Abstract
As the aging population grows, so too does the number of well-tolerated antimyeloma therapies. Physicians will see an increasing volume of patients for subsequent lines of therapy, which could now extend this relationship for over a decade. For younger patients, treatment choices are infrequently impacted by concerns of fitness, but instead about effecting the deepest, most durable response. Older adults, in contrast, are more likely to experience under- than overtreatment, and therefore more objective (and ideally straightforward) ways to evaluate their fitness and ability to tolerate therapy will increasingly assist in decision-making. Post hoc analyses categorizing the fitness of trial patients in the modern treatment era globally demonstrate that even in highly selected populations, those that are recategorized as less fit or frail are consistently at higher risk of inferior outcomes and increased toxicities. Real-world data are comparatively lacking but do demonstrate that most patients with myeloma are not representative of those enrolled on clinical trials, generally more heavily burdened by comorbidities and more likely to be categorized as “less than fit.” Simultaneously, the number of therapeutic options open to patients in the relapsed setting continues to grow, now including T-cell engagers and cellular therapies, with their unique toxicity profiles. The aim of this review is to summarize the available data, highlight some of the approaches possible to easily assess fitness and how results might inform treatment selection, and illustrate ways that patients' condition can be optimized rather than lead to exclusion from the more complex therapies newly available.
Learning Objectives
Overview the variety of ways that fitness for antimyeloma therapy can be assessed and potentially influence treatment choice
How these tools may be utilized to optimize a patient's journey with myeloma, which can span sequential lines of therapy
Introduction
Multiple myeloma (MM) is the second leading hematologic malignancy in the United States.1 Individuals diagnosed with MM tend to be older, with a median age of 69 years at diagnosis and the majority over age 65.1 Improvements in disease management have led to impressive gains in progression-free and overall survival, so that greater numbers of individuals are living with their disease and receiving antimyeloma treatment for longer durations.2-4 Novel agent combination therapies have dramatically improved outcomes, but they are not without toxicity. Depending on the criteria used, up to 58% of patients will be considered burdened with significant comorbidities.5 So how can we best evaluate patients in the current treatment era for fitness or frailty and tailor their treatment appropriately?
CLINICAL CASE
A 68-year-old African American retired lawyer presents with fatigue, anemia, and hypercalcemia. Investigations demonstrated IgG κ, Revised International Scoring System II MM with 70% plasma cell infiltration in the bone marrow, and amplification of Ch1q. Her prior medical history is significant for atrial fibrillation and hypertension, for which she takes rivaroxaban 20 mg and amlodipine 10 mg once daily. She lives with her husband and adult son. Her Eastern Cooperative Oncology Group (ECOG) performance status is 1. She has received an intravenous bisphosphonate to correct her hypercalcemia, is feeling better, and wants to discuss her treatment options. Her sister underwent an autologous stem cell transplant (ASCT) for relapsed diffuse large B-cell lymphoma and asks if this is the right treatment for her. She wants to spend as much time with her grandchild as possible and is keen to receive the “best” treatment option available even if that comes with an increased risk of toxicity.
Frailty vs age for the initial selection of therapy
Despite multiple reports that older patients with myeloma are frequently undertreated, age continues to be a key factor in oncologists' decision-making6 ; it has heavy weighting in most frailty assessment tools,7,8 restricts the inclusion of older patients from clinical trials,9,10 and appears in guidelines limiting who should be offered upfront transplantation.11 Better tools are emerging that allow for assessment of biological rather than chronological aging.8,12 Some can depict immunosenescence,8 body composition,13 nutritional status,14 and track physical activity.15 In many countries where longevity is becoming the norm, older age should not simply equal unfit for treatment.
Formal frailty assessment can assist with decision-making, and a number of scoring systems have been proposed, mostly developed in those deemed transplant ineligible. Other measures that assess physical or cognitive performance or nutritional status can be very informative but are not universally incorporated into clinical practice (Table 1). The International Myeloma Working Group (IMWG) score16 is still considered the gold standard for the assessment of transplant-ineligible patients (although not always routinely performed), and it includes age, the Katz activities of daily living index, the Lawton Instrumental Activities of Daily Living scale, and the Charlson Comorbidity index. This and other simplified scores can predict survival outcomes as well as treatment-related toxicity and discontinuation rates.
Frailty scoring/outcome scoring systems myeloma patients
| Score . | Reference . | Variables . | Frailty/outcome groups defined . |
|---|---|---|---|
| Transplant assessment scoring systems | |||
| HCT-CI | 5 | Based on criteria reported by Sorror et al49 | 0 1-2 >2 |
| Frailty scoring/outcome scoring systems for transplant-ineligible patients | |||
| IMWG* | 16 | Age CCI IADL ADL | Fit Intermediate-fit Frail |
| Simplified IMWG | 89 | Age CCI ECOG | Nonfrail Frail |
| R-MCI | 90 | Age eGFR Lung disease KPS Frailty Cytogenetics | Fit Intermediate-fit Frail |
| UK MRP | 91 | Age PS CRP ISS | Low risk Medium risk High risk |
| Mayo frailty | 92 | Age PS NT-BNP | I II III IV |
| Objective and patient-reported outcome measures | |||
| Objective measures | |||
| Timed up and go85 | Subjects rise from a standard armchair, walk 3 meters, walk back, and sit down again | ||
| Hand grip strength93 | Measured using a dynamometer (static force hand can squeeze around a dynamometer) | ||
| Short Physical Performance Battery94 | Combines gait speed, chair stand, and balance tests | ||
| Score . | Reference . | Variables . | Frailty/outcome groups defined . |
|---|---|---|---|
| Transplant assessment scoring systems | |||
| HCT-CI | 5 | Based on criteria reported by Sorror et al49 | 0 1-2 >2 |
| Frailty scoring/outcome scoring systems for transplant-ineligible patients | |||
| IMWG* | 16 | Age CCI IADL ADL | Fit Intermediate-fit Frail |
| Simplified IMWG | 89 | Age CCI ECOG | Nonfrail Frail |
| R-MCI | 90 | Age eGFR Lung disease KPS Frailty Cytogenetics | Fit Intermediate-fit Frail |
| UK MRP | 91 | Age PS CRP ISS | Low risk Medium risk High risk |
| Mayo frailty | 92 | Age PS NT-BNP | I II III IV |
| Objective and patient-reported outcome measures | |||
| Objective measures | |||
| Timed up and go85 | Subjects rise from a standard armchair, walk 3 meters, walk back, and sit down again | ||
| Hand grip strength93 | Measured using a dynamometer (static force hand can squeeze around a dynamometer) | ||
| Short Physical Performance Battery94 | Combines gait speed, chair stand, and balance tests | ||
ADL, activities of daily living; CCI, Charleson Comorbidity Index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; IADL, instrumental activities of daily living; ISS, International Scoring System; KPS, Karnofsky Performance Scale; MRP, UK Myeloma Research Alliance Risk Profile; NT-BNP, B-natriuretic peptide; PS, performance status; R-MCI, revised Myeloma Comorbidity Index.
Although there is mounting evidence that geriatric assessments are efficient and cost-effective ways to personalize therapy and reduce the adverse effects experienced by patients, they continue to be perceived as time-consuming by physicians.17,18 Shorter, highly efficient screening tools such as the Geriatric 8 (G8) questionaire19,20 (available at https://www.siog.org/files/public/g8_english_0.pdf), Vulnerable Elders Survey 13,21 or senior-adult oncology questionnaire22 can be completed in under 10 minutes. Combined with other rapid screening tools of cognitive function such as the Mini-Cog™ and timed-up-and-go (TUG) test, this brief assessment can provide highly relevant and actionable information that can influence therapy (and generally will outperform clinical judgment with the exception of categorizing the very fit and very frail).20,23 A proposed approach is suggested in Table 2. Simplified geriatric screening tools such as those outlined can spare the efforts of full geriatric assessment in 20% to 40% of patients and prompt referrals for patient optimization in 42%.22,23 These screening assessments, combined with a Mini-Cog™ and TUG, typically add no more than 15 to 27 minutes to standard of care, with the majority of this time spent by the patient and caregiver and only 5 to 6 minutes by the health care provider.17
Proposed brief and simplified approach to screening patients with myeloma for frailty
| Measures . | Options . | Comments . |
|---|---|---|
| Performance status* | • ECOG • KPS | Consider patient-reported PS—can highlight subjective differences and prompt further evaluation95 |
| Documentation of comorbidities* | • CCI • HCT-CI • CIRS | Consider a validated index to quantify—can be useful to compare with published data and CCI incorporated into IMWG scoring system |
| Medication history* | Approaches to tackle polypharmacy broken down by health care setting in older adults with cancer (review)96 | Rather than simple documentation, greater emphasis on minimizing potential drug-drug interactions, dose reductions, and removing any unnecessary medications |
| Nutritional evaluation* | • Weight • HbA1C | Consider intervention for patients with significant weight loss >10% and those with comorbid or emergent diabetes |
| Geriatric screening tool | • G8 • Vulnerable Elders Survey 13 • Senior Adult Oncology Program | If abnormal—consider comprehensive geriatric screening or referral action based on low scoring domain19,22 |
| Cognitive screening | • Mini-Cog™ • Mini-mental state exam | |
| Physical functioning | • TUG | No specific training is needed to perform this test. Example available here: https://www.youtube.com/watch?v=tNay64Mab78 |
| Measures . | Options . | Comments . |
|---|---|---|
| Performance status* | • ECOG • KPS | Consider patient-reported PS—can highlight subjective differences and prompt further evaluation95 |
| Documentation of comorbidities* | • CCI • HCT-CI • CIRS | Consider a validated index to quantify—can be useful to compare with published data and CCI incorporated into IMWG scoring system |
| Medication history* | Approaches to tackle polypharmacy broken down by health care setting in older adults with cancer (review)96 | Rather than simple documentation, greater emphasis on minimizing potential drug-drug interactions, dose reductions, and removing any unnecessary medications |
| Nutritional evaluation* | • Weight • HbA1C | Consider intervention for patients with significant weight loss >10% and those with comorbid or emergent diabetes |
| Geriatric screening tool | • G8 • Vulnerable Elders Survey 13 • Senior Adult Oncology Program | If abnormal—consider comprehensive geriatric screening or referral action based on low scoring domain19,22 |
| Cognitive screening | • Mini-Cog™ • Mini-mental state exam | |
| Physical functioning | • TUG | No specific training is needed to perform this test. Example available here: https://www.youtube.com/watch?v=tNay64Mab78 |
Considered standard care. Adapted from Loh et al.23
CIRS, cumulative comorbidity rating score; HbA1c, hemoglobin A1c.
Frailty should be considered a dynamic rather than static process, and tailored dose adjustment of induction therapy should be considered, as illustrated in Figure 1.8,24 Prospective validation of this approach is under evaluation in the Myeloma XIV FiTNEss study comparing IMWG frailty-adjusted vs standard dosing approaches (NCT03720041). De-escalation of steroids should be considered upon disease control, as continuous dexamethasone until disease progression did not improve outcomes compared to time-limited dexamethasone in IMWG intermediate-fit patients.25
Induction strategies based on age and fitness
Long-term survivors with MM are more likely to have received triplet novel combinations and consolidative transplant.26 For patients in whom transplant fitness is uncertain, ideally optimal induction should not differ from that of younger patients although could be initially dose modified and adjusted with each cycle based on tolerance8 as fitness may improve with disease control and should be reexamined at each cycle (Figure 1).
For those newly diagnosed patients who are definitely transplant ineligible from the outset, the updated results of the MAIA study demonstrated unprecedented efficacy for upfront daratumumab, lenalidomide, and dexamethasone (DRd) (60-month progression-free survival [PFS] 52.5% vs 28.7% for lenalidomide and dexamethasone [Rd]).27 Longer follow-up also demonstrated an overall survival (OS) benefit—median not reached for DRd vs 55.7 months for Rd alone, P = .0013. The benefit was consistent across age and frailty subgroups (with significant numbers of frailer patients recruited and tolerating treatment well).28 The ALCYONE trial also demonstrated the benefit of the addition of daratumumab to bortezomib, melphalan, and prednisolone, regardless of frailty status.29 Other options for treatment include the combination bortezomib, lenalidomide, and dexamethasone based on SWOG S077730 or as the RVd-lite combination (dose-modified RVd).31 For fit older patients such as one considered in the Clinical Case, immunotherapeutic therapies are also being studied in the upfront setting, such as the CARTITUDE-5 trial comparing RVd continued to progression vs RVd followed by chimeric antigen receptor T-cell (CAR-T) (ciltacabtagene autoleucel) consolidation (NCT04923893), provided patients have a frailty index of 0 to 1 according to the IMWG scoring system.16 In the case of patients much frailer than that discussed here, options for all-oral therapy are also available such as Rd32 or ixazomib + Rd.33
Role of transplantation as consolidation in older patients
ASCT after induction therapy remains standard of care for fit patients with newly diagnosed myeloma. In the era of novel agents, this is based on results from several trials demonstrating a significant PFS benefit with transplant. Although age was restricted to <65 years34-37 in most trials, many centers offer ASCT up to age 70 or older in carefully selected fit patients.38-40 For these age groups, there are no randomized data in the modern era, but registry studies suggest benefit is conferred albeit with the caveats common to all such analyses.38,41 One retrospective, post hoc analysis of the Myeloma XI trial compared ASCT to no ASCT in matched patients from the transplant-eligible and transplant-ineligible pathways and found a significant progression-free and overall survival benefit in favor of transplant.42 There was no significant increase in morbidity or mortality associated with transplant in patients aged 65 to 70 or even >70 years. This study also randomly assigned patients to lenalidomide maintenance or observation after ASCT, confirming a benefit in older patients with a similar hazard ratio to younger patients.43 For those with comorbid concerns such as renal impairment or for those >70 years of age, dose adjustment of melphalan from 200 mg to 140 mg/m2 appears to be a reasonable approach to improve tolerability.44,45
Studies investigating the use of prehabilitation physiotherapy prior to ASCT are under way and may be of particular importance in the older cohort of patients who are frequently sarcopenic at presentation.46-48 Determining whether older patients are fit for transplant remains largely based on clinician assessment and patient preference. Scoring strategies such as the Hematopoietic Cell Transplantation–specific Comorbidity Index (HCT-CI, Table 1) may assist with decision-making but should not be considered a comprehensive assessment of an individual patient's ability to tolerate ASCT.49 A single- center experience of ~1800 patients treated over a 10-year period reported no difference in median OS (108.2 vs 106.25 months [P = .13] for those with an HCT-CI of ≥3 vs <3). There was similarly no difference in 3-year OS for patients <70 vs ≥70 years of age.119 The IMWG frailty score has been investigated prospectively in 1 study of transplant patients, assessed prior to physicians choosing a transplant or no-transplant approach.50 The IMWG frailty score predicted inferior PFS with ASCT for unfit patients aged 70 to 75 and no difference between ASCT and no ASCT, suggesting a potential role in this group. There was, however, no additional information provided by the score for fit, unfit aged 65 to 69, or frail patients, but this may have been limited by a relatively small sample size.
Several transplant centers are incorporating a more comprehensive assessment of older adults coming forward for transplant to optimize their outcomes, including several measures included in Table 2.51 Suggested additional assessments for patients under consideration include documentation of current and prior infections, creatinine clearance calculation, cardiac evaluation by electrocardiogram, echocardiogram or multiple gated cardiac blood pool imaging, and others, as indicated based on prior history or symptoms. Given the strong influence of pulmonary function tests in the revised myeloma comorbidity index/HCT-CI, many consider them also to be part of standard workup, including diffusing capacity of the lungs for carbon monoxide.49,52
CLINICAL CASE (Continued)
The patient was deemed fit to tolerate induction therapy with daratumumab-RVd, achieving a very good partial response after 4 induction cycles. At her pretransplant visit, her ECOG was 0, echocardiogram showed a normal left ventricular ejection fraction, electrocardiogram showed rate-controlled atrial fibrillation, pulmonary function tests were normal, and her creatinine clearance was 90 mL/min. Screening G8 was 17 (normal), TUG was 10 seconds, and Mini-Cog™ was unremarkable. She was counselled as to the potential risks and decided to proceed. She was referred for an exercise program with physiotherapy, underwent successful hematopoietic cell collection, and underwent transplant as an outpatient with 200 mg/m2 melphalan without incident. She achieved stringent complete response with minimal residual disease negativity and had a negative positron emission tomography scan at 3 months post-transplant reassessment (day 90) and therefore commenced lenalidomide maintenance therapy.
After 4 years of maintaining a good response, the patient was noted to have rising serum monoclonal protein, meeting the criteria for biochemical relapse, with no other symptoms. Repeat bone marrow confirmed persistent amplification of Ch1q and no other clonal evolution, with 40% plasma cell involvement. The patient was still able to walk her dog but less frequently attended active older adult fitness programs at the YMCA. The patient returned to the clinic to discuss treatment options.
How fitness might be incorporated at relapse
Despite the significant advances made in the treatment of MM, patients invariably relapse, and a number of factors have to be considered when deciding the next line of therapy for any patient, which includes their fitness for therapy (Figure 2). Therapy should be personalized and based on shared decision-making, but there are a few generalizations to be considered. Consistent with published retrospective analyses, our preference is to initiate alternative therapy when biochemical progression is established, given the potential negative consequences of waiting for clinical symptoms to emerge, which could worsen frailty and/or limit choice of therapy.53 Several published trials have demonstrated the clear superiority of 3-drug regimens over 2-drug regimens in terms of overall response rates (ORRs) and PFS; real-world data demonstrate attrition rates of 20% to 50% per line of therapy, so in principle, patients should be considered for the most effective combination they can tolerate, based on their disease status and fitness.54
Factors to consider when determining choice of therapy for RRMM. AE, adverse event; Tx, treatment.
Factors to consider when determining choice of therapy for RRMM. AE, adverse event; Tx, treatment.
Few trials have been conducted specifically for the frail patient population. Data can be extrapolated from trials in “elderly” patients, transplant-ineligible patients, or a subset analysis of larger studies (Table 3). It is important to remember that age and frailty are not synonymous terms, and all subset analyses have inherent limitations.
Breakdown of regimen options in the relapsed setting with subgroup analyses of older/frail populations
| Study . | Regimen . | Median prior lines of Tx (range) . | ORR, % . | ≥VGPR, % . | PFS . | OS . | Frail/elderly subgroup analysis . | No. (%) in triplet group . | PFS . | OS . |
|---|---|---|---|---|---|---|---|---|---|---|
| IMiD based | ||||||||||
| ASPIRE [Siegel et al, 2018]97 | KRd | 2 (1-3) | 87.1 | 69.9 | 26.1 mo* | 48.3 mo* | Facon et al, 202098 | 93 (23) frail by IMWG frailty index | 24.1 mo | 36.4 mo |
| TOURMALINE-MM1 [Richardson et al, 2021]99 | IxaRd | [1 prior, 62%; 2-3 prior, 38%]* | 79* | 52* | 20.6 mo | 53.6 mo* | Richardson et al, 202199 | 47 (13) >75 y | — | 36.9 mo* |
| POLLUX [Bahlis et al, 2020]100 | DRd | 1 (1-11)* | 92.9* | 80.4* | 44.5 mo* | @12 mo: 92.1% | Mateos et al, 2020101 | 29 (10) ≥75 y | 28.9 mo | Data immature |
| ELOQUENT-2 [Dimopoulos et al, 2020]102 | EloRd | 2 (1-4) | 79 | 33 | 19.4 mo* | 48.3 mo* | Dimopoulos et al, 2020102 | 68 (21) ≥75 y | — | 48.5 mo |
| ELOQUENT-3 [Dimopoulos et al, 2021]103 | EloPd | 3 (2-8) | 53 | 20 | 10.3 mo | @12 mo: 79%* | — | |||
| APOLLO [Dimopoulos et al, 2021]104 | DPd | 2 (1-5) | 69 | 51 | 12.4 mo | Data immature | Dimopoulos et al, 2021104 | 88 (58) ≥65 y | 14.2 mo | Data immature |
| ICARIA-MM [Attal et al, 2019]105 | IsaPd | 3 (2-4) | 60 | 32 | 11.5 mo | @12 mo: 72% | Schjesvold et al, 2021106 | 48 (31.2) frail by Facon score | 9.0 mo | @12 mo: 66.9% |
| PI based | ||||||||||
| OPTIMISMM [Richardson et al, 2019]107 | PVd | [1 prior, 40%; 2+ prior, 42%] | 82.2 | 53 | 11.2 mo | Data immature | Rocafiguera et al, 2022108 | 93 (33) frail by IMWG frailty index | 9.7 mo | — |
| PANORAMA1 [San-Miguel et al, 2016]109 | FVd | [1 prior, 51%; 2-3 prior, 49%] | 60.7 | 28 | 12.0 mo | 40.3 mo* | San-Miguel et al, 2016109 | 162 (42) ≥65 y | — | 37.3 mo* |
| BOSTON [Grosicki et al, 2020]110 | SeliVd | 2 (1-3) | 76.4 | 45 | 13.9 mo | Data immature | Auner et al111 | 66 (34) frail and 109 (56) >65 y | 13.93 mo for frail cohort | Data immature |
| CASTOR [Mateos et al, 2020]112 | DVd | 2 (1-9) | 85* | 63* | 16.7 mo* | Data immature | Mateos et al, 2020101 | 23 (9) ≥75 y | 17.9 mo* | Data immature |
| CANDOR [Usmani et al, 2022]113 | DKd | [1 prior, 46%; 2-3 prior, 54%] | 84* | 69* | 28.6 mo* | Data immature | Usmani et al, 2022113 | 134 (43) >65 y | 25.9 mo* | Data immature |
| IKEMA [Moreau et al, 2021]114 | IsaKd | 2 (1-2) | 87 | 73 | @24 mo: 69% | Data immature | Facon et al115 | 52 (28.5) ≥70 y | NR (24 mo PFS ~72%) | Data immature |
| Study . | Regimen . | Median prior lines of Tx (range) . | ORR, % . | ≥VGPR, % . | PFS . | OS . | Frail/elderly subgroup analysis . | No. (%) in triplet group . | PFS . | OS . |
|---|---|---|---|---|---|---|---|---|---|---|
| IMiD based | ||||||||||
| ASPIRE [Siegel et al, 2018]97 | KRd | 2 (1-3) | 87.1 | 69.9 | 26.1 mo* | 48.3 mo* | Facon et al, 202098 | 93 (23) frail by IMWG frailty index | 24.1 mo | 36.4 mo |
| TOURMALINE-MM1 [Richardson et al, 2021]99 | IxaRd | [1 prior, 62%; 2-3 prior, 38%]* | 79* | 52* | 20.6 mo | 53.6 mo* | Richardson et al, 202199 | 47 (13) >75 y | — | 36.9 mo* |
| POLLUX [Bahlis et al, 2020]100 | DRd | 1 (1-11)* | 92.9* | 80.4* | 44.5 mo* | @12 mo: 92.1% | Mateos et al, 2020101 | 29 (10) ≥75 y | 28.9 mo | Data immature |
| ELOQUENT-2 [Dimopoulos et al, 2020]102 | EloRd | 2 (1-4) | 79 | 33 | 19.4 mo* | 48.3 mo* | Dimopoulos et al, 2020102 | 68 (21) ≥75 y | — | 48.5 mo |
| ELOQUENT-3 [Dimopoulos et al, 2021]103 | EloPd | 3 (2-8) | 53 | 20 | 10.3 mo | @12 mo: 79%* | — | |||
| APOLLO [Dimopoulos et al, 2021]104 | DPd | 2 (1-5) | 69 | 51 | 12.4 mo | Data immature | Dimopoulos et al, 2021104 | 88 (58) ≥65 y | 14.2 mo | Data immature |
| ICARIA-MM [Attal et al, 2019]105 | IsaPd | 3 (2-4) | 60 | 32 | 11.5 mo | @12 mo: 72% | Schjesvold et al, 2021106 | 48 (31.2) frail by Facon score | 9.0 mo | @12 mo: 66.9% |
| PI based | ||||||||||
| OPTIMISMM [Richardson et al, 2019]107 | PVd | [1 prior, 40%; 2+ prior, 42%] | 82.2 | 53 | 11.2 mo | Data immature | Rocafiguera et al, 2022108 | 93 (33) frail by IMWG frailty index | 9.7 mo | — |
| PANORAMA1 [San-Miguel et al, 2016]109 | FVd | [1 prior, 51%; 2-3 prior, 49%] | 60.7 | 28 | 12.0 mo | 40.3 mo* | San-Miguel et al, 2016109 | 162 (42) ≥65 y | — | 37.3 mo* |
| BOSTON [Grosicki et al, 2020]110 | SeliVd | 2 (1-3) | 76.4 | 45 | 13.9 mo | Data immature | Auner et al111 | 66 (34) frail and 109 (56) >65 y | 13.93 mo for frail cohort | Data immature |
| CASTOR [Mateos et al, 2020]112 | DVd | 2 (1-9) | 85* | 63* | 16.7 mo* | Data immature | Mateos et al, 2020101 | 23 (9) ≥75 y | 17.9 mo* | Data immature |
| CANDOR [Usmani et al, 2022]113 | DKd | [1 prior, 46%; 2-3 prior, 54%] | 84* | 69* | 28.6 mo* | Data immature | Usmani et al, 2022113 | 134 (43) >65 y | 25.9 mo* | Data immature |
| IKEMA [Moreau et al, 2021]114 | IsaKd | 2 (1-2) | 87 | 73 | @24 mo: 69% | Data immature | Facon et al115 | 52 (28.5) ≥70 y | NR (24 mo PFS ~72%) | Data immature |
Data per updated analysis; otherwise, per initial publication.
DKd, daratumumab, carfilzomib, dexamethasone; DPd, daratumumab, pomalidomide, dexamethasone; DRd, daratumumab, lenalidomide, dexamethasone; DVd, daratumumab, bortezomib, dexamethasone; EloPd, elotuzumab, pomalidomide, dexamethasone; EloRd, elotuzumab, lenalidomide, dexamethasone; FVd, panobinostat, bortezomib, dexamethasone; IsaKd, isatuximab, carfilzomib, dexamethasone; IsaPd, isatuximab, pomalidomide, dexamethasone; IxaRd, ixazomib, lenalidomide, dexamethasone; KRd, carfilzomib, lenalidomide, dexamethasone; NR, not reported; Pd, pomalidomide; PI, proteosome inhibitor; PVd, pomalidomide, bortezomib, dexamethasone; SeliVd, selinexor, bortezomib, dexamethasone; Tx, treatment; VGPR, very good partial response.
For most patients whose disease has not progressed on monoclonal antibody therapies or did not have exposure to them as frontline therapy, an anti-CD38 monoclonal antibody should be considered in combination with immunomodulatory imide drugs (IMiDs) or proteasome inhibitors (PIs) and dexamethasone. Dose adjustments for age or frailty are not required for daratumumab or isatuximab but can be considered for the IMiD or PI partner.8 The universal theme from post hoc subanalyses performed (Table 3) suggests that even in older or frail patients, outcomes are better in those who receive the triplet combination arm vs the doublet, and discontinuation rates have been frequently higher in the doublet arms. Of course, treatment needs to be adapted to a patient's needs and special attention paid to cardiac risk, which may be higher in frail patients.55 Nonetheless, with careful monitoring and adapted dosing, authors conclude that novel combinations should not be restricted based upon frailty status.
CLINICAL CASE (Continued)
Repeat G8 screening revealed a score of 15, so a comprehensive geriatric assessment was deemed unnecessary. This patient had a normal B-natriuretic peptide, good ejection fraction (65%), and no other contraindications. Given the probable advantage of carfilzomib in patients with disease progression on lenalidomide, the combination daratumumab, carfilzomib, and dexamethasone using the once-weekly, dose-reduced carfilzomib (56 mg/m2)56,57 was discussed with the patient and commenced. Due to insomnia, dexamethasone dose was reduced to 10 mg on days of chemotherapy and discontinued after 1 year due to excellent disease control. She experienced a biochemical relapse 22 months later. Treatment was changed to elotuzumab, pomalidomide, and dexamethasone. Initial pomalidomide dosing was 3 mg but increased to 4 mg due to excellent tolerance. However, remission was less durable and 12 months later required changing therapy to cyclophosphamide, bortezomib, and dexamethasone. After 6 months, laboratory tests suggested slow biochemical progression. At that time, her G8 score decreased to 13 due to some weight loss and reduction in body mass index. Her simplified IMWG frailty score was 2 (scoring for ECOG = 1 and age) but her TUG was 11 seconds. Other comprehensive myeloma frailty score calculators are also available online (eg, http://www.myelomafrailtyscorecalculator.net/). She was referred for a comprehensive geriatric assessment. This patient is now 76 years old and triple-class exposed (PI, IMiD, and anti-CD38 antibody), and although her myeloma had attained a response on this latest regimen, she asks about what options she has if she were to subsequently experience relapse. She has been on therapy for over 8 years and is keen to learn about the potential for a “drug holiday.”
Fourth line and beyond—is this patient a fit enough candidate for complex immunotherapy?
Autologous T cells transduced with a chimeric antigen receptor (CAR) directed against B-cell maturation antigen (BCMA) have demonstrated unprecedented efficacy in patients with heavily pretreated relapsed and refractory MM.58-60 The impressive results achieved in pivotal studies using idecabtagene vicleucel and more recently ciltacabtagene autoleucel have led to approvals for commercial use.60-62 Patients referred for US Food and Drug Administration (FDA)–approved CAR-T are required to have received at least 4 prior lines of treatment. As patients experience disease progression through multiple lines of therapy, they become more debilitated and their quality of life diminishes.63,64 Monocentric experience suggests that at least 29% of patients treated with commercial CAR-T cells are ≥70 years of age, 84% have an HCT-CI of 1 or more (median, 3; range, 0-6), and 73% have baseline sarcopenia.120 Real-world consortium experience suggests that 77% of patients being treated with commercial idecabtagene vicleucel would not have met eligibility criteria for the pivotal clinical trial, 31% due to organ dysfunction and 17% due to poor performance status, albeit with comparable overall outcomes and toxicity when compared to the trial experience.65 A subgroup analysis of the KARMMA trial showed that those over 70 years derived equal benefit but may be at slightly higher risk of adverse events with increased rates of grade ≥3 cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity.66 In addition, the impressive results of T-cell engaging (TCE) BCMA-targeting teclistamab will likely also lead to imminent approval for use in patients with relapsed and refractory disease.67,68 The decision will then be on the clinician to evaluate fitness for BCMA targeting therapy—TCE vs CAR-T?69
Risk vs benefit discussion—establishing patient preference to proceed
When asked about their preferences, patients with myeloma have reported that durable disease response is a high priority and that they would even be willing to consider more significant side effects to achieve this goal.70 The achievement of deep and durable responses is associated with improved quality of life and has been confirmed in patient-reported outcomes from the pivotal CAR-T trials.71-73 So too has interruption of continuous therapy.64 Alternative available regimens at this stage in the disease generally yield short duration of disease control, with low ORRs of less than 30% and median PFS estimates in the region of 3 to 4 months.74 Responses from CAR-T are comparatively much better with higher ORR (73%-97%) and longer durations of response, even in matched comparisons with available data in triple-class refractory patients.58,60,74-76 Similar outperformance of matched alternatives has been shown for teclistamab.77,78 However, administration is not without risk, with high incidence of unique toxicities, including CRS, infections, and the potential for both neurotoxicity and prolonged cytopenias79 (Table 4). Nonetheless, the limited data that exist suggest that the administration of CD19-directed CAR-T products to older patients has thus far demonstrated similar (sometimes even improved) efficacy when compared with outcomes achieved by younger patients, although with suggestion that older patients may be at increased risks of toxicity vs their younger counterparts.66,80-83
Response rates and key toxicities reported from pivotal BCMA-targeting trials and real-world data
| Generic name . | Commercial name . | ORR, % . | Median DOR . | CRS rates: Any grade (≥3), % . | Neuro toxicity rates: Any grade (≥3), % . | FDA approved . | Reference . |
|---|---|---|---|---|---|---|---|
| Idecabtagene vicleucel (ide-cel, also called bb2121) | Abecma | 73 | 10.7 mo | 84 (5) | 18 (3) | Yes | 58,61 |
| Real-World Consortium Data (ide-cel) | Abecma | 83 | NR | 82 (5) | 15 (5) | NA | 116 |
| Ciltacabtagene autoleucel (cilta-cel) | Carvikty | 97 | 21.8 mo | 95 (4) | 21 (9) | Yes | 60,62 |
| Meta-analysis BCMA-directed CAR-T including those in trial phase | NA | 78-85 | 14 mo* | NR (6.4-6.6) | NR (2.2-3.5) | NA | 117,118 |
| Teclistamab | Tecvayli | 63 | 18.4 mo | 72.1 (0.6) | 14.5 (0.6) | Pending decision at time of writing | 67 |
| DOR, duration of response; FDA, Food and Drug Administration; NA, not applicable. | |||||||
| Generic name . | Commercial name . | ORR, % . | Median DOR . | CRS rates: Any grade (≥3), % . | Neuro toxicity rates: Any grade (≥3), % . | FDA approved . | Reference . |
|---|---|---|---|---|---|---|---|
| Idecabtagene vicleucel (ide-cel, also called bb2121) | Abecma | 73 | 10.7 mo | 84 (5) | 18 (3) | Yes | 58,61 |
| Real-World Consortium Data (ide-cel) | Abecma | 83 | NR | 82 (5) | 15 (5) | NA | 116 |
| Ciltacabtagene autoleucel (cilta-cel) | Carvikty | 97 | 21.8 mo | 95 (4) | 21 (9) | Yes | 60,62 |
| Meta-analysis BCMA-directed CAR-T including those in trial phase | NA | 78-85 | 14 mo* | NR (6.4-6.6) | NR (2.2-3.5) | NA | 117,118 |
| Teclistamab | Tecvayli | 63 | 18.4 mo | 72.1 (0.6) | 14.5 (0.6) | Pending decision at time of writing | 67 |
| DOR, duration of response; FDA, Food and Drug Administration; NA, not applicable. | |||||||
Median PFS, not DOR reported.
How can patients' fitness be optimized?
Given the known toxicity profile of these BCMA-targeting therapies as outlined in Table 4, ideally clinicians should aim to optimize patients' performance status, comorbidities, and physiologic reserve a priori in order to make this treatment safe and effective.84 Early referral for consideration and assessment is likely key, more so for CAR-T than TCE, as due to manufacturing delays and limited availability, waiting for patients' myeloma to meet criteria for disease progression with uncontrollable disease burden may not allow for patients to benefit optimally.
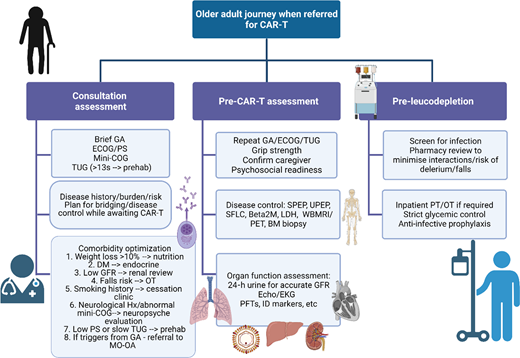
Patients with relapsed disease may be at risk of falls, may struggle to navigate the complexities of their care, and may be burdened by additional disability secondary to the disease itself that makes them even more vulnerable.52,85,86 Many have accumulated comorbidities, but these can be managed and do not necessarily need to prevent a patient from proceeding to CAR-T. For example, although patients with renal impairment were universally excluded from registration CAR-T trials, fludarabine can be dose adjusted and CAR-T has been safely administered to patients with reduced glomerular filtration rate, albeit with short duration of follow-up and a very small sample size (N = 7).87 Cardiac reserve will also be important in order to deal with the physiologic stress of CRS, but more widespread use of tocilizumab and low rates of severe-grade CRS could make this less of a key determinant of eligibility.88 A more comprehensive approach to the assessment of older adults has been suggested as imperative prior to CAR-T (Figure 3).51 This may also ultimately inform patient and product selection as more constructs or T-cell engaging therapies become available, and it also permits consideration for which patients' myeloma could be safely treated in the outpatient setting.
Comprehensive assessment of the older adult referred for CAR-T. Created with BioRender.com. BM, bone marrow; DM, diabetes mellitus; EKG, electrocardiogram; GA, geriatric assessment; GFR, glomerular filtration rate; Hx, history; ID, infectious disease; LDH, lactate dehydrogenase; MO-OA, medical oncology for older adults; OT, occupational therapy; PET, positron emission tomography; PFT, pulmonary function tests; PS, performance status; SFLC, serum free light chains; SPEP, serum protein electrophoresis; UPEP, urine protein electrophoresis; WBMRI, whole body magnetic resonance imaging.
Comprehensive assessment of the older adult referred for CAR-T. Created with BioRender.com. BM, bone marrow; DM, diabetes mellitus; EKG, electrocardiogram; GA, geriatric assessment; GFR, glomerular filtration rate; Hx, history; ID, infectious disease; LDH, lactate dehydrogenase; MO-OA, medical oncology for older adults; OT, occupational therapy; PET, positron emission tomography; PFT, pulmonary function tests; PS, performance status; SFLC, serum free light chains; SPEP, serum protein electrophoresis; UPEP, urine protein electrophoresis; WBMRI, whole body magnetic resonance imaging.
Conclusion
The available treatment options that can be accessed to control disease and prolong the life of patients with multiple myeloma continue to increase. Although all tools to assess fitness or frailty have their inherent limitations, some are efficient in their application and can identify areas for intervention and guide decision-making. Improved therapy can also lead to improved fitness and quality of life; thus, optimizing the patient for the best possible treatment should be considered the core principle wherever possible.
Conflict-of-interest disclosure
Charlotte Pawlyn: Abbvie, Amgen, Takeda. Janssen, Celgene, Sanofi: consultancy/honoraria/travel support.
Abdullah M. Khan: Amgen: speakers bureau; Janssen: honoraria; Sanofi: speakers bureau; Secura Bio: consultancy, research funding.
Ciara L. Freeman: BMS, Seattle Genetics, Celgene, Abbvie, Sanofi, Incyte, Amgen, and Janssen: honoraria/consulting; Teva, Janssen, and Roche/Genentech: research funding.
Off-label drug use
Charlotte Pawlyn: nothing to disclose.
Abdullah M. Khan: nothing to disclose.
Ciara L. Freeman: nothing to disclose.