Abstract
Based upon the development of highly effective therapies such as immunomodulatory drugs, proteasome inhibitors, and monoclonal antibodies that target plasma cell biology, a dramatic improvement in overall survival has been observed for most patients with multiple myeloma (MM) over the past 2 decades. Although it is now commonplace for many patients with myeloma to live in excess of 10 years after diagnosis, unfortunately a large subset of patients continues to experience an aggressive disease course marked by substantial morbidity and early mortality. Many clinical biomarkers and staging systems in use today can help with prognostication, but accurate risk assessment can be difficult due to the presence of many different biomarkers with variable prognostic value. Furthermore, with the implementation of novel therapies and unprecedented rates of deep and durable responses, it is becoming apparent that risk assessment is best envisioned as a dynamic process that requires ongoing reevaluation. As risk and response-adapted approaches are becoming more commonplace, it is essential that clinicians understand the biological and prognostic implications of clinical, genomic, and response-based biomarkers in order to promote management strategies that will help improve both survival and quality of life for patients across the risk spectrum.
Learning Objectives
Appreciate the importance and applications of accurate risk assessment in multiple myeloma
Understand the clinical features and cytogenetic abnormalities that can identify patients with high-risk disease at the time of diagnosis
Recognize that risk assessment is a dynamic process that involves ongoing assessment of response to therapy and detection of early relapse
CLINICAL CASE
A 57-year-old woman with no medical history presents with progressive dyspnea and fatigue and is found to have macrocytic anemia with a hemoglobin concentration of 8.5 g/dL, platelet concentration of 87 K/µL, creatinine of 0.9 mg/dL, calcium of 9.5 mg/dL, albumin of 3.7 g/dL, and a total protein level of 8.5 g/dL. Additional workup revealed a β2- microglobulin (β2M) of 4.9 mg/L, lactate dehydrogenase (LDH) of 267 U/L, and an IgA κ monoclonal protein measuring 3.2 g/dL, free κ of 56 mg/dL, and a free light chain ratio of 70.4. Positron emission tomography/computed tomography showed diffuse uptake within the osseous structures without discrete lytic bone lesions. A bone marrow biopsy identified 70% κ-restricted plasma cells with large, atypical appearance. Karyotype was normal in 20 cells, and fluorescence in situ hybridization (FISH) panel identified t(4;14), gain(1q), and −13.
Concepts of risk stratification
Multiple myeloma (MM) is a complex disease with variable clinical features and outcomes. In recognition that outcomes remain heterogeneous, many attempts have been made to determine risk factors that can help to identify patients who are at risk for early treatment failure and mortality. Among risk stratification systems in MM, “standard- risk” disease generally expected to follow a pattern of early and consistent response to therapy with longer periods of disease control. “High-risk” disease, on the other hand, may respond well to initial therapy but develops early drug resistance, which often leads to rapid and increasingly frequent relapses that can result in substantial morbidity and early mortality. The International Myeloma Working Group has defined high-risk myeloma as having an expected overall survival of 2 years or less despite the use of novel agents.1 This benchmark was defined prior to the widespread implementation of many of the drugs that are commonly used today, but the concept remains pertinent and arguably carries increased significance within the context of modern therapy and the prospect of risk and response-adapted management.
It is essential for clinicians to have a foundational understanding of risk stratification in MM for several reasons. First, this information helps to set realistic expectations about prognosis and the likelihood of response duration with various therapies. Second, knowledge of risk may assist in contextualizing management decisions such as whether to add a fourth drug to induction, whether delayed transplant is reasonable, or if adjustments to treatment regimens are warranted. Finally, because patients with high-risk disease typically derive less long-term benefit from current therapies compared with patients with standard-risk disease, it will be essential to accurately identify patients with high-risk disease who are appropriate for clinical trials using novel approaches to improve outcomes in this at-risk population.
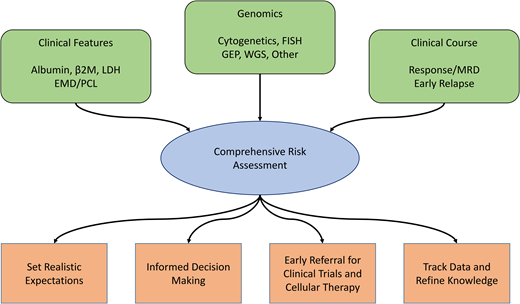
Many factors contribute to disease biology and risk in MM, including clinical features such as disease burden and manifestations, proliferative ability, molecular biology and genomic aberrations in myeloma cells, and depth of response to therapy. A comprehensive approach to risk stratification incorporates all of these features while also recognizing that many other factors influence survival such as age, medical comorbidities, frailty, social support, access to care, and health care disparities.
Risk stratification and staging at diagnosis
Clinical risk factors
The first validated prognostic staging system in MM was the International Staging System (ISS).2 This large international collaboration identified β2M and albumin, 2 easily measured and highly reproducible laboratory tests, as powerful biomarkers that could stratify risk of mortality into 3 distinct stages. In the revised ISS (R-ISS), cytogenetic abnormalities and LDH— biomarkers that represent the biological factors of proliferation and genomics—were added to the model.3 The major advantage of the R-ISS is that it more accurately identifies patients on the extremes of the risk stratification schema. However, more than half of patients are now grouped into the R-ISS stage 2 classification, and outcomes of patients in this group remain highly variable. Furthermore, the R-ISS includes only t(4;14), t(14;16), and del(17p) as high-risk cytogenetic abnormalities and does not include other genomic factors that have more recently been determined to be important prognostic biomarkers.
On a conceptual level, it is somewhat intuitive that patients with ISS stage 3 disease due to high tumor burden or who are more acutely ill at diagnosis are more likely to have aggressive disease biology. However, β2M, albumin, and LDH are all nonspecific and can be affected by many different (and often reversible) clinical variables. As such, the ISS and R-ISS do not always reflect the underlying disease biology. Cytogenetics, on the other hand, define the abnormal molecular profile of malignant plasma cells and carry inherent potential risk, regardless of the manner of presentation. Certainly, patients with extramedullary disease and/or plasma cell leukemia (PCL)—conditions that are usually seen only in late relapse and in which myeloma cells have become independent of the bone marrow niche for survival and proliferation—should be considered high risk, regardless of cytogenetics.4,5 However, cytogenetic abnormalities, typically determined by FISH, have become the dominant biomarker used for risk stratification and management decisions in MM.
Cytogenetics
In current practice, both karyotyping and FISH are commonly used for assessing cytogenetics in MM. By FISH, large structural aberrancies can be identified in nearly all patients,6 and therefore FISH is the preferred means for identifying cytogenetic abnormalities in MM today. Although karyotype can identify copy number changes and translocations among cells in metaphase, it appears falsely normal in approximately two-thirds of patients with newly diagnosed MM,7 primarily because of the relatively low proliferative rate of most myeloma cells. As such, abnormal and/or complex karyotype at diagnosis is best considered a surrogate for increased proliferation, which is a hallmark of aggressive disease,8 but is not recommended as a standalone test for cytogenetic analysis.
Approximately 50% of MM cases are characterized by copy gains of odd-numbered chromosomes (3, 5, 7, 9, 11, 15, 19, and 21)—commonly referred to as “hyperdiploidy.” Most remaining cases of MM are characterized by translocations involving the immunoglobulin heavy chain locus at 14q32 and rarely coexist with hyperdiploidy.9 Aside from these primary abnormalities, which are believed to occur at the initiation of the plasma cell clone, many secondary cytogenetic abnormalities have also been identified, which have been implicated in the progression from precursors to malignancy or during clonal evolution during the course of the disease. A summary of the most common primary and secondary cytogenetic abnormalities is provided in Table 1.
Common primary and secondary cytogenetic abnormalities in multiple myeloma
| . | Abnormality/locus . | Genes involved . | Frequency, % . | Characteristics . | Prognosis/risk . |
|---|---|---|---|---|---|
| Primary abnormalities | Hyperdiploidy | Many/unknown | 50 | Highly associated with lytic bone disease, often as the only disease manifestation Associated with “exceptional response” to lenalidomide | Standard/low |
| t(6;14)(p21;q32) | CCND3 | <5 | Similar to hyperdiploidy | Standard/low | |
| t(11;14)(q13;q32) | CCND1 | 15-20 | Lymphoplasmacytoid appearance, often with B-cell expression profile High rates of free light chain only, oligosecretory, nonsecretory, and IgM/IgD myeloma Most common abnormality in primary PCL and AL amyloidosis | Intermediate | |
| t(4;14)(p16;q32) | MMSET (WHSC1) | 10-15 | Large, atypical appearance of plasma cells Frequently co-occurs with +1q May derive particular benefit from proteasome inhibitors compared to chemo | High | |
| t(14;16)(q32;q23) | MAF | 5 | Frequently associated with high circulating free light chains and renal failure Frequent association with APOBEC mutations Second most common abnormality in PCL | High | |
| t(14;20)(q32;q12) | MAFb | <5 | Similar to t(14;16) | High | |
| Secondary abnormalities | +1q Gain(1q) [3 copies] Amp(1q) [4 copies] | Many (CKS1B, MCL1, IL-6R, PBX-1, ADAR1, SLAMF7, FcHR5) | 40 30 10 | Can co-occur with standard-risk or high-risk cytogenetics; more strongly associated with high risk “Jumping 1q” syndrome associated with genomic instability Copy number can increase over time | High Risk increases as copy number rises |
| Del(1p) | CDKN2C, FAM46C | 5-10 | High | ||
| Del(17p) | TP53 | 5-20 | Can be acquired at any time in disease process Higher proliferative rate and LDH Prognosis may be dependent on clonal fraction and/or coexistent TP53 mutation | High | |
| MYC rearrangement | MYC | 40 (all SV types) 5-10 (immunoglobulin translocation) | Associated with higher disease burden and β2M Commonly seen in hyperdiploid subset, rarely with t(11;14) | High Prognosis depends on type of rearrangement, worse for IgH/IgL translocations | |
| −13 | RB1, DIS3, others | ~50 | Very commonly identified in both standard and high-risk patients, more frequent in high risk | No impact; possibly high risk if found on karyotype | |
| Hypodiploidy | Many | ~20 | Defined as loss of chromosomal material On karyotype, 45 or fewer chromosomes; on FISH, can manifest with loss of IgH, MAF, or other probes | High |
| . | Abnormality/locus . | Genes involved . | Frequency, % . | Characteristics . | Prognosis/risk . |
|---|---|---|---|---|---|
| Primary abnormalities | Hyperdiploidy | Many/unknown | 50 | Highly associated with lytic bone disease, often as the only disease manifestation Associated with “exceptional response” to lenalidomide | Standard/low |
| t(6;14)(p21;q32) | CCND3 | <5 | Similar to hyperdiploidy | Standard/low | |
| t(11;14)(q13;q32) | CCND1 | 15-20 | Lymphoplasmacytoid appearance, often with B-cell expression profile High rates of free light chain only, oligosecretory, nonsecretory, and IgM/IgD myeloma Most common abnormality in primary PCL and AL amyloidosis | Intermediate | |
| t(4;14)(p16;q32) | MMSET (WHSC1) | 10-15 | Large, atypical appearance of plasma cells Frequently co-occurs with +1q May derive particular benefit from proteasome inhibitors compared to chemo | High | |
| t(14;16)(q32;q23) | MAF | 5 | Frequently associated with high circulating free light chains and renal failure Frequent association with APOBEC mutations Second most common abnormality in PCL | High | |
| t(14;20)(q32;q12) | MAFb | <5 | Similar to t(14;16) | High | |
| Secondary abnormalities | +1q Gain(1q) [3 copies] Amp(1q) [4 copies] | Many (CKS1B, MCL1, IL-6R, PBX-1, ADAR1, SLAMF7, FcHR5) | 40 30 10 | Can co-occur with standard-risk or high-risk cytogenetics; more strongly associated with high risk “Jumping 1q” syndrome associated with genomic instability Copy number can increase over time | High Risk increases as copy number rises |
| Del(1p) | CDKN2C, FAM46C | 5-10 | High | ||
| Del(17p) | TP53 | 5-20 | Can be acquired at any time in disease process Higher proliferative rate and LDH Prognosis may be dependent on clonal fraction and/or coexistent TP53 mutation | High | |
| MYC rearrangement | MYC | 40 (all SV types) 5-10 (immunoglobulin translocation) | Associated with higher disease burden and β2M Commonly seen in hyperdiploid subset, rarely with t(11;14) | High Prognosis depends on type of rearrangement, worse for IgH/IgL translocations | |
| −13 | RB1, DIS3, others | ~50 | Very commonly identified in both standard and high-risk patients, more frequent in high risk | No impact; possibly high risk if found on karyotype | |
| Hypodiploidy | Many | ~20 | Defined as loss of chromosomal material On karyotype, 45 or fewer chromosomes; on FISH, can manifest with loss of IgH, MAF, or other probes | High |
AL, amyloid light chain; SV, structural variant.
Conventionally, t(4;14) and t(14;16), as well as del(17p), are considered high-risk abnormalities, and all other abnormalities have been considered “standard risk.” However, over time, new insights have led to some shifts in the cytogenetic risk stratification schema. Hyperdiploidy continues to be the cytogenetic abnormality associated with the best overall prognosis,10 and patients with hyperdiploidy are more likely to be “exceptional responders” to lenalidomide-based therapy and/or autologous stem cell transplant.11,12 Importantly, it must be noted that outcomes among hyperdiploid patients remain heterogeneous due to the occurrence of secondary cytogenetic abnormalities and other complex structural changes that may affect disease biology in this large subset.13,14 Although t(11;14) was initially considered to have a similar prognosis to hyperdiploidy, this abnormality now appears to portend intermediate risk,15 likely because it has not derived as much benefit from therapies targeting plasma cell biology compared with those with other subsets of standard-risk myeloma. However, patients with t(11;14) generally have a better prognosis than those with high-risk biomarkers, and it remains to be seen whether the implementation of B-cell lymphoma 2 (BCL-2) inhibitors in this subset will affect survival, based on the efficacy of venetoclax-based therapy for patients with t(11;14).16
One of the biggest developments in cytogenetic risk stratification is the clarification of the prognostic impact of copy gains of chromosome 1q (+1q), one of the most common cytogenetic abnormalities among patients with MM. Although it has long been known that many genes involved in high-risk gene expression profiling are present at 1q21,17 it was only recently that +1q has been clarified as an independent prognostic factor for poor survival in MM.18-20 The prognostic impact of +1q does appear to be somewhat context dependent, specifically regarding copy number, for which terminology should be clarified. Although no consensus definition exists, gain(1q) should be considered to indicate the presence of 3 copies of 1q, whereas “amplification” or amp(1q) should be reserved for patients with 4 or more extra copies of the 1q probe.21 Whereas the impact of gain(1q) may depend on choice of therapy and coexisting cytogenetic abnormalities, amp(1q) is universally associated with poor survival.18,20,22 In recognition of the impact of +1q on survival, multiple new risk classification systems, including the Mayo Additive Staging System23 and Second Revision of the ISS,24 have been proposed, incorporating +1q alongside the conventional R-ISS criteria.
Rearrangements involving MYC at 8q24.1 have been implicated as key events that drive progression from precursor states to symptomatic disease25,26 and have been proposed to portend a poor prognosis among patients with MM.27 Through the use of next-generation sequencing in the Clinical Outcomes in Multiple Myeloma to Personal Assessment study, approximately 40% of patients with MM have structural variants involving MYC, but the poor prognostic impact appears to be limited to those with an immunoglobulin locus translocation.28 Although MYC rearrangements can be seen in conjunction with any other abnormalities, they are highly associated with hyperdiploidy,27,28 and this may partially explain some of the unexpected early relapses in that subset.
Several recent studies strongly suggest that high-risk disease might not be defined by any one genomic abnormality but rather that the high-risk definition might be context dependent and/or require combinations of high-risk factors. Indeed, through whole-genome sequencing, 2 “double-hit” profiles were identified—namely, biallelic inactivation of TP53 and the combination of amp(1q) and ISS stage 3 disease—that resulted in a dismal prognosis.29 Similar findings have been demonstrated in analyses of +1q—although gain(1q) may impart worse progression- free survival, the worst prognosis is clearly among patients who have amp(1q) and/or +1q in combination with t(4;14), t(14;16), del(17p), del(1p), and/or MYC rearrangements.18,20,30 In fact, it is becoming more apparent that the traditional high-risk markers may not always portend high-risk disease.31-33 A very rational explanation for this is the requirement of a “second hit” or the accumulation of multiple high-risk genomic aberrancies in order to produce true high-risk disease that manifests with biologically unstable and aggressive myeloma that is likely to develop rapid drug resistance and early treatment failure.
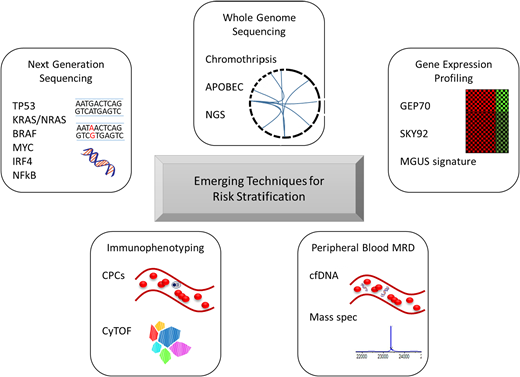
Although FISH is able to accurately risk stratify many patients, the biological processes in myeloma cells are dependent not only on large structural variations but also on point mutations (not captured by FISH), transcription profiling, proteomics, and the interactions between MM and the microenvironment, among others. Technologies evaluating these elements of myeloma cell biology have been studied and do appear to add prognostic value in many instances. A summary of new techniques with potential prognostic value is provided in Figure 1. In particular, high-risk signatures from the GEP70 and SKY92 gene expression profiles17,34 and the presence of chromothripsis and APOBEC mutational signatures detected on whole-genome sequencing appear to be very powerful tools to identify high-risk patients.35,36 Furthermore, detection of low-level circulating plasma cells by flow cytometry can also identify patients with otherwise standard- risk features who have a worse prognosis.37,38 Comprehensive immune profiling may also allow for improved risk stratification and/or prediction of outcomes to initial therapy.39,40 Integration of these tools into routine risk stratification will help to further characterize the spectrum of risk profiles that can be observed in MM and hopefully will help to clarify which risk factors can be overcome through the use of certain therapies and which will require novel strategies.
Emerging techniques for risk stratification in multiple myeloma. Each of these tools has potential for researching disease biology and for implementation in the clinic for real-time assessment of disease control and/or risk. cfDNA, cell-free DNA; CPCs, circulating plasma cells; CyTOF, mass cytometry time of flight; MGUS, monoclonal gammopathy of undetermined significance; NGS, next-generation sequencing.
Emerging techniques for risk stratification in multiple myeloma. Each of these tools has potential for researching disease biology and for implementation in the clinic for real-time assessment of disease control and/or risk. cfDNA, cell-free DNA; CPCs, circulating plasma cells; CyTOF, mass cytometry time of flight; MGUS, monoclonal gammopathy of undetermined significance; NGS, next-generation sequencing.
CLINICAL CASE (Continued)
The patient was diagnosed with ISS stage 2, R-ISS stage 2 MM and considered to have high-risk cytogenetics due to t(4;14) and gain(1q). She was started on daratumumab, bortezomib, lenalidomide, and dexamethasone, achieving a complete response prior to undergoing high-dose therapy and autologous stem cell transplant. Restaging evaluation at day +90 showed that she had achieved a stringent complete response, and measurable residual disease (MRD) analysis by flow cytometry showed no evidence of residual disease at a depth of 10−5.
Dynamic risk stratification
Response
Beyond genomic factors, an established risk factor in MM is achievement of a deep response. Specifically, it is now recognized that achievement of MRD negativity is a powerful prognostic factor—and in fact, multiple studies have shown that this is a more important prognostic factor than cytogenetic abnormalities.41,42 In the PETHEMA/GEM2012MENOS study, patients who achieved MRD negativity by flow cytometry at a depth of 10−6 had almost nonexistent rates of progression, regardless of cytogenetic risk, arguing that achievement of MRD negativity may overcome the adverse effect of high-risk cytogenetics.43 Furthermore, in that study, detectable MRD was associated with the same risk of progression, regardless of depth of response by standard International Myeloma Working Group criteria.44
Based on these data regarding the importance of MRD, this provides an opportunity for dynamic risk assessment and modification of risk assessment over the course of the disease. Standard-risk patients who fail to achieve MRD negativity have a higher risk of progression compared with those who are MRD negative. Similarly, high-risk patients who become MRD negative may still derive the long-term benefits seen among patients with MRD negativity and perhaps experience survival in excess of patients previously believed to have standard-risk disease who fail to respond well to initial therapy. In addition, MRD assessment should be considered a quantitative variable monitored over time rather than a singular categorical assessment. Among patients who are MRD positive, sequential testing with deepening of response is associated with better outcomes compared with stable or increasing disease burden,45 and sustainment of MRD negativity over time has been well described as a more important prognostic factor than a solitary assessment.45-47
The powerful prognostic data provided by MRD assessment must be taken into account for accurate dynamic risk stratification in MM. However, care should be taken not to abandon the context provided by risk stratification at diagnosis. Certainly, some patients with standard-risk disease experience years of survival without MRD negativity—as seen for those with a monoclonal gammopathy of undetermined significance–like expression profile48 and many of the “exceptional responders” to lenalidomide therapy.11 By contrast, despite sustained MRD negativity, in the MASTER trial, much higher rates of MRD resurgence or progression were seen after treatment discontinuation among patients with ≥2 high-risk FISH abnormalities.49 As such, dynamic risk assessment allows for improved prognostication and provides additional context for management decisions in MM but is best implemented alongside, rather than as a replacement for, risk stratification at diagnosis.
CLINICAL CASE (Continued)
After discussion of possible maintenance regimens, the patient elects for risk-adapted treatment with lenalidomide and bortezomib. After 6 months, bortezomib is discontinued. One year after transplant, she is noted to have rising free κ. She is started on second-line therapy with isatuximab, carfilzomib, and dexamethasone. She has a rapid response and tolerates therapy well. After 4 cycles, she develops severe fatigue with worsening of anemia and dark stools. A large, bleeding mass in the stomach is identified and determined to be a κ-restricted plasmacytoma. Positron emission tomography/computed tomography shows bright uptake in the stomach, as well as additional sites throughout the gastrointestinal tract and several pleural-based nodules. Free κ measures 4 mg/dL and free λ is undetectable, with an M-spike of 0.2. Bone marrow biopsy specimen shows 30% plasma cells, with t(4;14), amp(1q) (4-5 copies of CKS1B), and −13 and a complex karyotype with hypodiploidy.
Progression and clonal evolution
In addition to MRD assessment, ongoing surveillance remains important. Progression within 18 months of diagnosis after receipt of autologous stem cell transplant is a very poor prognostic sign, regardless of cytogenetic risk.50 In addition, among patients achieving MRD negativity, resurgence of detectable MRD is a harbinger of progression and carries a worse prognosis than never having achieved MRD negativity.46 It remains unknown whether interventions for early relapse and/or MRD resurgence can overcome the prognostic impact of these events, but identification of early relapse should prompt the treating clinician to consider referral for clinical trials using novel treatment strategies to overcome high-risk disease.
Management of relapsed/refractory MM can be very challenging. Although some patients may experience long durations of disease control with second- and third-line therapies, duration of disease control often shortens with successive therapies, and attrition rates are high.51 This pattern is reflective of genomic instability and is a relatively common pathway of end-stage MM. Patients with high-risk cytogenetics may reach this phase more rapidly, sometimes with development of additional cytogenetic abnormalities such as amp(1q) or del(17p).52,53 Furthermore, as myeloma cells become refractory to therapies that are primarily inhibiting pathways upregulated in normal plasma cells, the malignant clone may develop a more poorly differentiated phenotype with higher rates of extramedullary disease, secondary PCL, and nonsecretory/oligosecretory disease.
Although there is reason for optimism due to novel therapeutics, including chimeric antigen receptor-T cells and bispecific antibodies, the unfortunate reality is that many patients, particularly with high-risk disease, are unable to receive such therapies due to poor access or inability to sustain disease control to allow for enrollment on clinical trials or to successfully undergo chimeric antigen receptor-T cell manufacturing. Development of PCL or oligosecretory disease can also prevent patients from being included in important clinical trials. There is a critical need to identify patients with high-risk MM early in order to facilitate referrals for innovative clinical trials that seek to overcome high-risk biology through the early implementation of cellular therapies at a time when this is more likely to be feasible and possibly more effective, before the development of end-stage myeloma.
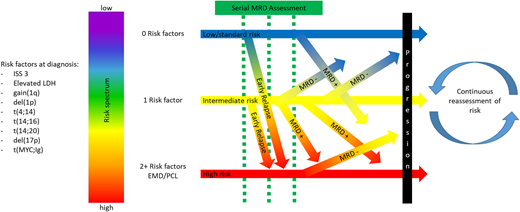
In conclusion, comprehensive risk assessment of patients with MM is essential both at diagnosis and through dynamic monitoring. Risk in MM is best considered on a spectrum, with the consideration that myeloma is a complex disease through which multiple clinical and genomic factors, as well as clinical course, affect patient outcomes. A visual characterization of this concept is depicted in Figure 2. Knowledge of clinical and cytogenetic risk provides the opportunity to set realistic expectations with patients and contextualize management decisions. High- sensitivity MRD testing and monitoring for early progression allows for the identification of patients who need to be considered for more aggressive interventions early in the relapsed setting. As our understanding evolves regarding the interactions between genomics, biological manifestations of disease, and response to therapy, new insights will be developed that might help to identify patients in need of different treatment strategies early in the disease course and also to avoid overtreatment of patients who may not require such an approach. The future for patients with MM is bright, and comprehensive risk evaluation will help to individualize management and improve the chances of long-term high-quality survival.
Dynamics of risk assessment in multiple myeloma. Considering all of the potential clinical and genomic risk factors, including but not limited to those in this figure, risk stratification at diagnosis should be considered a spectrum, with risk factors being additive rather than absolute. After initial stratification, risk should be reassessed based on the clinical course, with early relapse indicating a poor prognosis, regardless of initial risk considerations, and with recalibration of risk permitted by serial response assessment, with the most reliable indicator being MRD status. EMD, extramedullary disease.
Dynamics of risk assessment in multiple myeloma. Considering all of the potential clinical and genomic risk factors, including but not limited to those in this figure, risk stratification at diagnosis should be considered a spectrum, with risk factors being additive rather than absolute. After initial stratification, risk should be reassessed based on the clinical course, with early relapse indicating a poor prognosis, regardless of initial risk considerations, and with recalibration of risk permitted by serial response assessment, with the most reliable indicator being MRD status. EMD, extramedullary disease.
Conflict-of-interest disclosure
Timothy Martin Schmidt has received honoraria as a consultant for Janssen and Sanofi.
Off-label drug use
Timothy Martin Schmidt: nothing to disclose.