Abstract
Despite significant improvement in the treatment of multiple myeloma (MM), a cure remains elusive, and patients failing proteasome inhibitors, immunomodulatory drugs, and anti-CD38 monoclonal antibodies remain a challenge due to a lack of standard of care treatment and a dismal survival rate. The development of T-cell redirecting therapies, including bispecific T-cell engagers and chimeric antigen receptor (CAR) T cells, have transformed the outcome of triple-class exposed relapsed and refractory MM (RRMM). B-cell maturation antigen (BCMA) has proven to be an important target in MM, and BCMA-directed CAR T cells have shown unprecedented efficacy with a prolonged duration of response in a population with advanced RRMM, leading to the approval of 2 different BCMA CAR T-cell products. Still, and in contrast to prior experience in the field of CD19-directed CARs, no plateau has been seen in the survival curves, and relapses continue to occur. Therefore, further improvement is needed. Early use in the course of the disease as well as of next- generation CARs may further augment the efficacy of these therapies. In this review we address current state-of-the-art approved BCMA-directed CAR T-cell therapy in RRMM, as well as potential future developments focused on optimizing patient care and novel CAR designs.
Learning Objectives
Summarize the current evidence regarding BCMA-directed CAR T cells for RRMM, focusing on approved constructs
Discuss potential limitations of current CAR T-cell therapy in myeloma
Describe potential strategies for improving current results, with a focus on the clinical perspective
CLINICAL CASE
A 56-year-old male was diagnosed in September 2019 with immunoglobulin A lambda multiple myeloma (MM). At diagnosis he presented with diffuse osteolytic lesions and a revised International Staging System (R-ISS) 3 status (high lactose dehydrogenase + t(4;14)). He received frontline treatment with daratumumab in combination with bortezomib, lenalidomide, and dexamethasone (D-VRD) in the context of a randomized clinical trial. He completed 4 planned induction cycles followed by autologous stem cell transplant and 2 additional consolidation cycles. He achieved a very good partial response after consolidation and started maintenance treatment with daratumumab plus lenalidomide in June 2020. Unfortunately, 1 month later he progressed with the presence of a lumbar plasmacytoma. As of this moment, he has been transferred to our department to be considered for a B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell clinical trial enrolling patients with early relapse after frontline treatment.
Introduction
The treatment landscape of MM has significantly evolved in recent years, leading to unprecedented survival rates.1 Nevertheless, this improvement has not been uniform, and there are still patient populations with high-risk features who have not benefited enough from recently approved combinations and require innovative strategies to overcome their dismal prognosis. These populations are, among others, patients with extramedullary disease, a high number of circulating plasma cells, high-risk cytogenetic abnormalities (Cas), particularly double-hit myeloma, and patients who relapse early after optimized frontline therapy.2–4 Regarding the latter, patients with R-ISS 3 status at diagnosis who fail to achieve undetectable minimal residual disease (MRD) after frontline treatment are at a high risk of an early relapse with a median progression-free survival (PFS) of only 14 months and a median overall survival (OS) of fewer than 2 years.5 In addition, patients who have failed proteasome inhibitors, immunomodulatory drugs, and anti-CD38 monoclonal antibodies have very poor outcomes and limited treatment options.6 Therefore, we need new treatments with novel mechanisms of action able to rescue difficult-to-treat populations and improve patient survival.
BCMA CAR T cells approved: what is now
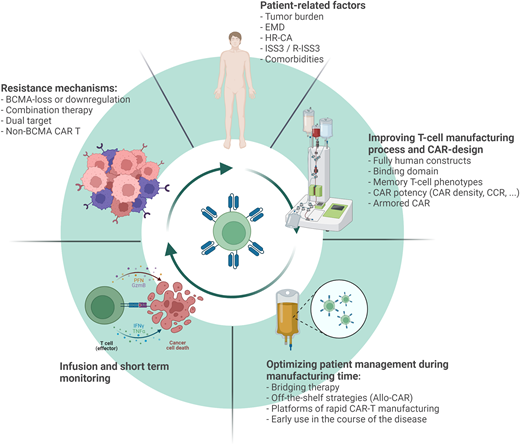
In this scenario CAR T-cell therapy has shown unprecedented response rates in heavily pretreated patients, leading to the approval of 2 BCMA-directed CAR T-cell products (ABECMA™ and CARVIKTY™) for the treatment of triple-class exposed relapsed/refractory (RR) MM who have received at least 4 prior lines of treatment. In this review we discuss the current state-of-the-art of approved CAR T-cell therapy in RRMM and future developments, with a focus on the clinical perspective (Figure 1).
Several strategies are being evaluated to improve the current clinical efficacy of CAR T-cell therapy in the context of RRMM. These strategies can be divided into patient-related factors, aspects related to the manufacturing process and CAR design, patient management during bridging time and infusion, and finally, understanding the mechanisms of resistance and CAR failure. CCR, chimeric costimulatory receptor; EMD, extramedullary disease; HR-CA, high-risk cytogenetic abnormalities.
Several strategies are being evaluated to improve the current clinical efficacy of CAR T-cell therapy in the context of RRMM. These strategies can be divided into patient-related factors, aspects related to the manufacturing process and CAR design, patient management during bridging time and infusion, and finally, understanding the mechanisms of resistance and CAR failure. CCR, chimeric costimulatory receptor; EMD, extramedullary disease; HR-CA, high-risk cytogenetic abnormalities.
Idecabtagene vicleucel (ide-cel, bb2121, ABECMA™) was the first cellular therapy approved for the treatment of RRMM. Ide-cel is a second-generation CAR with a 4-1BB costimulatory domain approved for the treatment of RRMM based on the data of the phase 2 single-arm pivotal KarMMa trial.7 Overall, 128 patients were infused with 2 different doses of ide-cel 150, 300, and 450 × 106 CAR T+ cells. The median number of prior lines was 6 (3-18), and 84% of patients were triple-class refractory. All patients received standard lymphodepleting chemotherapy with fludarabine and cyclophosphamide. The overall response rate (ORR) was 73% among all treated patients and 81% in those receiving the higher-dose level (450 × 106 ), with 33% and 39% of complete responses (CR), respectively. Responses were rapid, with a median time to first response of 1.0 month (range, 0.5-8.8 months). Median PFS was 8.8 months in the overall population and was longer in patients receiving the target dose (median PFS, 12.1 months) and in those achieving CR or better (median PFS, 20.2 months; 95% CI, 12.3-NE [not evaluable]). Median OS was 24.8 months (95% CI, 19.9-31.2). Safety overall was manageable, with cytokine release syndrome (CRS) reported in 84% of patients across the 3 doses and 96% at the target dose. The majority of events were grade 1 or 2 with a CRS grade higher than or equal to 3 in fewer than 6% of patients, with a median time to onset of 1 day (range, 1-12). Neurological complications were less common, were reported in 18% of patients, and were mostly grade 1 to 2. On the other hand, cytopenias were frequent and not dose related, with a median time to recovery from grade 3 or higher neutropenia and thrombocytopenia of 2 and 3 months, respectively (Table 1).7
Safety and efficacy data of the 2 FDA- and EMA-approved CARs
| . | Idecabtagene vicleucel KarMMa trial1–3 . | Ciltacabtagene autoleucelCARTITUDE 1 trial . |
|---|---|---|
| Number of patients infused, n | 128 (140 apheresed) | 97 (120 apheresed) |
| Phase | 2 | 1b/2 |
| Target/costimulation | BCMA/4-1BB | BCMA/4-1BB *2 BCMA-targeting heavy-chain antibody |
| scFv | Chimeric mouse | Chimeric llama |
| Specificity | Autologous | Autologous |
| Follow-up, median (range) | 13.3 mo (0.2-21.2) | 21.7 mo (not reported) |
| Prior lines, median (range) | 6 (3 to 16) | 6 (3-18) |
| Triple-class refractory, n (%) | 108 (84) | 85 (87.6) |
| Penta-exposed, n (%) | 77 (60) | 81 (83.5) |
| Bridging therapy, n (%) | 112 (88) | 73 (75) |
| Response to bridging therapy, n (%) | 5/112 (4) | 33/73 (45) |
| EMD, n (%) | 50 (39) | 13 (13) |
| CAR T-cell dose | 150, 300, 450 × 106 CAR + T | 0.75 × 106 CAR T+/kg |
| LD chemotherapy | Fludarabine 30 mg/m2 × 3 d Cyclo 300 mg/m2 × 3 d | Fludarabine 30 mg/m2 × 3 d Cyclo 300 mg/m2 × 3 d |
| ORR, n (%) | 94 (73) At 450 × 106 (n = 54): 44 (81%) | 95 (97.9) |
| CR or sCR, n (%) | 42 (33) At 450 × 106 (n = 54): 21 (39) | 82.5% (sCR) |
| Time to first response, median (range) | 1.0 mo (0.5-8.8) | 1.0 mo (IQR 0.9-1.0) |
| MRD in CR, n (%) | 33/42 MRD neg (10−5) | 61 evaluable 92% MRD negative (10−5) |
| DOR, median (95% CI) | 10.7 mo (9.0-11.3) | Not reported |
| PFS, median (95% CI) | 8.8 mo (5.6-11.6) At 450 × 106 : 12.1 mo (8.8-12.3) | NR (16.8-NE) 2-y PFS: 60.5% (48.5-70.4) |
| PFS in high-risk, median (95% CI) | PFS at 2y, % (95% CI) | |
| ISS 3/R-ISS 3 | 4.9 mo (1.8-8.2)a | NE (NE-NE) |
| High-risk CA | 8.2 mo (4.8-11.9) | 48.4% (25.1-68.4) |
| Plasmacytomas | 7.9 mo (5.1-10.9) | 47.4% (24.4-67.3) |
| OS, median (95% CI) | 24.8 mo (19.9-31.2) | NR (27.2-NE) |
| CRS, n (%) | ||
| Overall | 107 (84) | 92 (95) |
| Grade 3-4 | 7 (5) | 4 (4) |
| Time to CRS onset, median (range) | 1 d (1-12) | 7 d (5-8) |
| Duration of CRS, median (range) | 5 d (1-63) | 4 d (3-6) |
| Neurotoxicity, n (%) | ||
| Overall | 23 (18) | 20 (21)b |
| Grade 3-4 | 4 (3) | 9 (9) |
| Time to neurotoxicity onset, median (range) | 2 d (1-10) | 8 d (6-8) for ICANs Other: CAR-T cell neurotoxicites: 26.5 d (11-108)b |
| Grade 3-4 neutropenia, n (%) | 114 (89) | 92 (95) |
| Time to recovery, median (range) | 1.9 mo (1.2-5.6) | Not reported |
| Grade 3-4 thrombocytopenia, n (%) | 67 (52) | 58 (60) |
| Time to recovery, median (range) | 2.1 mo (1.2-13.8) | Not reported |
| Infections, n (%) | 88 (69) | 56 (58) |
| Grade 3-4 infections, n (%) | 28 (22) | 19 (20) |
| Death, n (%) | 44 (34) | 14 |
| Reference | (1) Munshi et al9 (2) Anderson et al.34 (3) Oriol et al.35 | (4) Berdeja et al8 (5) Martin et al.36 (6) Jakubowiak et al11 |
| . | Idecabtagene vicleucel KarMMa trial1–3 . | Ciltacabtagene autoleucelCARTITUDE 1 trial . |
|---|---|---|
| Number of patients infused, n | 128 (140 apheresed) | 97 (120 apheresed) |
| Phase | 2 | 1b/2 |
| Target/costimulation | BCMA/4-1BB | BCMA/4-1BB *2 BCMA-targeting heavy-chain antibody |
| scFv | Chimeric mouse | Chimeric llama |
| Specificity | Autologous | Autologous |
| Follow-up, median (range) | 13.3 mo (0.2-21.2) | 21.7 mo (not reported) |
| Prior lines, median (range) | 6 (3 to 16) | 6 (3-18) |
| Triple-class refractory, n (%) | 108 (84) | 85 (87.6) |
| Penta-exposed, n (%) | 77 (60) | 81 (83.5) |
| Bridging therapy, n (%) | 112 (88) | 73 (75) |
| Response to bridging therapy, n (%) | 5/112 (4) | 33/73 (45) |
| EMD, n (%) | 50 (39) | 13 (13) |
| CAR T-cell dose | 150, 300, 450 × 106 CAR + T | 0.75 × 106 CAR T+/kg |
| LD chemotherapy | Fludarabine 30 mg/m2 × 3 d Cyclo 300 mg/m2 × 3 d | Fludarabine 30 mg/m2 × 3 d Cyclo 300 mg/m2 × 3 d |
| ORR, n (%) | 94 (73) At 450 × 106 (n = 54): 44 (81%) | 95 (97.9) |
| CR or sCR, n (%) | 42 (33) At 450 × 106 (n = 54): 21 (39) | 82.5% (sCR) |
| Time to first response, median (range) | 1.0 mo (0.5-8.8) | 1.0 mo (IQR 0.9-1.0) |
| MRD in CR, n (%) | 33/42 MRD neg (10−5) | 61 evaluable 92% MRD negative (10−5) |
| DOR, median (95% CI) | 10.7 mo (9.0-11.3) | Not reported |
| PFS, median (95% CI) | 8.8 mo (5.6-11.6) At 450 × 106 : 12.1 mo (8.8-12.3) | NR (16.8-NE) 2-y PFS: 60.5% (48.5-70.4) |
| PFS in high-risk, median (95% CI) | PFS at 2y, % (95% CI) | |
| ISS 3/R-ISS 3 | 4.9 mo (1.8-8.2)a | NE (NE-NE) |
| High-risk CA | 8.2 mo (4.8-11.9) | 48.4% (25.1-68.4) |
| Plasmacytomas | 7.9 mo (5.1-10.9) | 47.4% (24.4-67.3) |
| OS, median (95% CI) | 24.8 mo (19.9-31.2) | NR (27.2-NE) |
| CRS, n (%) | ||
| Overall | 107 (84) | 92 (95) |
| Grade 3-4 | 7 (5) | 4 (4) |
| Time to CRS onset, median (range) | 1 d (1-12) | 7 d (5-8) |
| Duration of CRS, median (range) | 5 d (1-63) | 4 d (3-6) |
| Neurotoxicity, n (%) | ||
| Overall | 23 (18) | 20 (21)b |
| Grade 3-4 | 4 (3) | 9 (9) |
| Time to neurotoxicity onset, median (range) | 2 d (1-10) | 8 d (6-8) for ICANs Other: CAR-T cell neurotoxicites: 26.5 d (11-108)b |
| Grade 3-4 neutropenia, n (%) | 114 (89) | 92 (95) |
| Time to recovery, median (range) | 1.9 mo (1.2-5.6) | Not reported |
| Grade 3-4 thrombocytopenia, n (%) | 67 (52) | 58 (60) |
| Time to recovery, median (range) | 2.1 mo (1.2-13.8) | Not reported |
| Infections, n (%) | 88 (69) | 56 (58) |
| Grade 3-4 infections, n (%) | 28 (22) | 19 (20) |
| Death, n (%) | 44 (34) | 14 |
| Reference | (1) Munshi et al9 (2) Anderson et al.34 (3) Oriol et al.35 | (4) Berdeja et al8 (5) Martin et al.36 (6) Jakubowiak et al11 |
Data from the KarMMa and CARTITUDE-1 pivotal trials with ide-cel and cilta-cel, respectively, are summarized.
PFS in R-ISS 3.
ICANS occurred in 16 (17%) patients. Other neurotoxicities occurred in 12 patients, with a median time to onset of 27 days. Five patients had a cluster of movement and neurocognitive treatment–emergent adverse events.
EMA: European Medicines Agency; EMD, extramedullary disease; FDA: US Food and Drug Administration.
Ciltacabtagene autoleucel (cilta-cel; former LCAR-B38M, CARVIKTY™), another second-generation CAR T cell containing 2 BCMA-binding domains, was investigated in the CARTITUDE-1 study (and previously evaluated in the LEGEND-2 phase 1 study). Overall, 97 patients were infused with a median number of 6 prior lines and 87.6% were triple class refractory. The ORR was 97.9% with 82.5% of patients achieving a stringent CR (sCR). With a median follow-up of 2 years, outstanding survival rates have been reported, with a 2-year PFS of 60.5% (95% CI, 48.5-70.4) and 71.0% (95% CI, 57.6-80.9) in sCR patients. Two-year OS was 71.0% (95% CI, 57.6-80.9). Regarding safety, cytopenias and CRS were the most frequent treatment-related adverse events reported. Any grade of neutropenia was present in 96% of patients, with 95% having grade 3 or higher. The incidence of CRS was 95%, mostly grade 1 to 2, with a median time to onset of 7 days (range, 1-12). Neurological events were reported in 20 patients. Among these, 16 presented immune-effector cell–associated neurotoxicity syndrome (ICANS), and 12 patients presented with other neurotoxicities. Of these, 5 experienced a movement and neurocognitive disorder with a median time to onset of 27.0 days (IQR 16.0-73.0) (Table 1).8
Other BCMA-directed CARs are under development, and the status of those studies are summarized in Table 2.
Main reported clinical trials of BCMA-directed CAR T cells in RRMM
| . | BB21217 CRB-402 (n = 72) . | Orva-cel EVOLVE (n = 62) . | CT053 LUMMICAR (n = 18) . | CT103A FUMANBA-1 (n = 79) . | C-CAR088 (n = 23) . | P-BCMA-101 PRIME (n = 53) . | ALLO-715 UNIVERSAL (n = 43) . | ARI-002h (n = 30) . |
|---|---|---|---|---|---|---|---|---|
| Phase | 1 | 1 | 1b/2 | 1/2 | 1 | 1/2 | 1 | 1 |
| Follow-up, median (range) | 23 (9-46) | 9.5 mo | 6 mo (2-11) | 25.3 mo (4.1-36.7) | 6.2 mo (0.7-16.1) | NA | 4 mo | 13 mo (7-13) |
| Target/costimulation | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB |
| scFv | Mouse | Mouse | Human | Human | Human | Mouse | Human | Human |
| Specificity | Autologous | Autologous | Autologous | Autologous | Autologous | Autologous | Allogeneic | Autologous |
| CAR T-cell dose | 150-450 M | 300-600 M | 1.5-3.0 × 108 cells | 1.0 × 106/kg | 1.0-6.0 × 106/kg | 51-1178 × 106 | 40-480 × 106 | 3 × 106/kg |
| Population | ||||||||
| Age, median (range) | 62 (33-76) | 61 (33-77) | 62 (36-78) | 56 (39-70) | 60 (45-74) | 60 (42-74) | 64 (46-77) | 61 (36-74) |
| Prior lines, median (range) | 6 (3-17) | 6 (3-18) | 5 (3-11) | 5 (3-13) | 4 (2-12) | 8 (2-18) | 5 (3-11) | 4 (2-10) |
| Triple-class refractory, n (%) | 50 (69) | 58 (94) | 85% | 13 (16.5) | NR | 60% | NR | 61% |
| Penta-refractory, n (%) | NR | 30 (48) | 50% | NR | NR | NR | 42% | NR |
| Efficacy | At 320 × 106 | |||||||
| ORR, n (%) | 69% | 92% | 94% | 75 (94.9) | 22 (95.7) | 50%-75% | 71% | 100% |
| CR, n (%) | 36% | 36% | 27.8% | 58.2% | 43.5% | NR | 25% | 60% |
| PFS (mo), median (95% CI) | 12.8 mo (7.3-18.6) | 9.3 mo (300 M) | NR | 25.3 mo (3.0-NE) | 6 mo PFS: 65.1% | NR | NR | 15.8 mo (12.9-NE) |
| CRS | ||||||||
| All grade, n (%) | 54 (75) | 55 (89) | 15/18 (83.3) | 72 (92.4) | 21 (91.3) | 17% | 24 (56) | 87% |
| Grade 3-4, n (%) | 3 (2 grade 5) | 2 (3) | 0 (0) | 2 (2.5) | 1 (4.3) | 0% | 1 (2) | 0 (0) |
| Onset (d), median (range) | 2 d (1-20) | 2 (1-4) | 2 (DL0) & 1 (DL1) | 6 (1-12) | 6 (1-11) | NR | NR | NR |
| Duration (d), median (range) | 4 d | 4 (1-10) | 4 (DL0) & 3 (DL1) | 5 (1-30) | 5 (2-9) | NR | NR | 4 (1-12) |
| Tocilizumab/steroids %/% | 53/17 | 76/52 | ≈30/20 | 20/34.7 | 26/9 | 7/6 | 23/14 | 76/12 |
| ICANS | ||||||||
| All grade, n (%) | 11 (15) | 8 (13) | 2 (DL0) & 1 (DL1) | 1 (1.3) | 1 (4.3) | 4% | 6 (14) | 0 |
| Grade 3-4, n (%) | 3 (4) | 2 (3) | 1 patient | 0 | 0 (0) | 4% | 0 (0) | 0 |
| Onset (d), median (range) | 7 d (2-24) | 4 (1-6) | NR | 10 | 8 | NR | NR | — |
| Duration (d), median (range) | 2 d | 4 (1-10) | NR | 1 | 1 | NR | NR | — |
| Status | Not further developed | Not further developed | Ongoing | Ongoing | Ongoing | Not further developed | Ongoing | Ongoing |
| Reference | Raje et al27 | Mailankody et al.37 | Chen et al.38 | Chunrui et al.39 | Gan An, et al.40 | Costello et al.41 | Mailankody et al.42 | de Larrea et al24 |
| Identification | NCT03274219 | NCT03430011 | NCT03915184 | NCT05066646 | NCT05066646 | NCT03288493 | NCT04093596 | NCT04309981 |
| . | BB21217 CRB-402 (n = 72) . | Orva-cel EVOLVE (n = 62) . | CT053 LUMMICAR (n = 18) . | CT103A FUMANBA-1 (n = 79) . | C-CAR088 (n = 23) . | P-BCMA-101 PRIME (n = 53) . | ALLO-715 UNIVERSAL (n = 43) . | ARI-002h (n = 30) . |
|---|---|---|---|---|---|---|---|---|
| Phase | 1 | 1 | 1b/2 | 1/2 | 1 | 1/2 | 1 | 1 |
| Follow-up, median (range) | 23 (9-46) | 9.5 mo | 6 mo (2-11) | 25.3 mo (4.1-36.7) | 6.2 mo (0.7-16.1) | NA | 4 mo | 13 mo (7-13) |
| Target/costimulation | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB | BCMA/4-1BB |
| scFv | Mouse | Mouse | Human | Human | Human | Mouse | Human | Human |
| Specificity | Autologous | Autologous | Autologous | Autologous | Autologous | Autologous | Allogeneic | Autologous |
| CAR T-cell dose | 150-450 M | 300-600 M | 1.5-3.0 × 108 cells | 1.0 × 106/kg | 1.0-6.0 × 106/kg | 51-1178 × 106 | 40-480 × 106 | 3 × 106/kg |
| Population | ||||||||
| Age, median (range) | 62 (33-76) | 61 (33-77) | 62 (36-78) | 56 (39-70) | 60 (45-74) | 60 (42-74) | 64 (46-77) | 61 (36-74) |
| Prior lines, median (range) | 6 (3-17) | 6 (3-18) | 5 (3-11) | 5 (3-13) | 4 (2-12) | 8 (2-18) | 5 (3-11) | 4 (2-10) |
| Triple-class refractory, n (%) | 50 (69) | 58 (94) | 85% | 13 (16.5) | NR | 60% | NR | 61% |
| Penta-refractory, n (%) | NR | 30 (48) | 50% | NR | NR | NR | 42% | NR |
| Efficacy | At 320 × 106 | |||||||
| ORR, n (%) | 69% | 92% | 94% | 75 (94.9) | 22 (95.7) | 50%-75% | 71% | 100% |
| CR, n (%) | 36% | 36% | 27.8% | 58.2% | 43.5% | NR | 25% | 60% |
| PFS (mo), median (95% CI) | 12.8 mo (7.3-18.6) | 9.3 mo (300 M) | NR | 25.3 mo (3.0-NE) | 6 mo PFS: 65.1% | NR | NR | 15.8 mo (12.9-NE) |
| CRS | ||||||||
| All grade, n (%) | 54 (75) | 55 (89) | 15/18 (83.3) | 72 (92.4) | 21 (91.3) | 17% | 24 (56) | 87% |
| Grade 3-4, n (%) | 3 (2 grade 5) | 2 (3) | 0 (0) | 2 (2.5) | 1 (4.3) | 0% | 1 (2) | 0 (0) |
| Onset (d), median (range) | 2 d (1-20) | 2 (1-4) | 2 (DL0) & 1 (DL1) | 6 (1-12) | 6 (1-11) | NR | NR | NR |
| Duration (d), median (range) | 4 d | 4 (1-10) | 4 (DL0) & 3 (DL1) | 5 (1-30) | 5 (2-9) | NR | NR | 4 (1-12) |
| Tocilizumab/steroids %/% | 53/17 | 76/52 | ≈30/20 | 20/34.7 | 26/9 | 7/6 | 23/14 | 76/12 |
| ICANS | ||||||||
| All grade, n (%) | 11 (15) | 8 (13) | 2 (DL0) & 1 (DL1) | 1 (1.3) | 1 (4.3) | 4% | 6 (14) | 0 |
| Grade 3-4, n (%) | 3 (4) | 2 (3) | 1 patient | 0 | 0 (0) | 4% | 0 (0) | 0 |
| Onset (d), median (range) | 7 d (2-24) | 4 (1-6) | NR | 10 | 8 | NR | NR | — |
| Duration (d), median (range) | 2 d | 4 (1-10) | NR | 1 | 1 | NR | NR | — |
| Status | Not further developed | Not further developed | Ongoing | Ongoing | Ongoing | Not further developed | Ongoing | Ongoing |
| Reference | Raje et al27 | Mailankody et al.37 | Chen et al.38 | Chunrui et al.39 | Gan An, et al.40 | Costello et al.41 | Mailankody et al.42 | de Larrea et al24 |
| Identification | NCT03274219 | NCT03430011 | NCT03915184 | NCT05066646 | NCT05066646 | NCT03288493 | NCT04093596 | NCT04309981 |
Data of safety and efficacy are shown.
NR, not reached.
What is next and how to improve current results
Despite the impressive clinical results obtained thus far and given the short follow-up in some studies, no plateau has yet been seen in the survival curves, and relapses continue to occur. Therefore, further improvement is needed, and several points remain to be discussed.
Improving patient selection
Patient selection is a critical aspect of CAR T-cell therapy. Indeed, it is of particular relevance if we consider the present and future availability of other off-the-shelf BCMA-directed therapies. Although high and deep responses are achieved with both approved therapies, outcomes in selected high-risk populations remain inferior compared to standard-risk patients. In the KarMMa trial, patients with extramedullary disease, high-risk CAs, and R-ISS 3 showed lower CR rates and shorter PFS compared to patients without high-risk disease (Table 1).9,10 Likewise, in the CARTITUDE-1 trial, patients with high-risk CAs, ISS 3, and the presence of plasmacytomas showed a lower PFS at 2 years compared to the intention-to-treat population, suggesting that further improvement is needed to abrogate the adverse outcome of patients with high-risk features even in the context of potent therapies such as CAR T cell (Table 1).11
On top of this, data from the different studies suggest that the achievement of deep responses (CR or MRD negativity) is of utmost importance in the context of “one-shot” CAR T-cell therapy and is associated with prolonged duration of response (DoR) and PFS.8,9 Therefore, understanding the patient characteristics associated with a higher likelihood of achieving CR is of great importance. A recent analysis of the KarMMa trial showed that the presence of high tumor burden (soluble BCMA) and high inflammation (D-dimer, ferritin) along with the myeloma subtype (immunoglobulin G heavy chain) had a negative association with CR/sCR achievement, whereas a high vector copy number in the drug product showed a positive correlation with the CR/sCR rate.12 These factors suggest that optimal and timely patient selection may be relevant for CAR T-cell outcomes since it is not an off-the-shelf therapy, and rapidly progressive patients with high-risk features or limited bridging options may be difficult to manage given the manufacturing time. In fact, between 10% and 20% of the patients who underwent apheresis in the different trials did not reach infusion due to complications during the manufacturing time or disease progression.
In this regard, adequate selection of bridging therapy is of utmost importance to maintain the patient's condition during manufacturing. Whenever possible, agents to which the patient has not been previously exposed should be prioritized. In this sense, and although the data are limited, BCMA-targeted agents are probably not the best option during the bridging period. Indeed, patients with prior BCMA treatment were excluded from the pivotal CAR T studies, and limited data are therefore available. Recent evidence from the cohort C of the CARTITUDE-2 trial that enrolled patients previously treated with BCMA-antibody drug conjugates (BCMA-ADC) and BCMA biespecific antibodies (BCMA-BsAb) showed that responses to cilta-cel were inferior to that of the CARTITUDE-1 trial (ORR: 62% in the BCMA-ADC and 57% in the BCMA-BsAb groups, respectively). Factors related to response were a shorter duration of prior BCMA treatment and a longer median time between last BCMA treatment and apheresis, suggesting a potential negative impact of prior BCMA treatment in outcome with CAR T-cell therapies.13 On the other hand, in the recently reported real-world experience, 22% of patients had prior anti-BCMA therapy, and the ORR was consistent with that of the KarMMa trial.14 Even so, both the downregulation of BCMA expression and the biallelic loss of the BCMA locus have been reported after anti-BCMA treatment, with the potential to limit BCMA CAR T-cell efficacy.15–17
Additionally, strategies to shorten vein-to-vein time are being developed, such as platforms for rapid CAR T-cell manufacturing or universal off-the-shelf allogeneic CAR. ALLO-715 is an allogeneic BCMA-targeting CAR T cell. It is manufactured with a knockout of the T-cell receptor alpha constant and CD52 to minimize the risk of graft-versus-host disease and to allow profound T-cell depletion using an anti-CD52 antibody to improve engraftment of the CAR T cells, respectively.18 The UNIVERSAL phase 1 study tested ALLO-715 in 43 RRMM patients using escalating doses and different lympho-depleting regimens. The best clinical results were obtained with 320 × 106 cells in combination with fludarabine, cyclophosphamide, and anti-CD52 antibody (ALLO-647). In this cohort, 24 patients were infused. The ORR was 71%, and the median DoR was 8.3 months.19 Potential immunogenicity and short persistence are the main challenges when using allogenic CAR T cells, and other cellular platforms are under investigation, such as natural killer CAR or γδ T cells.18
Treating patients in earlier lines of therapy with less refractory disease may help overcome some of the aforementioned problems. Therefore, different studies are indeed evaluating the feasibility and efficacy of this therapy in the context of early relapse or frontline disease. In cohort A from the phase 2 CARTITUDE-2 study, patients with 1 to 3 prior lines and lenalidomide-refractory disease were included and treated with cilta-cel. The median number of prior lines was 2 (range, 1-3). Interestingly, 40% of the patients were already triple-class refractory. Nineteen out of 20 patients responded (95%), and 85% achieved at least CR. The median PFS has not yet been reached, with a PFS at 12 months of 84% (95% CI, 59.1-94.7). Safety was comparable to that of the CARTITUDE-1 trial without new safety signals.20 Likewise, in cohort B of the same study, patients with early relapse (within the first 12 months) after frontline therapy with a proteasome inhibitor and immunomodulatory drugs were included. A total of 19 patients were infused. The ORR was 95%, and 79% of patients achieved at least CR, with a 12-month PFS of 84% (95% CI, 57.9-94.5) and a safety profile comparable to that of the CARTITUDE-1 trial.21 Altogether, these results suggest that CAR T-cell therapy in earlier lines of therapy is safe and may yield high and deep responses in different unmet-need populations. Still, its efficacy needs to be confirmed in the context of large phase 3 studies both in early relapse (KarMMa-3, NCT03651128; CARTITUDE-4, NCT04181827) and the frontline setting (BMTCTN1902, NCT05032820; CARTITUDE-5, NCT04923893; CARTITUDE-6, NCT04181827) (Table 3).
Summary of ongoing studies evaluating CAR T cell therapy in earlier lines of therapy or in combinations with standard of care treatment
| Trial . | Study population . | Study design . | CAR name . | ScFv origin . | Phase . | n . | Antigen . | Cell source . | End point . | Dose . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FUMANBA-2 (NCT05181501) | NDMM high-risk | VRD/PAD/PCD × 3-ASCT (if eligible) >> CT103A | CT103A | Human | 1 | 20 | BCMA | Autologous | MRD negativity 2-year PFS | 1 × 106/kg | Not yet enrolling |
| NCT04287660 | NDMM | BiRD + BCMA CAR T cell | NA | NA | 3 | 20 | BCMA | Autologous | ORR at 4 weeks | 2-3 × 107/kg | Recruiting |
| CARTITUDE-5 NCT04923893 | NDMM not intended for ASCT | A: VRD × 8 + Rd B: VRD (6 + 2) + cilta-cel | Cilta-cel | Llama | 3 randomized | 650 | BCMA | Autologous | PFS | 0.75 × 106/kg | Recruiting |
| NCT04935580 | NDMM-TE high-risk | 2 cycles induction + CAR T + Len maintenance | GC012F | Human | 1/2 | 20 | BCMA- CD19 | Autologous | Safety ORR, PFS, MRD | 3 × 105/kg | Recruiting |
| CARTITUDE-6 NCT05257083 | NDMM TE | A: DVRD × 4 + ASCT +2 × DVRD + Len B: DVRD × 6 + cilta-cel + Len (2 y) | Cilta-cel | Llama | 3 randomized | 750 | BCMA | Autologous | PFS | 0.75 × 106/kg | Not yet enrolling |
| KarMMa-4 NCT04196491 | NDMM high-risk (R-ISS 3) | Standard induction × 3 cycles + Ide-cel | Ide-cel | Murine | 1 | 13 | BCMA | Autologous | Safety | 450 × 106 | Active Not recruiting |
| CARTITUDE-2 NCT04133636 | Multicohort | Cohort A: 1-3 prior lines Len-ref Cohort B: early relapse after frontline treatment Cohort C: relapse after BCMA Cohort F: NDMM after frontline Cohort E: NDMM-Tx not planned (high-risk) | Cilta-cel | Llama | 2 | 157 | BCMA | Autologous | MRD negativity at 1 year | 0.75 × 106/kg | Recruiting |
| KarMMa-2 NCT03601078 | Multicohort | 2a + b: R-ISS 3 + early relapse 2c: suboptimal response to ASCT | Ide-cel | Murine | 2 | 181 | BCMA | Autologous | ORR | 150-450 × 106 | Recruiting |
| KarMMa-7 NCT04855136 | 1-3 PL + Len R ≥3 PL RRMM | (A): Ide-cel + iberdomide maintenance (B): Ide-cel + gamma secretase inhibitor | Ide-cel | Murine | 2 | 181 | BCMA | Autologous | ORR | 150-450 × 106 | Recruiting |
| KarMMa-3 NCT03651128 | 2-4 prior lines Len-ref & anti-CD38 exposed | A: DVd/DPd/EloPd/Ixa-Rd/Kd B: Ide-cel | Ide-cel | Murine | 3 Randomized | 381 | BCMA | Autologous | PFS | 450 × 106 | Recruiting |
| CARTITUDE-4 NCT04181827 | 1-3 prior lines Len refractory | A: PVd or DPd B: cilta-cel | Cilta-cel | Llama | 3 randomized | 419 | BCMA | Autologous | PFS | 0.75 × 106/kg | Active Not recruiting |
| BMTCTN1902 NCT05032820 | NDMM post-ASCT | Ide-cel + Len maintenance | Ide-cel | Murine | 2 | 40 | BCMA | Autologous | ORR | 450 × 106 | Recruiting |
| Trial . | Study population . | Study design . | CAR name . | ScFv origin . | Phase . | n . | Antigen . | Cell source . | End point . | Dose . | Status . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FUMANBA-2 (NCT05181501) | NDMM high-risk | VRD/PAD/PCD × 3-ASCT (if eligible) >> CT103A | CT103A | Human | 1 | 20 | BCMA | Autologous | MRD negativity 2-year PFS | 1 × 106/kg | Not yet enrolling |
| NCT04287660 | NDMM | BiRD + BCMA CAR T cell | NA | NA | 3 | 20 | BCMA | Autologous | ORR at 4 weeks | 2-3 × 107/kg | Recruiting |
| CARTITUDE-5 NCT04923893 | NDMM not intended for ASCT | A: VRD × 8 + Rd B: VRD (6 + 2) + cilta-cel | Cilta-cel | Llama | 3 randomized | 650 | BCMA | Autologous | PFS | 0.75 × 106/kg | Recruiting |
| NCT04935580 | NDMM-TE high-risk | 2 cycles induction + CAR T + Len maintenance | GC012F | Human | 1/2 | 20 | BCMA- CD19 | Autologous | Safety ORR, PFS, MRD | 3 × 105/kg | Recruiting |
| CARTITUDE-6 NCT05257083 | NDMM TE | A: DVRD × 4 + ASCT +2 × DVRD + Len B: DVRD × 6 + cilta-cel + Len (2 y) | Cilta-cel | Llama | 3 randomized | 750 | BCMA | Autologous | PFS | 0.75 × 106/kg | Not yet enrolling |
| KarMMa-4 NCT04196491 | NDMM high-risk (R-ISS 3) | Standard induction × 3 cycles + Ide-cel | Ide-cel | Murine | 1 | 13 | BCMA | Autologous | Safety | 450 × 106 | Active Not recruiting |
| CARTITUDE-2 NCT04133636 | Multicohort | Cohort A: 1-3 prior lines Len-ref Cohort B: early relapse after frontline treatment Cohort C: relapse after BCMA Cohort F: NDMM after frontline Cohort E: NDMM-Tx not planned (high-risk) | Cilta-cel | Llama | 2 | 157 | BCMA | Autologous | MRD negativity at 1 year | 0.75 × 106/kg | Recruiting |
| KarMMa-2 NCT03601078 | Multicohort | 2a + b: R-ISS 3 + early relapse 2c: suboptimal response to ASCT | Ide-cel | Murine | 2 | 181 | BCMA | Autologous | ORR | 150-450 × 106 | Recruiting |
| KarMMa-7 NCT04855136 | 1-3 PL + Len R ≥3 PL RRMM | (A): Ide-cel + iberdomide maintenance (B): Ide-cel + gamma secretase inhibitor | Ide-cel | Murine | 2 | 181 | BCMA | Autologous | ORR | 150-450 × 106 | Recruiting |
| KarMMa-3 NCT03651128 | 2-4 prior lines Len-ref & anti-CD38 exposed | A: DVd/DPd/EloPd/Ixa-Rd/Kd B: Ide-cel | Ide-cel | Murine | 3 Randomized | 381 | BCMA | Autologous | PFS | 450 × 106 | Recruiting |
| CARTITUDE-4 NCT04181827 | 1-3 prior lines Len refractory | A: PVd or DPd B: cilta-cel | Cilta-cel | Llama | 3 randomized | 419 | BCMA | Autologous | PFS | 0.75 × 106/kg | Active Not recruiting |
| BMTCTN1902 NCT05032820 | NDMM post-ASCT | Ide-cel + Len maintenance | Ide-cel | Murine | 2 | 40 | BCMA | Autologous | ORR | 450 × 106 | Recruiting |
ASCT, autologous stem cell transplantation; BiRD, bortezomib, claritromicine, lenalidomide, dexamethasone; DPd, daratumumab, pomalidomide, dexamethasone; DVd, daratumumab, bortezomib, dexamethasone; EloPd, elotuzumab, pomalidomide, dexamethasone; IxaRd, izaxomib, lenalidomide, dexamethasone; Kd, carfilzomib, dexamethasone; Len, lenalidomide; Len-ref, lenalidomide refractory; NA, not available; NDMM, newly diagnosed multiple myeloma; NDMM-TE, newly diagnosed transplant eligible; PAD, bortezomib, adryamicine, dexamethasone; PCD, bortezomib, cyclophosphamide, dexamethasone; PVd, pomalidomide, bortezomib, dexamethasone; Rd, lenalidomide-dexamethasone; Tx, transplant.
CLINICAL CASE (Continued)
Our patient underwent apheresis after the completion of screening tests and received bridging therapy, with progressive disease as the best response. The bridging period was complicated with acute kidney failure and several infectious complications. After recovery, he received standard fludarabine-cyclophosphamide conditioning before CAR T-cell infusion, which was performed in October 2020. He did not develop CRS or ICANs but did have grade 4 cytopenia requiring transfusion support, granulocyte colony-stimulating factor, erythropoietin-stimulating agents, and thrombopoietin analogues. After CAR T-cell treatment, the patient achieved a stringent CR with MRD negativity (10−6) as well as a complete metabolic response (Deauville 3), using an F-fluorodeoxyglucose emission tomography/computed tomography scan, that continues today. His cytopenia improved by month 12, and the patient no longer requires transfusions or medication.
Improving the T-cell manufacturing process
Several studies across different CAR T-cell products have shown a positive correlation between the depth and DoR and CAR T-cell expansion or persistence.9,17 Therefore, significant effort has been fostered to optimize the final CAR T-cell product. One relevant aspect to consider is the immunogenicity of approved animal-derived constructs. Indeed, in the KarMMa trial, patients with antidrug antibodies (ADAs) increased over time, from 21% (21 of 102) at month 3 to 65% (34 of 52) at month 12.9 Although these antibodies did not seem to influence CAR concentration or response, only patients without negative ADAs responded to CAR T-cell reinfusion, suggesting the potential role of these ADAs in CAR T-cell persistence or activity. Humanized or fully human constructs may be a way to overcome the negative impact of immunogenicity in CAR T-cell outcomes. Preliminary data from several phase 1 and 2 trials have shown encouraging clinical efficacy with high ORRs and deep and durable responses.22,23 Moreover, in 1 academic study evaluating ARI-002h, a humanized BCMA CAR T, patients with an ongoing response and the absence of significant toxicity to the first infusion were eligible for a reinfusion. Sixteen out of 30 patients received a second infusion, and 5 patients showed an improved response, suggesting an adequate expansion of the CAR T-cell product.24,25 Alternatively, the use of non–scFv-based CARs, such as engineered binding scaffolds, receptors, or natural ligands, might additionally serve to mitigate immunogenic reactions.26
Higher levels of memory T-cell phenotypes have been associated with better clinical outcomes, and several strategies, such as CD4/CD8 in a 1:1 ratio, may yield a higher proportion of these populations in the final product. An interesting approach was evaluated in the phase 1 CRB-402 study. In this trial, ide-cel was cultured with a PI3K inhibitor (bb007), resulting in a different CAR T-cell product (bb21217) enriched with T cells having a memory-like phenotype and a superior proliferative capacity upon adoptive transfer. Overall, 72 patients with a median of 6 prior lines were infused. The ORR was 69%, with 36% of the patients achieving a CR or better. The median DoR was 23.8 months and was not reached by patients in CR. Importantly, the persistence of CAR T cells was prolonged, with detectable CAR T cells in some patients up to 24 months.27
One limitation of targeted immunotherapies is the potential heterogeneity of antigen expression among tumor cells and the loss or downregulation of antigen after exposure.18 Simultaneously targeting different antigens could overcome this barrier, and several trials are evaluating this possibility. GC012F is a dual BCMA/CD19-targeted CAR T manufactured in 24 to 36 hours on the FASTCAR® platform. Sixteen patients with RRMM and a median of 5 prior lines of therapy were infused with GC012F (dose range: 100 000-300 000 CAR T cells per kilogram). The ORR was 94%, with 56.3% of patients achieving CR or better. The median DoR was not reached at 7.3 months of median follow-up.28
Likewise, other targets are being explored (Table 4). GPR5D (G protein–coupled receptor 5 family D)-directed CAR T cells are also under investigation. In a first-in-human study of MCARH109, 17 patients were infused. Four doses were tested (25, 50, 150, and 450 × 106 CAR + T cells). Fifty-nine percent of the patients had prior BCMA therapy, including 47% with prior CAR T-cell treatment. The ORR was 60% and 80% in patients with prior BCMA therapy. Data are still preliminary since the median follow-up was only 18 weeks.29
Clinical trials in multiple myeloma evaluating non–BCMA-directed CAR T-cell constructs
| Trial . | Sponsor . | CAR name . | ScFv origin . | Phase . | n . | Antigen . | Costimulatory domain . | Cell source . | Transfer method . | Dose . | Efficacy . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT04499339 | European Union & CARAMBA | CARAMBA | Murine | 1 | 38 | SLAMF7 | NR | Autologous | Sleeping beauty | NA | NR |
| NCT02135406 | University of Pennsylvania | CTL019 | Murine | 1 | 10 | CD19 | 4-1BB | Autologous | Lentiviral | 1.1-6 × 108 | VGPR: 6; PR: 2; PD: 2 |
| NCT01886976 | Chinese PLA General Hospital | CART-138 | Murine | 1/2 | 5 | CD138 | 4-1BB | Autologous | Retroviral | 0.44-1.51 × 107 | SD: 4; PD: 1 |
| NCT00881920 | Baylor College of Medicine | κ.CARTs | Murine | 1 | 7 | κ light chain | CD28 | Autologous | Retroviral | 2.0 × 108 | 4 SD |
| NCT03958656 | National Cancer Institute | NA | NA | 1 | 42 | SLAMF7 | CD28 or 4-1BB/ CD3z + inducible caspase 9 (IC9) cell suicide | Autologous | NA | 0.3-12.0 × 106 | NA |
| NCT04142619 | Cellectis S.A. | UCARTCS1 | NA | 1 | 18 | SLAMF7 (TALEN-targeted gene editing TCR and SLAMF7) | 4-1BB | Allogeneic | NA | NA | NA |
| NCT02203825 | Celyad | NKG2D-CAR | Human | 1 | 12 | NKG2D ligands | DAP10 | Autologous | Retroviral | 1-3 × 107 | NA |
| NCT03464916 | Sorrento Therapeutics | CAR2 anti- CD38 A2 | NA | 1 | 72 | CD38 | NA | Autologous | NA | NA | NA |
| EU-CART | EU Horizon 2020 Program | NA | NA | 1 | NA | CD44v6 (+HSV-TK suicide gene) | CD28 | Autologous | Retroviral | NA | NA |
| NCT01716364 | Peter MacCallum Cancer Centre, Australia | LeY | NA | 1 | 6 | Lewis Y | CD28 | Autologous | Retroviral | NA | NA |
| NCT05016778 | Zhejiang University | GPR5D-CAR T | NA | 1 | 15 | GPRC5d | NA | Autologous | NA | 1.0, 3.0, or 6.0 × 106/kg | NA |
| NCT05219721 | Tongji Hospital | CAR-GPR5D | Human | 1 | 18 | GPRC5d | 4-1BB | Autologous | NA | 0.5, 1.0, and 2.0 × 106/kg | NA |
| NCT04555551 | Memorial Sloan Kettering Cancer Center | MCARH109 | Human | 1 | 17 | GPRC5d | 4-1BB | Autologous | Lentiviral | 25, 50, 150, 450 × 106 | ORR 69% CR 25% |
| Trial . | Sponsor . | CAR name . | ScFv origin . | Phase . | n . | Antigen . | Costimulatory domain . | Cell source . | Transfer method . | Dose . | Efficacy . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT04499339 | European Union & CARAMBA | CARAMBA | Murine | 1 | 38 | SLAMF7 | NR | Autologous | Sleeping beauty | NA | NR |
| NCT02135406 | University of Pennsylvania | CTL019 | Murine | 1 | 10 | CD19 | 4-1BB | Autologous | Lentiviral | 1.1-6 × 108 | VGPR: 6; PR: 2; PD: 2 |
| NCT01886976 | Chinese PLA General Hospital | CART-138 | Murine | 1/2 | 5 | CD138 | 4-1BB | Autologous | Retroviral | 0.44-1.51 × 107 | SD: 4; PD: 1 |
| NCT00881920 | Baylor College of Medicine | κ.CARTs | Murine | 1 | 7 | κ light chain | CD28 | Autologous | Retroviral | 2.0 × 108 | 4 SD |
| NCT03958656 | National Cancer Institute | NA | NA | 1 | 42 | SLAMF7 | CD28 or 4-1BB/ CD3z + inducible caspase 9 (IC9) cell suicide | Autologous | NA | 0.3-12.0 × 106 | NA |
| NCT04142619 | Cellectis S.A. | UCARTCS1 | NA | 1 | 18 | SLAMF7 (TALEN-targeted gene editing TCR and SLAMF7) | 4-1BB | Allogeneic | NA | NA | NA |
| NCT02203825 | Celyad | NKG2D-CAR | Human | 1 | 12 | NKG2D ligands | DAP10 | Autologous | Retroviral | 1-3 × 107 | NA |
| NCT03464916 | Sorrento Therapeutics | CAR2 anti- CD38 A2 | NA | 1 | 72 | CD38 | NA | Autologous | NA | NA | NA |
| EU-CART | EU Horizon 2020 Program | NA | NA | 1 | NA | CD44v6 (+HSV-TK suicide gene) | CD28 | Autologous | Retroviral | NA | NA |
| NCT01716364 | Peter MacCallum Cancer Centre, Australia | LeY | NA | 1 | 6 | Lewis Y | CD28 | Autologous | Retroviral | NA | NA |
| NCT05016778 | Zhejiang University | GPR5D-CAR T | NA | 1 | 15 | GPRC5d | NA | Autologous | NA | 1.0, 3.0, or 6.0 × 106/kg | NA |
| NCT05219721 | Tongji Hospital | CAR-GPR5D | Human | 1 | 18 | GPRC5d | 4-1BB | Autologous | NA | 0.5, 1.0, and 2.0 × 106/kg | NA |
| NCT04555551 | Memorial Sloan Kettering Cancer Center | MCARH109 | Human | 1 | 17 | GPRC5d | 4-1BB | Autologous | Lentiviral | 25, 50, 150, 450 × 106 | ORR 69% CR 25% |
NA, not available; NR, not reached; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response.
In addition, different strategies aiming to improve CAR potency and sensitivity are under evaluation, and the possibilities are endless. Therefore, the fine-tuning of CAR density in the T cell,30 the co-infusion of CAR and chimeric costimulatory receptors,31 the use of HLA-independent T-cell receptors instead of CAR,32 armored CAR T, or a combination with gamma-secretase inhibitors to increase BCMA density in the tumor cell are potential venues to improve.33 Clinical data, however, are still scanty (Figure 2).
Possibilities for the future development of CAR T-cell therapy. Depicted are some of the novel new-generation CAR T constructs, including third-generation CARs incorporating 2 different costimulatory domains; fourth-generation CARs (also named “armored” CARs) incorporating transducible genes codifying for cytokines, antibodies, or receptors (among others); and novel target (non-BCMA) and dual targeting strategies, including tandem CAR and dual CAR.
Possibilities for the future development of CAR T-cell therapy. Depicted are some of the novel new-generation CAR T constructs, including third-generation CARs incorporating 2 different costimulatory domains; fourth-generation CARs (also named “armored” CARs) incorporating transducible genes codifying for cytokines, antibodies, or receptors (among others); and novel target (non-BCMA) and dual targeting strategies, including tandem CAR and dual CAR.
Conclusions
In conclusion, BCMA-directed CAR T-cell therapies have demonstrated impressive clinical results in the context of advanced triple-class exposed RRMM, leading to the approval of 2 CARs. New modalities are in development to overcome some of the current limitations of CAR T-cell therapy, aiming to improve outcomes, shorten vein-to-vein time, and reduce toxicity. The further use of BCMA-directed CAR T-cell treatment in earlier lines of therapy is sure to improve outcomes and, hopefully, find the way to curing MM patients.
Conflict-of-interest disclosure
Paula Rodriguez-Otero: honoraria: Abbvie, Amgen, Bristol Myers Squibb/Celgene, GSK, Janssen, Kite Pharma, Oncopeptides, Pfizer, Sanofi; consultancy: Celgene, GSK, Janssen, Pfizer.
Jesús F. San-Miguel: honoraria: Abbvie, Amgen, Bristol Myers Squibb, Celgene, Janssen, MSD, Novartis, Sanofi, Roche, Takeda.
Off-label drug use
Paula Rodriguez-Otero: nothing to disclose.
Jesús F. San-Miguel: nothing to disclose.