactic platelet transfusions when counts are below 10 × 109/L in patients with therapy-induced hypoproliferative thrombocytopenia (grade 1).18,19 Yet, in a prospective audit of 57 hospitals in Canada, nearly half of adult platelet product orders were deemed inappropriate, including 42% of prophylactic platelet transfusions requested at an inappropriate threshold.20 Furthermore, enhancing ethnic diversity among the donor pool for full representation of HLA subtypes will ensure more patients of ethnic minorities have access to HLA-matched platelets.
CLINICAL CASE (Continued)
No response is observed to first-line immunosuppressive therapy. Identification of a suitable hematopoietic stem cell transplant donor and desensitization are not feasible given the degree of HLA alloimmunization. Monthly HLA antibody screens continue to demonstrate detectable HLA alloantibodies with a cPRA of 100%. Intermittent epitope-matched and antigen-negative units are transfused as needed for severe thrombocytopenia while awaiting response to second-line immunosuppressive therapy.
Acknowledgments
The authors thank Erina He, Medical Arts, NIH for her assistance with manuscript figures and Sharon Adams, PhD, and Anh Dinh, MD, for their critical review of the manuscript. Debbie Jiang receives support from an institutional training grant from the National Heart, Lung, and Blood Institute (T32 HL007093).
Conflict-of-interest disclosure
Debbie Jiang: no competing financial interests to declare.
Sandhya R. Panch: no competing financial interests to declare.
Off-label drug use
Debbie Jiang: nothing to disclose.
Sandhya R. Panch: nothing to disclose.


![Diagnostic and management algorithm for severe alloimmune platelet refractoriness. Key factors involved in the identification, diagnosis, management, and prevention of alloimmune platelet refractoriness. The corrected count increment (CCI) may be calculated using the following formula: [Posttransfusion platelet count – Pretransfusion platelet count (/L)] × body surface area (m2)/platelets transfused (1011). The number of platelets transfused may be assumed to be 3 × 1011 when this information is unavailable. In clinical practice, refractoriness may be suspected when the posttransfusion platelet increment is less than 10 × 109/L. IVIG, intravenous immune globulin.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2022/1/10.1182_hematology.2022000416/5/m_hem2022000416figure1.png?Expires=1765039885&Signature=ALTUjuojVtQLRPJBcD76LlC86Jr-v4KvmiVNzGM9ujnorRfd-J4e9SlAnh7U5142jhycwhgyOdZaiYmXKbdU~kP6GLmPlG-VwfMemTxBszCnndsrXSYer4vKtlGE-C0z8rX6RXwX2mGCOqMFE7EHHcX8TrqD4CnGo0-JDlq5Ls08twMRR6DYX6XlUwdaZbW6sjuaAAP4TicaNTKPmzgLCDqLBS8fMn9bvbaC0Swxuao4NDYJCgETDlGa8erxTMFz6MEnAECEIRZMhfLLzrihaHfk1S0GPA~gN9DEDu0NzAhZV1sVRzspGwJGUD6lJw-b5fGGaZ5PpYXFxwJBfODpqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)