Abstract
Bleeding disorders with normal, borderline, or nondiagnostic coagulation tests represent a diagnostic challenge. Disorders of primary hemostasis can be further evaluated by additional platelet function testing modalities, platelet electron microscopy, repeat von Willebrand disease testing, and specialized von Willebrand factor testing beyond the usual initial panel. Secondary hemostasis is further evaluated by coagulation factor assays, and factor XIII assays are used to diagnose disorders of fibrin clot stabilization. Fibrinolytic disorders are particularly difficult to diagnose with current testing options. A significant number of patients remain unclassified after thorough testing; most unclassified patients have a clinically mild bleeding phenotype, and many may have undiagnosed platelet function disorders. High-throughput genetic testing using large gene panels for bleeding disorders may allow diagnosis of a larger number of these patients in the future, but more study is needed. A logical laboratory workup in the context of the clinical setting and with a high level of expertise regarding test interpretation and limitations facilitates a diagnosis for as many patients as possible.
Learning Objectives
Diagnose bleeding disorders that present with normal conventional coagulation tests
Identify disorders of primary hemostasis that present diagnostic challenges and recommend specialized testing that may be diagnostically informative
Explain a multistep approach for evaluating bleeding disorders of secondary hemostasis, clot stabilization, and fibrinolysis
Introduction
Laboratory coagulation testing plays an important role in the diagnosis and management of bleeding disorders. However, patients with a suspected bleeding disorder who have normal or nondiagnostic initial coagulation tests present a significant challenge. Both inherited and acquired abnormalities should be carefully considered, with close attention to bleeding assessment tool results, medical history, family history, medications, liver and renal function, and physical examination findings.1 In this review, we discuss how to best approach the laboratory workup beyond the common tests, including limitations and interpretation of laboratory testing. The article is organized by hemostasis component: primary hemostasis, secondary hemostasis, and fibrinolysis. Primary hemostasis refers to platelet plug formation that results from platelet adhesion to damaged vasculature via von Willebrand factor (VWF) and direct platelet binding, secretion of granules, and platelet aggregation, whereas secondary hemostasis refers to fibrin formation via the coagulation cascade that is subsequently stabilized by factor XIII (FXIII). Fibrinolysis is the normal breakdown of fibrin clot that occurs during healing.2
CASE 1
A 15-year-old girl was evaluated for easy bruising, frequent nosebleeds, and heavy menstrual bleeding. There was a vague family history of an uncharacterized mucocutaneous bleeding disorder. Her platelet count, blood smear morphology, basic clotting times, and fibrinogen were normal. Prior von Willebrand testing had been performed and showed similar values for VWF antigen (VWF:Ag) and ristocetin cofactor activity (VWF:RCo) that were either normal or slightly below the reference interval, not definitive for von Willebrand disease (VWD) and considered possible “low VWF.” Prior VWF multimer analysis was normal. She was referred to our laboratory for a repeat von Willebrand panel and platelet aggregation studies.
CASE 2
A 12-year-old boy sought evaluation from the hematology clinic after having multiple episodes of intramuscular bleeding that began after he joined his junior high school football team. He reported a prior episode of prolonged bleeding after a tooth extraction. Initial testing, including platelet count and peripheral smear review, basic clotting times, and a von Willebrand panel (VWF:Ag, VWF:RCo, and factor VIII [FVIII] activity), was normal. Due to the high clinical suspicion for a bleeding disorder, there were questions about what additional testing to perform.
Initial testing for a bleeding disorder
Coagulation testing is most useful in patients with an abnormal bleeding history, and thus a higher likelihood of a bleeding disorder, and is less useful for screening unselected patients (such as in the preoperative setting).3,4 Initial testing typically includes platelet count and morphology review, prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen activity.3,5-7 The most common fibrinogen assay available in hospital laboratories is the Clauss fibrinogen activity assay, which is a calibrated test based on the thrombin time. The test will be abnormal in hypofibrinogenemia and most cases of dysfibrinogenemia. Additional workup of dysfibrinogenemia may include fibrinogen antigen, thrombin time (TT), reptilase time, and genetic testing. Testing for VWD and platelet function disorders is sometimes included in the initial evaluation and, if not, should be the next step due to the frequency of these disorders and inability of routine tests to detect them.4,5 The aPTT is prolonged in VWD if the FVIII is sufficiently low, but the aPTT is normal in most cases, as is the platelet count.8 Platelet function disorders frequently have normal platelet count and morphology.
In a study designed to evaluate the utility of common coagulation screening tests, results from 800 patients referred for bleeding disorder evaluation were analyzed, and only 11% had 1 or more abnormalities of PT, aPTT, fibrinogen, or TT.3 The sensitivity of this group of tests to a clinically significant abnormality was only 3.7%, but this increased to 8.5% when a basic VWD panel was added and to 30% when both a VWD panel and platelet function testing (by light transmission aggregometry) were added, suggesting that these should be included in the initial testing.3 Early platelet aggregometry is recommended in a recent guideline from the International Society on Thrombosis and Haemostasis.9
Challenges in diagnosis of disorders of primary hemostasis
Patients with disorders of primary hemostasis demonstrate a mucocutaneous pattern of immediate bleeding (prolonged time to achieve initial hemostasis).2,10 Although the skin bleeding time is an outdated method for assessing primary hemostasis, it has been supplanted in many centers by devices such as the Platelet Function Analyzer (PFA)–100 (or the PFA-200 in some locations outside of the United States) as an initial screen of primary hemostasis, primarily due to accessibility and ease of use.11 Although the PFA-100 conceptually represents a global test for evaluating primary hemostasis, the presence of high shear (which results in a VWF conformational change that exposes the platelet binding domain) and redundant platelet agonists (including collagen that binds platelet collagen receptors and shear-activated VWF) tends to mask mild abnormalities, and a normal closure time result only excludes more severe VWD (such as types 2 and 3) or severe platelet dysfunction (such as Glanzmann thrombasthenia and Bernard-Soulier syndrome).12,13 The PFA-100 and other microfluidics-based assays are initiated by the adhesion functions of VWF and platelets and are thus more sensitive to disorders of adhesion. PFA-100 sensitivity to VWD is estimated at approximately 60% to 70% overall, with milder type 1 phenotypes and those with “low VWF” as a bleeding risk factor more commonly missed.5,12 Although storage pool deficiencies and secretion defects represent the most common heritable platelet function abnormalities, studies suggest that up to half of these cases are missed by PFA-100 testing.5,12 If there is strong suspicion for a bleeding disorder of primary hemostasis, specific VWD testing panels and platelet aggregometry should be used despite the increased technical and interpretive complexity of aggregometry.1,12 Collagen disorders, such as Ehlers-Danlos syndrome, should also be considered and are evaluated by joint mobility studies and genetic testing in some cases. Because a significant number of patients with Ehlers-Danlos syndrome who have an elevated bleeding score have been shown to also have platelet dysfunction, platelet function testing is considered useful in this setting.14
Comprehensive investigation for VWD is beyond the scope of this review, but testing should include VWF antigen and activity (such as VWF:RCo or newer assays), FVIII, and additional specialized testing as needed to confirm the diagnosis and subtype.15 Moderate/severe VWD cases are detected most readily due to their more pronounced bleeding and laboratory abnormalities. Mild type 1 cases can be challenging to diagnose, sometimes presenting with a normal panel due to the acute phase nature of VWF.8,15,16 If there is high clinical suspicion for VWD (history of immediate mucocutaneous bleeding), repeated measurements may be necessary to uncover the true baseline values.8,15,17 Due to estrogen's effects on VWF levels, women with heavy menstrual bleeding are often tested at the onset of menses when estrogen is lowest, and VWD screening should be avoided in women on estrogen therapy or during or immediately after pregnancy. A recent study of initial and follow-up VWF testing in women seen in an emergency department for heavy menstrual bleeding showed higher median VWF and FVIII levels during acute bleeding as opposed to follow-up, highlighting the limited diagnostic utility during acute bleeding.15 The authors also noted that if the initial FVIII value was near the normal range, the associated VWF levels were closer to baseline, suggesting that because FVIII also has acute phases, close attention to the FVIII value may help determine if an acute phase response is present, but findings should be interpreted with caution.18 Another recent retrospective study of 811 pediatric patients with suspected VWD indicated that 70% of these patients had von Willebrand (VW) panel results diagnostic of VWD in the first testing episode. Patients with VW panel values between 30 and 50 IU/dL are likely most at risk for repeat testing prior to diagnosis; higher VW panel values are more likely to exclude VWD (negative predictive value for VWF antigen and activity >75 IU/dL was 94.1% and 97.4%, respectively).15,19
Other settings in which an initial VWD panel may be unrevealing include rare subtypes of type 2M VWD due to collagen binding defects because routine panels do not typically assess this function.20 A collagen binding assay could be performed (VWF:CB) and would demonstrate decreased collagen binding out of proportion to the VWF protein level.5,21 A subset of type 2B cases can also appear normal in basic VWD panels and have normal platelet count and VWF multimers as opposed to the classic type 2B profile of thrombocytopenia, missing large VWF multimers, and decreased VWF activity with a low activity/antigen ratio.22 Low-dose ristocetin-induced platelet aggregation can uncover the diagnosis by demonstrating the abnormally high affinity of mutant VWF for platelets. Cases with normal multimers have been shown to have different type 2B mutations and milder bleeding as opposed to type 2B patients with multimeric defects.3,22 Genetic testing for VWD is also available and has proven most useful for type 2 subtypes with challenging laboratory phenotypes or where phenotypic testing is not available.20
Acquired von Willebrand syndrome (AVWS), which is a heterogeneous disorder due to antibody-mediated clearance, adsorption to platelets or neoplastic cells, or proteolysis due to increased shear stress, can be diagnostically challenging. Specific clinical circumstances frequently associated with AVWS include patients treated with extracorporeal membrane oxygenation, heart pump (such as Abiomed's Impella), and left ventricular assist devices.23,24 VWF values are often in the normal range due to adequate VWF production and acute phasing. Even if the absolute values are normal, a decreased activity/antigen ratio (such as VWF:RCo/Ag or VWF:CB/Ag) is helpful and may indicate clearance of large multimers, which occurs in many, but not all, cases of AVWS.25 Subtle loss of large multimers by VWF electrophoresis may be the only abnormality observed, and upfront multimer analysis is reasonable if AVWS is suspected.25,26
Although platelet aggregation is the preferred initial test for assessing platelet function, it is not sensitive to all disorders and suffers in practice from nonstandardization despite literature supporting standardization and harmonization.4,9,10,27,28 Platelet dense granule deficiency is thought to be as common as VWD, may demonstrate normal aggregation results, and is best assessed by dense granule counts using whole-mount platelet electron microscopy.4,5,13,29 Although electron microscopy testing has limited availability, it demonstrates superior performance characteristics compared with the more widely available lumiaggregometry methods that measure adenosine triphosphate release from platelet dense granules.4,13,29-31 However, adenosine triphosphate secretion analysis may identify secretion defects that are not due to granule deficiency.32 Scott syndrome (platelet factor 3 deficiency) is a rare recessive bleeding disorder caused by abnormalities in platelet procoagulant activity due to lack of phosphatidylserine (PS) exposure after platelet activation. Calcium-dependent coagulation factors require binding to negatively charged phospholipids, such as PS, promoting formation of the tenase and prothrombinase complexes of coagulation and resulting in normal thrombin and fibrin generation. Scott syndrome is not identified by routine coagulation testing or platelet aggregation and requires either flow cytometric testing of platelet PS exposure or genetic testing for ANO6 mutations.33 More than 30 inherited platelet disorders, due to abnormalities in more than 50 genes, have been described, although not all affect platelet function.7 Next-generation sequencing has been used to interrogate patients with platelet function disorders, highlighting genetic complexity, but is not commonly performed.32
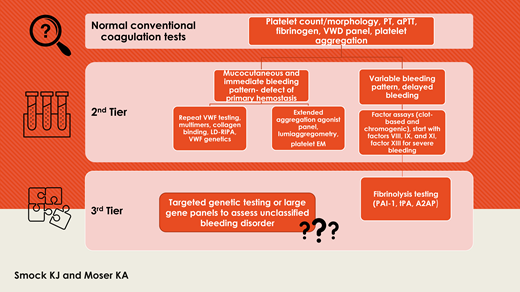
Laboratory considerations for patients suspected to have a bleeding disorder of primary hemostasis are shown in Table 1.
Laboratory considerations for patients with a suspected bleeding disorder of primary hemostasis and normal or nondiagnostic initial tests (normal platelet count/morphology, routine clotting times, VWD panel, and platelet function testing)
| Problem . | Laboratory considerations . |
|---|---|
| VWD | |
| Normal vs low VWF vs VWD masked by acute phase response | PFA-100 lacks sensitivity and specificity for VWD; initial panel should include VWF antigen, activity, and FVIII activity. Avoid testing during acute bleeding episodes, illness, and stress. Use care to avoid traumatic phlebotomy. Repeated measurements may be necessary due to normal biologic variability of VWF, particularly with VWF antigen and activity values between 30 IU/dL and 50 IU/dL. Consider values of other acute phase reactants drawn concurrently (FVIII, fibrinogen). |
| Less common VWD subtypes/phenotypes | Consider type 2M due to a collagen binding defect. First-line activity tests assess platelet binding function rather than collagen binding function. Order VWF:CB to assess for decreased VWF:CB/antigen ratio. Consider type 2B due to a mutation that results in a nonclassic laboratory phenotype (relatively normal activity and antigen without decreased activity/antigen ratio, normal multimers and platelet count). Perform LD-RIPA to assess for abnormally high affinity for platelets. LD-RIPA mixing studies (if available) can be used to differentiate type 2B from platelet-type VWD. VWD genetic testing can be performed and is most useful for type 2 cases with challenging laboratory phenotypes or when phenotypic testing is not available (LD-RIPA not possible if local laboratories do not perform aggregation testing and LD-RIPA mixing studies are not widely performed). Also useful to differentiate type 2B from platelet-type VWD and type 2N from HA. |
| Acquired VWS | Consideration of and appropriate testing for associated disorders such as autoimmune, lymphoproliferative or myeloproliferative neoplasms, plasma cell dyscrasia, and so on. Absolute VWF activity and antigen values often in the normal range; look for decreased activity/antigen ratio. VWF multimer analysis recommended as a first-line test since subtle defects of large multimers may be the only abnormality observed. |
| Platelet function | |
| Platelet function tests are nonstandardized and variably sensitive to the most common disorders | PFA-100 lacks sensitivity and specificity for the most common platelet function disorders. Platelet aggregometry is considered the gold-standard test but has more limited availability. Some laboratories offer aggregometry with an extended agonist panel. Aggregometry is often normal in common disorders such as dense granule deficiency and secretion defects. Consider platelet EM and lumiaggregometry. Mild PFDs remain difficult to diagnose; genetic testing has some clinical utility but is not in widespread clinical use. Consider treating undiagnosed disorders with tranexamic acid and desmopressin. |
| Problem . | Laboratory considerations . |
|---|---|
| VWD | |
| Normal vs low VWF vs VWD masked by acute phase response | PFA-100 lacks sensitivity and specificity for VWD; initial panel should include VWF antigen, activity, and FVIII activity. Avoid testing during acute bleeding episodes, illness, and stress. Use care to avoid traumatic phlebotomy. Repeated measurements may be necessary due to normal biologic variability of VWF, particularly with VWF antigen and activity values between 30 IU/dL and 50 IU/dL. Consider values of other acute phase reactants drawn concurrently (FVIII, fibrinogen). |
| Less common VWD subtypes/phenotypes | Consider type 2M due to a collagen binding defect. First-line activity tests assess platelet binding function rather than collagen binding function. Order VWF:CB to assess for decreased VWF:CB/antigen ratio. Consider type 2B due to a mutation that results in a nonclassic laboratory phenotype (relatively normal activity and antigen without decreased activity/antigen ratio, normal multimers and platelet count). Perform LD-RIPA to assess for abnormally high affinity for platelets. LD-RIPA mixing studies (if available) can be used to differentiate type 2B from platelet-type VWD. VWD genetic testing can be performed and is most useful for type 2 cases with challenging laboratory phenotypes or when phenotypic testing is not available (LD-RIPA not possible if local laboratories do not perform aggregation testing and LD-RIPA mixing studies are not widely performed). Also useful to differentiate type 2B from platelet-type VWD and type 2N from HA. |
| Acquired VWS | Consideration of and appropriate testing for associated disorders such as autoimmune, lymphoproliferative or myeloproliferative neoplasms, plasma cell dyscrasia, and so on. Absolute VWF activity and antigen values often in the normal range; look for decreased activity/antigen ratio. VWF multimer analysis recommended as a first-line test since subtle defects of large multimers may be the only abnormality observed. |
| Platelet function | |
| Platelet function tests are nonstandardized and variably sensitive to the most common disorders | PFA-100 lacks sensitivity and specificity for the most common platelet function disorders. Platelet aggregometry is considered the gold-standard test but has more limited availability. Some laboratories offer aggregometry with an extended agonist panel. Aggregometry is often normal in common disorders such as dense granule deficiency and secretion defects. Consider platelet EM and lumiaggregometry. Mild PFDs remain difficult to diagnose; genetic testing has some clinical utility but is not in widespread clinical use. Consider treating undiagnosed disorders with tranexamic acid and desmopressin. |
Abbreviations: EM, electron microscopy; LD-RIPA, low-dose ristocetin-induced platelet activation; PFD, platelet function disorder.
Challenges in diagnosis of disorders of secondary hemostasis
Patients with disorders of secondary hemostasis demonstrate variable bleeding patterns that can include muscle and soft tissue bleeding and delayed bleeding after achieving initial hemostasis.4,6 Conventional clotting times such as PT and aPTT are prolonged by moderate or severe factor deficiencies but frequently lack sensitivity to milder deficiencies that may be clinically significant with bleeding challenges.4,34,35 Coagulation factor assays can be ordered in a stepwise approach focusing on more common disorders first with assessment of factors VIII, IX, and XI via clot-based activity assays. Because some cases of nonsevere hemophilia A (HA) demonstrate discrepancies between clot-based and chromogenic FVIII assays based on how specific mutations affect the FVIII protein, it is useful to perform both types of FVIII assays.36 It is estimated that 40% of mild HA cases have significant assay discrepancies and that 5% to 10% of mild HA cases have normal FVIII activity by either assay, which can mask the diagnosis.5,36 Clinical phenotypes have correlated most closely with the lowest results observed. A similar phenomenon has been seen in patients with nonsevere hemophilia B (HB) when clot-based and chromogenic factor IX activity assays are compared, which supports a similar approach.37 Chromogenic factor IX assays are less widely available than chromogenic FVIII assays. Because factor activities may be borderline in mild hemophilia, genetic testing (gene sequencing) to confirm an HA or HB pathogenic mutation is often helpful to diagnose mild cases or female carriers.38,39
Additional assays for uncommon mild deficiencies of factors II, V, VII, and X could be considered if the initial factor assays are normal. Alternatively, a large battery of factor assays undertaken at once can save time and minimize blood draws. Some laboratories may offer reflexive bleeding disorder panels to assist with timely evaluation. Acquired factor deficiencies are usually multiple (due to vitamin K deficiency, liver disease, consumptive coagulopathies, etc.), which has a more pronounced effect on the PT and aPTT, making them more likely to be identified in the initial testing. It should also be remembered that clotting time prolongations due to a lupus anticoagulant may mask or confound identification of a factor deficiency or inhibitor. Current laboratory testing guidelines for lupus anticoagulant identification indicate that factor assays should be performed if there is suspicion for a factor deficiency (such as a positive bleeding history or clinical condition associated with factor deficiencies).40
Table 2 demonstrates an example of aPTT reagent sensitivity to progressive single FVIII deficiency or pan-deficiency of all coagulation factors. The upper limit of normal for this aPTT reagent is 35 seconds, and the aPTT is more sensitive to multiple deficiencies as opposed to a single deficiency. Mild HA with FVIII activity in the 20% to 40% range may demonstrate a normal or minimally prolonged aPTT. Different aPTT reagents may differ in these patterns.
aPTT reagent sensitivity to progressive single FVIII deficiency or pan-deficiency of all factors
| FVIII activity, % . | aPTT, s* . | All factor activity, % . | aPTT, s* . |
|---|---|---|---|
| 100 | 28.6 | 100 | 29.4 |
| 90 | 28.6 | 90 | 30.1 |
| 80 | 29.6 | 80 | 31.9 |
| 70 | 30.7 | 70 | 33.9** |
| 60 | 31.5 | 60 | 37.4** |
| 50 | 32.5 | 50 | 43.0 |
| 40 | 34.3** | 40 | 51.9 |
| 30 | 36.1** | 30 | 69.9 |
| 20 | 39.1 | 20 | 108.0 |
| 15 | 40.5 | 15 | No clot |
| 10 | 44.5 | 10 | No clot |
| 5 | 49.6 | 5 | No clot |
| 1 | 61.1 | 1 | No clot |
| 0.001 | 79.6 | 0.001 | No clot |
| FVIII activity, % . | aPTT, s* . | All factor activity, % . | aPTT, s* . |
|---|---|---|---|
| 100 | 28.6 | 100 | 29.4 |
| 90 | 28.6 | 90 | 30.1 |
| 80 | 29.6 | 80 | 31.9 |
| 70 | 30.7 | 70 | 33.9** |
| 60 | 31.5 | 60 | 37.4** |
| 50 | 32.5 | 50 | 43.0 |
| 40 | 34.3** | 40 | 51.9 |
| 30 | 36.1** | 30 | 69.9 |
| 20 | 39.1 | 20 | 108.0 |
| 15 | 40.5 | 15 | No clot |
| 10 | 44.5 | 10 | No clot |
| 5 | 49.6 | 5 | No clot |
| 1 | 61.1 | 1 | No clot |
| 0.001 | 79.6 | 0.001 | No clot |
Reference interval for this aPTT reagent (24-35 seconds).
The bold font indicates when the aPTT seconds begin to exceed the reference interval.
Challenges in diagnosis of FXIII deficiency
FXIII deficiency is a rare recessive bleeding disorder characterized by severe bleeding with a delayed bleeding pattern and normal standard coagulation tests. FXIII cross-links fibrin and incorporates the fibrinolytic inhibitor α2-antiplasmin (A2AP) to prevent premature degradation.41 Qualitative clot solubility tests are frequently used for screening but have significant limitations. Solubility tests are nonstandardized between laboratories and sensitive to only the most severe deficiencies (abnormal with <1% activity in our laboratory).41 They also demonstrate nonspecificity because abnormal results occur with hypo- or dysfibrinogenemia, although these conditions would likely be identified prior to FXIII testing by fibrinogen testing.41 Activity assays are preferred because they detect both quantitative and qualitative abnormalities and are more specific, but they may have suboptimal lower limits of quantitation (our assay can reliably quantify down to 5% activity) or use methods that may overestimate FXIII activity.41,42 Some laboratories use FXIII antigen tests for initial evaluation. FXIII deficiency should be subclassified as either A or B subunit subtypes due to treatment implications (a commonly used recombinant therapy contains only the A subunits). Subtyping can be performed using antigen assays or genetic testing. Ultimately, accurate diagnosis and classification of FXIII deficiency typically requires a combination of tests to be used.42
Challenges in diagnosis of fibrinolytic disorders
Rare patients have inherited recessive defects in the fibrinolytic inhibitors A2AP and plasminogen activator inhibitor 1 (PAI-1), resulting in a bleeding disorder with delayed bleeding due to increased lysis of fibrin clots.1,43 Routine clotting times are normal.1,44 Testing for A2AP and PAI-1 should be reserved until abnormalities that are more common have been excluded. Testing for fibrinolytic disorders can be difficult to interpret due to complex interactions between fibrinolytic activators and inhibitors and effects of preanalytical variables related to blood draw quality and timing. Clinically available PAI-1 assays have technical limitations in the low range and may not be able to definitively identify deficiencies.44 PAI-1 is an acute phase reactant, which may mask mild deficiency. PAI-1–deficient patients have reduced tissue plasminogen activator antigen due to faster clearance, and tissue plasminogen activator antigen should be tested concurrently.
Dominantly inherited profibrinolytic platelets due to increased urokinase-type plasminogen activator in platelet alpha granules result in a bleeding disorder known as Quebec platelet disorder. Bleeding is due to intraplatelet plasmin generation and proteolysis of platelet proteins and coagulation factors in the alpha granules.44 Coagulation and platelet aggregation testing is normal in this condition (with the exception of absent epinephrine response), and genetic testing for PLAU gene duplication should be performed for diagnosis.44
Because viscoelastic testing methods have become more commonplace, it is useful understand the expected patterns in patients with fibrinolytic disorders. These assays are often insensitive to lysis caused by small changes in plasminogen activator levels but can show evidence of lysis when there are activator levels significant enough to overcome PAI-1, including severe trauma, cardiopulmonary bypass, and the anhepatic phase of liver transplant. Viscoelastic testing methods have been used to optimize transfusion regimens or other therapies in these settings, but there is also controversy around the degree of benefit and around sensitivity and specificity in other types of patients.45
Laboratory considerations for patients suspected to have a bleeding disorder of secondary hemostasis, clot stabilization (FXIII), or fibrinolysis are shown in Table 3.
Laboratory considerations for patients with a suspected bleeding disorder of secondary hemostasis, clot stabilization, or fibrinolysis and normal or nondiagnostic initial tests (routine clotting times, fibrinogen)
| Problem . | Laboratory considerations . |
|---|---|
| Factor deficiencies | |
| Mild factor deficiencies may not prolong clotting times | - Perform factor activity assays with a stepwise approach focusing on more common deficiencies first (factors VIII, IX, and XI), followed by less common deficiencies (factors II, V, VII, and X). - Consider chromogenic factor VIII and IX assays due to potential for clinically significant discrepant results in nonsevere hemophilia; chromogenic factor IX assays are less widely available. - In discrepant nonsevere HA, a VWD panel may provide a clue in the form of a decreased FVIII activity/VWF:Ag ratio, even if the absolute FVIII result is normal; this is also seen in type 2N VWD. - Factor activities may be borderline, confirm mild cases and female carriers of HA and HB with genetic testing. |
| Prolongation of clotting times by a lupus anticoagulant may mask a factor deficiency or inhibitor | - Perform factor assays if there is a history of bleeding or a clinical condition strongly associated with factor deficiencies. Lupus anticoagulants often interfere with quantification of factor activity in clot-based factor assays; exercise caution in interpretation. |
| FXIII deficiency does not prolong clotting times | - Clot solubility tests are most widely available worldwide but lack sensitivity (pick up only the most severe deficiencies) and specificity (abnormal with fibrinogen and other abnormalities). - Activity assays are the preferred first-line tests but may not accurately quantify the most severe deficiencies or may overestimate FXIII activity. - Consider FXIII antigen testing (sensitive to severe deficiency at the cost of missing qualitative abnormalities) and use of testing combinations for diagnosis. - FXIII deficiency subtyping should be performed by antigen testing or genetic testing due to important treatment implications. |
| Fibrinolytic disorders are poorly defined and do not prolong clotting times | - Conditions are rare, and testing has limited availability. - Results are significantly affected by blood draw timing and quality. - Results are difficult to interpret due to acute phase responses, complex interactions between fibrinolytic system components, and technical limitations of assays (deficiencies are difficult to identify). - Test PAI-1 (activity and/or antigen) and tissue plasminogen activator antigen concurrently to look for expected patterns. - Platelet aggregation is mostly normal in Quebec platelet syndrome; perform genetic testing for PLAU duplication. - Viscoelastic studies are sensitive to severe defects but may lack sensitivity for mild or moderate abnormalities. - Fibrinolytic disorders remain difficult to diagnose; genetic testing has some clinical utility but is not in widespread clinical use. Consider treating undiagnosed disorders with tranexamic acid and desmopressin |
| Problem . | Laboratory considerations . |
|---|---|
| Factor deficiencies | |
| Mild factor deficiencies may not prolong clotting times | - Perform factor activity assays with a stepwise approach focusing on more common deficiencies first (factors VIII, IX, and XI), followed by less common deficiencies (factors II, V, VII, and X). - Consider chromogenic factor VIII and IX assays due to potential for clinically significant discrepant results in nonsevere hemophilia; chromogenic factor IX assays are less widely available. - In discrepant nonsevere HA, a VWD panel may provide a clue in the form of a decreased FVIII activity/VWF:Ag ratio, even if the absolute FVIII result is normal; this is also seen in type 2N VWD. - Factor activities may be borderline, confirm mild cases and female carriers of HA and HB with genetic testing. |
| Prolongation of clotting times by a lupus anticoagulant may mask a factor deficiency or inhibitor | - Perform factor assays if there is a history of bleeding or a clinical condition strongly associated with factor deficiencies. Lupus anticoagulants often interfere with quantification of factor activity in clot-based factor assays; exercise caution in interpretation. |
| FXIII deficiency does not prolong clotting times | - Clot solubility tests are most widely available worldwide but lack sensitivity (pick up only the most severe deficiencies) and specificity (abnormal with fibrinogen and other abnormalities). - Activity assays are the preferred first-line tests but may not accurately quantify the most severe deficiencies or may overestimate FXIII activity. - Consider FXIII antigen testing (sensitive to severe deficiency at the cost of missing qualitative abnormalities) and use of testing combinations for diagnosis. - FXIII deficiency subtyping should be performed by antigen testing or genetic testing due to important treatment implications. |
| Fibrinolytic disorders are poorly defined and do not prolong clotting times | - Conditions are rare, and testing has limited availability. - Results are significantly affected by blood draw timing and quality. - Results are difficult to interpret due to acute phase responses, complex interactions between fibrinolytic system components, and technical limitations of assays (deficiencies are difficult to identify). - Test PAI-1 (activity and/or antigen) and tissue plasminogen activator antigen concurrently to look for expected patterns. - Platelet aggregation is mostly normal in Quebec platelet syndrome; perform genetic testing for PLAU duplication. - Viscoelastic studies are sensitive to severe defects but may lack sensitivity for mild or moderate abnormalities. - Fibrinolytic disorders remain difficult to diagnose; genetic testing has some clinical utility but is not in widespread clinical use. Consider treating undiagnosed disorders with tranexamic acid and desmopressin |
Commentary on other testing modalities
Optimized laboratory testing approaches can maximize the likelihood of a definitive diagnosis in patients with a bleeding disorder. Although some patients are diagnosed after thorough laboratory investigation, others remain undiagnosed after an extensive search and fall into the category of unclassified bleeding disorders (UBDs), also known as bleeding of unknown cause (BUC).16 Mild bleeding disorders are overrepresented in this group, and mild platelet function disorders are underdiagnosed and remain diagnostically challenging.43 Testing such as viscoelastic studies, thrombin generation, or other currently research-based testing do not yet have a clear role in this setting due to variable performance in the literature or lack of clinical availability.1,5
Genetic testing using large gene panels has the potential to identify rare abnormalities not adequately assessed by clinically available tests and may be useful for certain patients, although variant interpretation may be difficult.7,43,46,47 Genetic abnormalities may correlate poorly with the clinical bleeding tendency due to multiple gene interactions or interaction with acquired factors.32 These panels may increase diagnosis and understanding of rare disorders, such as fibrinolytic disorders, in which currently available testing is not widely available, complex to perform or interpret, or limited by poor assay performance characteristics.16,42,44 When large panels were studied in the setting of UBD/BUC, the overall diagnostic yield was low (3.2% for the ThromboGenomics panel in a recent study of 2396 patients), and more study of high-throughput genetic modalities is needed.43,46 Currently, the American Thrombosis and Hemostasis Network, a network of 140 hemostasis centers across all 50 states, is offering genetic testing (whole-gene sequencing) for 30 rare clotting and platelet disorder genes, including FXIII deficiency. This represents a true network of diagnostic and therapeutic options for patients and families with rare and ultra-rare bleeding disorders, and the testing is currently provided free of charge. Without a specific diagnosis to tailor treatment, UBC/BUC is often treated with tranexamic acid and desmopressin with reported success.1
Case resolution and conclusion
Case 1
In this female patient with a mucocutaneous bleeding history, the repeat VWD panel results again showed borderline low activity and antigen results without clear discordance and a normal multimer pattern (VWF:Ag, 55%; VWF:RCo, 48%; activity/antigen ratio, 0.87). Her platelets responded normally to most platelet agonists in the aggregation study, although the low-dose ristocetin-induced platelet aggregation showed an abnormally increased response to low-dose ristocetin (0.5 mg/mL) with complete platelet aggregation. Type 2B VWD was confirmed by identification of a mutation in VWF exon 28 (p.R1379C).22
Case 2
In this male patient with recent onset of pronounced bleeding after mild to moderate trauma, additional testing included platelet aggregation studies (normal), a comprehensive panel of clot-based factor assays (normal), and FXIII activity testing (normal). A chromogenic FVIII assay showed a decreased FVIII activity of 30% (compared with the normal clot-based [1-stage] result of 65%). Mild HA was confirmed by identification of an F8 gene mutation (p.R546W).48 On rereview, his FVIII activity/VWF:Ag ratio was decreased on the initial VWD panel (65%/115%, ratio 0.57), which may have provided a clue to the diagnosis. A discordant FVIII activity/VWF:Ag ratio is also seen in type 2N VWD.49
In conclusion, in-depth knowledge of the variability of bleeding disorder phenotypes and laboratory testing performance characteristics and limitations resulted in a conclusive diagnosis for these patients.
Conflict-of-interest disclosure
Kristi J. Smock: no competing financial interests to declare.
Karen A. Moser: no competing financial interests to declare.
Off-label drug use
Kristi J. Smock: Off-label use of tranexamic acid and DDAVP for bleeding of unknown cause is discussed.
Karen A. Moser: Off-label use of tranexamic acid and DDAVP for bleeding of unknown cause is discussed.