Abstract
As a key member of the innate and adaptive immune response, neutrophils provide insights into the hematopoietic and inflammatory manifestations of inborn errors of immunity (IEI) and the consequences of immunotherapy. The facile recognition of IEI presenting with neutropenia provides an avenue for hematologists to facilitate early diagnosis and expedite biologically rationale care. Moreover, enhancing the understanding of the molecular mechanisms driving neutropenia in IEI—decreased bone marrow reserves, diminished egress from the bone marrow, and decreased survival—offers an opportunity to further dissect the pathophysiology driving neutropenia secondary to iatrogenic immune dysregulation, eg, immune checkpoint inhibitors and chimeric antigen receptor T-cell therapy.
Learning Objectives
Identify neutropenia as a potential first sign of underlying inborn errors of immunity and utilize a targeted history and physical exam to assess the need for additional evaluation
Describe peripheral blood smear and bone marrow findings associated with IEI
Discuss the benefits of early IEI diagnosis in relation to patient-specific, biologically rationale therapeutic decision-making
Explain the pathophysiology of iatrogenic neutropenia secondary to immunotherapy
Illustrate potential correlations between neutropenia secondary to immunotherapy and known immune disorders
Introduction
While neutrophils have long been recognized as critical mediators of first-line host defense and inflammation-induced injury, emerging evidence suggests a more nuanced and extensive role for neutrophils in both the innate and adaptive immune response.1 In parallel, neutropenia, defined as an absolute neutrophil count (ANC) <1500 cells per microliter for patients over 1 year of age, is increasingly appreciated as a clinical feature of inborn errors of immunity (IEI) and immune dysregulation.2 Patients with IEI also frequently present with other hematologic clinical features, eg, cytopenias, bone marrow failure, splenomegaly, and malignancy.3,4
Neutropenia in IEI and other immune dysregulation states can be a consequence of infection or a drug effect; however, for several disorders neutropenia is the result of an intrinsic defect of myelopoiesis or an outcome of an improper immune response. Understanding the pathophysiology driving neutropenia in IEI and immune dysregulation provides an opportunity to intervene in a biologically rational manner. Hematologists adept at distinguishing neutropenia secondary to IEI or immune dysregulation from other etiologies can facilitate early diagnosis. Additionally, investigations into IEI-mediated neutropenia provide insights into the mechanism and management of neutropenia secondary to the immune-related therapies used by oncologists that leverage the same molecular pathways to enhance immunologic antitumor activity.
Distinguishing patients with neutropenia related to IEI from other etiologies
Differentiating neutropenia associated with IEI from more common postinfectious, drug-related, nutritional, or primary autoimmune etiologies can be challenging, particularly as the number of IEI are rapidly expanding and may present with variable or incomplete clinical features.4 The history should be inclusive of screening for phagocyte disorders and humoral and combined immunodeficiency as well as specific questions to probe for bone marrow failure or immune dysregulation syndromes (see Table 1). Careful attention to the exam, particularly the oral cavity (eg, oral ulcers, gingivitis, abnormal dentition, periodontal disease), integumentary (eg, eczema, hemangiomas, hypopigmentation, warts, prominent superficial veins, vasculitis, etc), and hematologic/lymphatic systems, can provide clues to an underlying IEI. Notably, the hematologist can also raise concern for an underlying IEI by review of the peripheral blood smear and, if completed, bone marrow biopsy for signs suggestive of disrupted neutrophil development/function or marrow stress/infiltration (see Table 2). Functional laboratory evaluation tailored to the patient presentation can provide supportive evidence of an IEI. The Clinical Immunology Society maintains a DLI Laboratory Directory that is publicly accessible and can assist with the identification of labs currently providing Clinical Laboratory Improvement Amendments-certified immune testing (Accessed 30 September 2021. https://clinimmsoc.org/CIS/Resources/DLI-Lab-Directory.htm). A definitive diagnosis generally requires the identification of pathogenic variants via genetic sequencing.
Targeted screening by history for IEI in patients presenting with neutropenia
| Targeted screening by history for IEI . | Additional studies to consider . |
|---|---|
| Phagocyte disorders | |
| Recurrent fevers Persistent or recurrent oral ulcers Bleeding and/or receding gums Gingivitis Dental anomalies and/or dental infections Frequent or deep-seated skin infections Perirectal infections or inflammatory bowel disease Developmental delay Cardiac, renal, or skeletal anomalies Personal or family history of MDS/AML | Serial CBCPDs DHR flow Genetic sequencing Echocardiogram Renal ultrasound Skeletal survey Genetic sequencing |
| Bone marrow failure syndromes | |
| Personal or family history of bone marrow failure, MDS, AML, sarcomas, fat malabsorption, warts, lymphedema, pulmonary or hepatic fibrosis, early graying of hair, nail abnormalities, stroke, vasculitis | Pancreatic amylase Fecal elastase Pancreatic ultrasound Telomere length Chromosomal breakage ADA2 level Quantitative immunoglobulins T/B/NK-cell enumeration Genetic sequencing |
| Humoral deficiency | |
| Four or more new ear infections in 1 year Two or more sinus infections or pneumonias requiring antibiotics within the past 1 year Requirement for prolonged course or multiple courses of oral antibiotics to clear “simple” infections Need for intravenous antibiotics and/or hospitalizations for infection treatment | Quantitative immunoglobulins T/B/NK-cell enumeration Vaccine antibody responses BTK flow cytometry Genetic sequencing |
| Combined immunodeficiency | |
| Abnormal TREC newborn screen Failure to thrive Developmental delay Persistent oral thrush or fungal skin infections Two or more deep-seated infections Atypical or opportunistic infections | Quantitative immunoglobulins T/B/NK-cell enumeration Vaccine antibody responses Lymphocyte mitogen proliferation Lymphocyte antigen proliferation TRECs CD40L flow cytometry STAT1 functional screening Toll-like receptor-mediated signaling screening Genetic sequencing |
| Immune dysregulation | |
| Personal or family history of inflammatory bowel disease, particularly with onset prior to age 6, autoimmune cytopenias, progressive neurological dysfunction, hemophagocytic lymphohistiocytosis Evidence of nonmalignant lymphoproliferation with chronic lymphadenopathy or splenomegaly Differences in hair or skin pigmentation, particularly in relation to immediate family members | Ferritin TCR alpha-beta CD3+CD4−CD8− T cells Genetic sequencing |
| Targeted screening by history for IEI . | Additional studies to consider . |
|---|---|
| Phagocyte disorders | |
| Recurrent fevers Persistent or recurrent oral ulcers Bleeding and/or receding gums Gingivitis Dental anomalies and/or dental infections Frequent or deep-seated skin infections Perirectal infections or inflammatory bowel disease Developmental delay Cardiac, renal, or skeletal anomalies Personal or family history of MDS/AML | Serial CBCPDs DHR flow Genetic sequencing Echocardiogram Renal ultrasound Skeletal survey Genetic sequencing |
| Bone marrow failure syndromes | |
| Personal or family history of bone marrow failure, MDS, AML, sarcomas, fat malabsorption, warts, lymphedema, pulmonary or hepatic fibrosis, early graying of hair, nail abnormalities, stroke, vasculitis | Pancreatic amylase Fecal elastase Pancreatic ultrasound Telomere length Chromosomal breakage ADA2 level Quantitative immunoglobulins T/B/NK-cell enumeration Genetic sequencing |
| Humoral deficiency | |
| Four or more new ear infections in 1 year Two or more sinus infections or pneumonias requiring antibiotics within the past 1 year Requirement for prolonged course or multiple courses of oral antibiotics to clear “simple” infections Need for intravenous antibiotics and/or hospitalizations for infection treatment | Quantitative immunoglobulins T/B/NK-cell enumeration Vaccine antibody responses BTK flow cytometry Genetic sequencing |
| Combined immunodeficiency | |
| Abnormal TREC newborn screen Failure to thrive Developmental delay Persistent oral thrush or fungal skin infections Two or more deep-seated infections Atypical or opportunistic infections | Quantitative immunoglobulins T/B/NK-cell enumeration Vaccine antibody responses Lymphocyte mitogen proliferation Lymphocyte antigen proliferation TRECs CD40L flow cytometry STAT1 functional screening Toll-like receptor-mediated signaling screening Genetic sequencing |
| Immune dysregulation | |
| Personal or family history of inflammatory bowel disease, particularly with onset prior to age 6, autoimmune cytopenias, progressive neurological dysfunction, hemophagocytic lymphohistiocytosis Evidence of nonmalignant lymphoproliferation with chronic lymphadenopathy or splenomegaly Differences in hair or skin pigmentation, particularly in relation to immediate family members | Ferritin TCR alpha-beta CD3+CD4−CD8− T cells Genetic sequencing |
AML, acute myeloid leukemia; CBCPD, complete blood count with platelets and differential; DHR, dihydrorhodamine; MDS, myelodysplastic syndrome; TCR, T-cell receptor; TREC, T-cell receptor excision circle.
Peripheral blood smear and bone marrow findings in IEI associated with neutropenia
| Hematopathologic finding . | IEI to consider in differential . | Additional comments . |
|---|---|---|
| Absent or decreased neutrophil granules | GFI1-related neutropenia JAGN1-related neutropenia Specific granule deficiency | In specific granule deficiency, neutrophils with bilobed nuclei are commonly observed. |
| Atypical megakaryocytes | GATA2 haploinsufficiency | Megakaryocytes may be small or mononuclear or may have separated nuclear lobes. Often associated with marrow fibrosis.21 |
| Giant cytoplasmic granules | Chediak-Higashi syndrome | Giant azurophilic granules within the lysosomes are considered pathognomonic for the disorder and are easily visible in neutrophils, eosinophils, and other granulocytes.51 Additional findings in the marrow include extensive vacuolization, acidophilic inclusion (esp in promyelocytes), specific granules easily recognized as early as the myelocyte stage.9 |
| Myelofibrosis | DADA215 GATA2 haploinsufficiency21 VPS45-related neutropenia52 | DADA2 is also associated with lymphoid aggregates in the marrow. |
| Myelokathexis | WHIM syndrome23 G6PC3-related neutropenia53 | Neutrophils are also often hypersegmented with thin, long isthmi, pyknotic nuclei, and cytoplasmic vacuoles in WHIM syndrome. |
| Neutrophil nuclei herniation | WDR1 deficiency54 | In addition to herniation of the nuclear lobes, neutrophils also have agranular regions within the cytosol. |
| PHA | NBAS deficiency | Pseudo-PHA is associated with various medications (eg, immunosuppressive agents, antimicrobials, growth factors). The pseudo-PHA is generally reversible with medication cessation.54 PHA also seen in association with MDS. |
| Promyelocyte arrest | Severe, permanent arrest:55 ELANE-related neutropenia (severe congenital phenotype) HAX-1-related neutropenia CSF3R-related neutropenia G6PC3-related neutropenia WAS-related neutropenia JAGN1-related neutropenia56 Mild, intermittent arrest: GFI1-related neutropenia Neutropenia associated with poikiloderma | Copper deficiency can also result in maturation arrest; reversible with copper administration. Promyelocytic AML can mimic the promyelocytic arrest associated with IEI but is easily differentiated by additional flow and/or cytogenetic studies. |
| Hematopathologic finding . | IEI to consider in differential . | Additional comments . |
|---|---|---|
| Absent or decreased neutrophil granules | GFI1-related neutropenia JAGN1-related neutropenia Specific granule deficiency | In specific granule deficiency, neutrophils with bilobed nuclei are commonly observed. |
| Atypical megakaryocytes | GATA2 haploinsufficiency | Megakaryocytes may be small or mononuclear or may have separated nuclear lobes. Often associated with marrow fibrosis.21 |
| Giant cytoplasmic granules | Chediak-Higashi syndrome | Giant azurophilic granules within the lysosomes are considered pathognomonic for the disorder and are easily visible in neutrophils, eosinophils, and other granulocytes.51 Additional findings in the marrow include extensive vacuolization, acidophilic inclusion (esp in promyelocytes), specific granules easily recognized as early as the myelocyte stage.9 |
| Myelofibrosis | DADA215 GATA2 haploinsufficiency21 VPS45-related neutropenia52 | DADA2 is also associated with lymphoid aggregates in the marrow. |
| Myelokathexis | WHIM syndrome23 G6PC3-related neutropenia53 | Neutrophils are also often hypersegmented with thin, long isthmi, pyknotic nuclei, and cytoplasmic vacuoles in WHIM syndrome. |
| Neutrophil nuclei herniation | WDR1 deficiency54 | In addition to herniation of the nuclear lobes, neutrophils also have agranular regions within the cytosol. |
| PHA | NBAS deficiency | Pseudo-PHA is associated with various medications (eg, immunosuppressive agents, antimicrobials, growth factors). The pseudo-PHA is generally reversible with medication cessation.54 PHA also seen in association with MDS. |
| Promyelocyte arrest | Severe, permanent arrest:55 ELANE-related neutropenia (severe congenital phenotype) HAX-1-related neutropenia CSF3R-related neutropenia G6PC3-related neutropenia WAS-related neutropenia JAGN1-related neutropenia56 Mild, intermittent arrest: GFI1-related neutropenia Neutropenia associated with poikiloderma | Copper deficiency can also result in maturation arrest; reversible with copper administration. Promyelocytic AML can mimic the promyelocytic arrest associated with IEI but is easily differentiated by additional flow and/or cytogenetic studies. |
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; PHA, Pelger-Huet anomaly.
With recent advancements in sequencing methods and accessibility, genetic testing is increasingly promoted as a key tool in the definitive diagnosis of IEI.5-7 A molecular diagnosis is valuable for unequivocally establishing the IEI diagnosis, permitting appropriate genetic counseling to identify other at-risk family members and future pregnancies, facilitating genotype/phenotype correlations to inform patient-specific clinical expectations, and identifying patients most likely to benefit from gene-specific therapies.
Pathophysiology of neutropenia in IEI
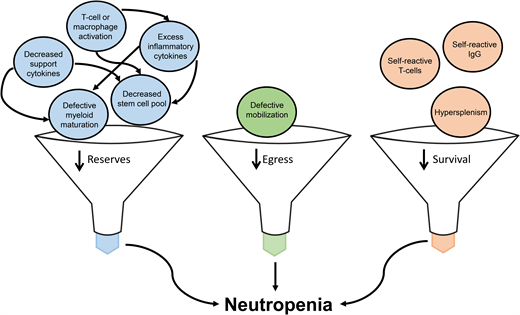
While the general mechanisms for neutropenia in IEI have been proposed, ie, diminished survival, flawed development, physical replacement, or failure to egress from the marrow,8 definitive mechanisms for neutropenia in the majority of IEI outside of the congenital disorders of isolated neutropenia (eg, ELANE- related neutropenia) have not been established. As such, this article focuses on IEI with well-established associations with neutropenia accompanied by the additional immune deficits most likely to be encountered by hematologists (Table 3).
Characteristics of neutropenia in IEI commonly encountered by hematologists
| IEI . | Frequency of neutropenia . | Proposed mechanism of neutropenia . | Neutropenia-directed therapy . | Comments . |
|---|---|---|---|---|
| Chediak-Higashi syndrome | Common57 | -Accelerated granulocyte turnover secondary to intramedullary granulocyte destruction compounded by hypersplenism.9 -Abnormal bone marrow reserves with defective granulocyte mobilization from the marrow space.9 | G-CSF Antimicrobials HSCT58 | -Phagocyte intracellular killing and NK cytotoxicity is impaired. |
| CVID | 1%-8.4%10,12 | -Autoimmune clearance as supported by the identification of antineutrophil antibodies and evidence of hyperplastic yet inefficient germinal center responses.11,59 -Hypersplenism -Postinfectious -Drug-related | Corticosteroids G-CSF Rapamycin | -Neutropenia does not improve with IgG replacement -Neutropenia is associated with increased infections, increased polyclonal lymphoproliferation, and autoimmunity.12 -Increased mortality rate associated with neutropenia in CVID patients.11,12 |
| DADA2 | 7%-15%14,60 | -Decreased ADA2 protein function in severely deleterious variants is proposed to lead to decreased marrow production.17 -Immune-mediated destruction is also hypothesized. | Corticosteroids Rituximab Anti-TNF agents HSCT61 | -ADA2 is highly expressed in myeloid cells and produced by activated macrophages, monocytes, and dendritic cells when stimulated by an inflammatory response.14 |
| Hyper IgM syndrome | 41%18 | -Decreased CD16 expression and dysregulated transcriptome resulting in impaired differentiation.62 -Disrupted cytokine or growth factor support in the bone marrow.63 | G-CSF | -Neutropenia may be chronic or intermittent. -Bone marrow evaluation may demonstrate maturation arrest.62 |
| GATA2 haploinsufficiency | 47%21 | -Reduction of the primitive HSC pool20 | G-CSF HSCT | -Mild chronic neutropenia may be the first manifestation of disease with other clinical features, eg, monocytopenia or MDS/AML presenting later. -Maturation of neutrophils in the bone marrow is generally preserved.20 |
| WHIM syndrome | Near universal | -Diminished egress from the bone marrow secondary to gain of function mutations in CXCR423 | G-CSF CXCR4 antagonists | -Bone marrow is hypercellular with full maturation and classic pyknotic nuclei. |
| XLA | 10%-26%64-66 | -Decreased bone marrow precursors25 -Decreased maturation of myeloid precursors secondary to changes in BTK-related signal transduction25 -Decreased cytokine/chemokine production from monocytes, esp decreased IL-1825 | G-CSF IVIG | -BTK expressed in myeloid and B-cell differentiation (limited to hematopoietic cells). -Neutropenia may be a presenting sign of XLA. -Neutropenia generally resolves with initiation of IVIG, allowing G-CSF withdrawal. -Neutropenia generally documented only in conjunction with an active infection. |
| IEI . | Frequency of neutropenia . | Proposed mechanism of neutropenia . | Neutropenia-directed therapy . | Comments . |
|---|---|---|---|---|
| Chediak-Higashi syndrome | Common57 | -Accelerated granulocyte turnover secondary to intramedullary granulocyte destruction compounded by hypersplenism.9 -Abnormal bone marrow reserves with defective granulocyte mobilization from the marrow space.9 | G-CSF Antimicrobials HSCT58 | -Phagocyte intracellular killing and NK cytotoxicity is impaired. |
| CVID | 1%-8.4%10,12 | -Autoimmune clearance as supported by the identification of antineutrophil antibodies and evidence of hyperplastic yet inefficient germinal center responses.11,59 -Hypersplenism -Postinfectious -Drug-related | Corticosteroids G-CSF Rapamycin | -Neutropenia does not improve with IgG replacement -Neutropenia is associated with increased infections, increased polyclonal lymphoproliferation, and autoimmunity.12 -Increased mortality rate associated with neutropenia in CVID patients.11,12 |
| DADA2 | 7%-15%14,60 | -Decreased ADA2 protein function in severely deleterious variants is proposed to lead to decreased marrow production.17 -Immune-mediated destruction is also hypothesized. | Corticosteroids Rituximab Anti-TNF agents HSCT61 | -ADA2 is highly expressed in myeloid cells and produced by activated macrophages, monocytes, and dendritic cells when stimulated by an inflammatory response.14 |
| Hyper IgM syndrome | 41%18 | -Decreased CD16 expression and dysregulated transcriptome resulting in impaired differentiation.62 -Disrupted cytokine or growth factor support in the bone marrow.63 | G-CSF | -Neutropenia may be chronic or intermittent. -Bone marrow evaluation may demonstrate maturation arrest.62 |
| GATA2 haploinsufficiency | 47%21 | -Reduction of the primitive HSC pool20 | G-CSF HSCT | -Mild chronic neutropenia may be the first manifestation of disease with other clinical features, eg, monocytopenia or MDS/AML presenting later. -Maturation of neutrophils in the bone marrow is generally preserved.20 |
| WHIM syndrome | Near universal | -Diminished egress from the bone marrow secondary to gain of function mutations in CXCR423 | G-CSF CXCR4 antagonists | -Bone marrow is hypercellular with full maturation and classic pyknotic nuclei. |
| XLA | 10%-26%64-66 | -Decreased bone marrow precursors25 -Decreased maturation of myeloid precursors secondary to changes in BTK-related signal transduction25 -Decreased cytokine/chemokine production from monocytes, esp decreased IL-1825 | G-CSF IVIG | -BTK expressed in myeloid and B-cell differentiation (limited to hematopoietic cells). -Neutropenia may be a presenting sign of XLA. -Neutropenia generally resolves with initiation of IVIG, allowing G-CSF withdrawal. -Neutropenia generally documented only in conjunction with an active infection. |
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.
Chediak-Higashi syndrome
Chediak-Higashi syndrome is an autosomal recessive disorder secondary to pathogenic variants in CHS1/LYST. Patients classically present with oculocutaneous albinism, recurrent pyogenic infections, bleeding diathesis, and progressive neurological dysfunction and are at high risk of developing hemophagocytic lymphohistiocytosis (HLH) secondary to defective lysosomal trafficking. Neutropenia is commonly observed and is attributed to abnormal bone marrow reserves and defective granulocyte mobilization from the marrow space compounded by intramedullary granulocyte destruction.9 While not explicitly studied, it is likely that infection-related marrow suppression and/or accelerated immune clearance also contributes to the neutropenia. The peripheral smear classically demonstrates giant azurophilic granules within granulocyte lysosomes. The treatment of neutropenia is primarily supportive with antimicrobials and granulocyte colony-stimulating factor (G-CSF), although hematopoietic stem cell transplant (HSCT) can resolve the hematopoietic aspects of the disorder.
Common variable immunodeficiency
Common variable immunodeficiency (CVID) is a heterogeneous disorder characterized by impaired B-cell differentiation and defective immunoglobulin (Ig) production. Autoimmune cytopenias are a common clinical feature of CVID, with immune thrombocytopenia and autoimmune hemolytic anemia more prevalent than autoimmune neutropenia.10 Cytopenias, including isolated neutropenia, frequently precede the diagnosis of CVID and herald a worse outcome.11,12 Autoimmune clearance secondary to self-reactive IgG to hematopoietic antigens is the primary suspected etiology of neutropenia, although hypersplenism and drug-related (eg, secondary to rituximab) and infection-related neutropenia is documented. Patients may be treated with corticosteroids and/or G-CSF. Splenectomy and rituximab are not useful,11 but rapamycin is emerging as an effective therapy.13
Deficiency of adenosine deaminase 2
A deficiency of adenosine deaminase 2 (DADA2) results from homozygous or compound heterozygous pathogenic variants that result in a loss of function of ADA2. Originally described as an etiology for monogenic vasculitis and early-onset stroke, the phenotype is now recognized to be highly variable and inclusive of hematologic and immune deficits.14 Hematologic presentations include cytopenias—eg, pure red blood cell aplasia and bone marrow failure—splenomegaly, and lymphadenopathy,15 with cytopenias often the first sign of disease. Age of presentation is also variable, with 24% of cases diagnosed in infancy and 77% before the age of 10, but adult-onset symptoms are increasingly recognized.14,16 The mechanism of neutropenia in DADA2 is unclear; however, both immune-mediated destruction and bone marrow failure are suspected to contribute. Genotype-phenotype correlations are emerging, with more deleterious variants resulting in hematologic disease with variable response to anti-tumor necrosis factor (TNF) agents.17 Corticosteroids, rituximab, and HSCT have been utilized for the treatment of cytopenias.
Hyper IgM syndrome
Hyper IgM syndrome is a genetic heterogeneous group of disorders typified by the inability to produce class-switched immunoglobulin. X-linked pathogenic variants in CD40L are the most common etiology of hyper IgM syndrome. Patients often present in early infancy or childhood with recurrent sinopulmonary infections and/or opportunistic infections such as Pneumocystis jirovecii, histoplasmosis, or cryptosporidium. Neutropenia can be chronic or intermittent in CD40L deficiency and is associated with an increased risk of oral and rectal ulcers, gingivitis, and proctitis.18 Neutropenia can be the initial clinical sign of hyper IgM syndrome and can mimic severe congenital neutropenia secondary to the presence of maturation arrest in the bone marrow. The exact mechanism of neutropenia remains obscure, although immune clearance, defective myeloid differentiation, and/or flawed bone marrow egress have been hypothesized.19 Patients are generally responsive to G-CSF. Of note, lymphomas and gastrointestinal-related malignancies are common in patients with hyper IgM syndrome.
GATA2 haploinsufficiency
GATA2 haploinsufficiency occurs secondary to autosomal dominant pathogenic variants in GATA2, an early hematopoietic transcription factor active in myeloid development. Neutropenia occurs secondary to negative impacts on the hematopoietic stem cell (HSC) pool.20 Patients with GATA2 haploinsufficiency have variable clinical presentations inclusive of neutropenia; monocytopenia; B-cell, CD4+ T-cell and NK-cell lymphopenia; myelodysplastic syndrome; acute myeloid leukemia; nontuberculous mycobacterial infections; viral infections (especially varicella, Epstein-Barr virus [EBV], and human papilloma virus [HPV]); and/or lymphedema.21 Chronic neutropenia may be the initial sign of GATA2 haploinsufficiency, particularly in pediatric patients.22 G-CSF and consideration of HSCT are therapeutic options to manage neutropenia.
Warts, hypogammaglobulinemia, infections, myelokathexis syndrome
The clinical tetrad of warts, hypogammaglobulinemia, infection, and myelokathexis (WHIM) syndrome is due to autosomal dominant pathogenic variants in CXCR4 that result in the abnormal retention of neutrophils in the bone marrow and subsequent peripheral neutropenia. Besides neutropenia, patients with WHIM syndrome often have quantitative defects in monocytes, B cells, and CD4+ T cells. Patients are susceptible to sinopulmonary infections due to hypogammaglobulinemia and HPV-related disease, including warts and malignancy.23 The majority of WHIM patients will spontaneously release neutrophils from the bone marrow during times of “stress,” eg, infection, and do not require prophylactic G-CSF. However, for patients who suffer recurrent infections and/or fail to demonstrate an adequate release of neutrophils from the bone marrow during infectious challenges, G-CSF is recommended.24 CXCR4 antagonists are demonstrating promise as therapeutic options to improve both neutrophil and lymphocyte counts.
X-linked agammaglobulinemia
Bruton's X-linked agammaglobulinemia (XLA) is secondary to pathogenic variants in the signal transduction gene BTK that result in the near absence of CD19+ B cells and severe hypogammaglobulinemia. Neutropenia is common and is most often identified during periods of marrow “stress,” eg, during an active infection. The exact etiology of neutropenia in XLA is uncertain; however, given that Bruton's tyrosine kinase (BTK) is expressed in myeloid and B-cell differentiation, proposed mechanisms include decreased maturation of myeloid precursors secondary to changes in BTK-related signal transduction compounded by changes in monocyte IL-18 production.25 Neutropenia can be the first clinical sign of XLA and is responsive to G-CSF. Intravenous immunoglobulin (IVIG) is the mainstay of therapy for patients with XLA, often allowing for the withdrawal of G-CSF.
CLINICAL CASE 1
An 8-month-old boy is admitted with severe neutropenia (ANC, 300/µL) and thrombocytosis (platelet count, 650,000/µL) in the setting of fever and rash. The physical exam is notable for extensive impetigo without lymphadenopathy and the absence of tonsillar tissue. A comprehensive metabolic panel reveals low total protein with normal albumin. Flow cytometry for B-cell enumeration demonstrates a near absence of CD19+ B cells. G-CSF and IVIG are initiated. Genetic testing confirms a pathogenic variant in BTK consistent with XLA. Following several IVIG infusions, his ANC normalizes, and the G-CSF is withdrawn.
Author commentary: Humoral deficiencies are often masked in early infancy due to the placental transfer of maternal IgG. A lack of localized lymphadenopathy despite an extensive skin infection, an absence of tonsillar tissue, and a low total protein (surmised to be due to low immunoglobulin, as albumin is normal) raise concern for a B-cell defect. Understanding that the neutropenia in X-linked agammaglobulinemia generally occurs during periods of marrow stress and is responsive to G-CSF is key to early management. Additionally, awareness that the neutropenia in XLA resolves with IVIG initiation lends confidence to withdrawing the G-CSF post infection resolution.
CLINICAL CASE 2
A 16-year-old-girl presents with isolated mild neutropenia (ANC, 1100/µL) incidentally identified on a screening sports physical. She has no family history of neutropenia, and her physical exam is unremarkable. Serial testing confirms chronic neutropenia. Her bone marrow is moderately hypocellular, prompting next-generation genetic screening, but no pathogenic variants are identified. Subsequently, she is admitted twice for dehydration and cytopenias related to a significant bout of mononucleosis. Post discharge, her absolute lymphocyte and absolute monocyte counts remain low. Following discussion with a geneticist, additional testing is sent to evaluate for intronic variants in GATA2, and a pathogenic variant is identified consistent with GATA2 haploinsufficiency.
Author commentary: This case highlights that many IEI have subtle presentations and clinical symptoms that evolve over time, necessitating the need for the hematologist to remain vigilant and to consider gaps in testing, eg, the limitations of genetic-sequencing techniques such as with intronic regions or pseudogenes, in suspicious cases. The unusual requirement for multiple admissions for a primary EBV infection should raise concern for immune dysfunction. In GATA2 haploinsufficiency, severe EBV infections are frequently encountered as dendritic cells, which are reduced or absent in GATA2 haploinsufficiency, are required for EBV antigen presentation to T cells. Early diagnosis of GATA2 haploinsufficiency in this patient greatly affects her supportive care plan, with recommendations for G-CSF and other preventative measures, eg, HPV vaccination and malignancy surveillance. Additionally, early diagnosis affords her the opportunity to consider curative therapy with HSCT.
Pathophysiology of neutropenia secondary to immunotherapy
The role of the immune system in tumor surveillance has long been appreciated, but only recently have scientists and clinicians harnessed the power of T cells to treat cancer. Two modern immune therapies include the use of genetically engineered T cells programmed to attack malignant cells, eg, chimeric antigen receptor (CAR) T cells, and blockade of signals that drive T-cell exhaustion in the tumor microenvironment. The overall toxicity from immunotherapy is less compared to conventional chemotherapy, but the development of immune-related adverse events (irAEs), including immune-related neutropenia, can result in severe toxicity. The understanding of the immunologic mechanisms underpinning immune-related neutropenia is incomplete, but our knowledge of neutropenia driven by IEI may provide helpful clues to uncover the pathophysiology and suggest potential treatments (see Table 4).
Neutropenia secondary to ICIs and CAR T-cells with correlation to known immune disorders
| Treatment . | irAE . | Proposed mechanism . | Treatment of cytopenias in irAE . | Correlate to known immune disorders . | Treatment of cytopenias in IEI . |
|---|---|---|---|---|---|
| ICI (PD-1/PD-L1 and CTLA-4 blockade) | Hypoplastic neutropenia/aplastic anemia | T-cell infiltration of the bone marrow with subsequent destruction of hematopoietic precursors26 | Corticosteroids Cyclosporine ATG G-CSF | Immune-mediated aplastic anemia67 | Cyclosporine ATG |
| Immune-mediated dysfunction of HSC maturation and proliferation68 | Corticosteroids G-CSF | HSC dysfunction in ADA2 deficiency69 | TNF inhibition15 | ||
| Hyperplastic/peripheral neutropenia | Peripheral destruction of neutrophils mediated by autoantibodies31 | Corticosteroids IVIG G-CSF | Fas/Fas ligand deficiency and development of autoimmune cytopenias70 | Corticosteroids Rapamycin Mycophenolate Mofetil | |
| HLH | T-cell and macrophage activation, perhaps from immune cell tumor invasion and malignant cell apoptosis71 | Corticosteroids Etoposide | HLH and MAS disorders with normal phagocytic and cytotoxic function; malignancy-associated HLH72 | Corticosteroids Etoposide IFNG antibody IL-1 antibody IL-6R antibody Jak inhibitors | |
| ICI CTLA-4 blockade | Multiple irAE, including neutropenia | Regulatory T-cell inhibition and/or depletion73,74 | Corticosteroids (to suppress expanded effector T cells) G-CSF | CTLA-4 haploinsufficiency LRBA deficiency IPEX syndrome (FOXP3 deficiency) CD25 deficiency | Corticosteroids CTLA-4-Ig Rapamycin Calcineurin inhibitors |
| CAR-T | Early neutropenia | Lymphodepleting therapy and CRS75 | IL-6R antibody Corticosteroids | HLH and MAS syndromes with normal phagocytic and cytotoxic function | Corticosteroids Etoposide IFNG antibody IL-1 antibody IL-6R antibody Jak inhibitors |
| Late neutropenia | Disrupted CXCL12 bone marrow gradient inhibiting neutrophil egress46 | G-CSF | WHIM syndrome (CXCR4-GOF) Late-onset neutropenia secondary to rituximab48 | G-CSF CXCR4 inhibition |
| Treatment . | irAE . | Proposed mechanism . | Treatment of cytopenias in irAE . | Correlate to known immune disorders . | Treatment of cytopenias in IEI . |
|---|---|---|---|---|---|
| ICI (PD-1/PD-L1 and CTLA-4 blockade) | Hypoplastic neutropenia/aplastic anemia | T-cell infiltration of the bone marrow with subsequent destruction of hematopoietic precursors26 | Corticosteroids Cyclosporine ATG G-CSF | Immune-mediated aplastic anemia67 | Cyclosporine ATG |
| Immune-mediated dysfunction of HSC maturation and proliferation68 | Corticosteroids G-CSF | HSC dysfunction in ADA2 deficiency69 | TNF inhibition15 | ||
| Hyperplastic/peripheral neutropenia | Peripheral destruction of neutrophils mediated by autoantibodies31 | Corticosteroids IVIG G-CSF | Fas/Fas ligand deficiency and development of autoimmune cytopenias70 | Corticosteroids Rapamycin Mycophenolate Mofetil | |
| HLH | T-cell and macrophage activation, perhaps from immune cell tumor invasion and malignant cell apoptosis71 | Corticosteroids Etoposide | HLH and MAS disorders with normal phagocytic and cytotoxic function; malignancy-associated HLH72 | Corticosteroids Etoposide IFNG antibody IL-1 antibody IL-6R antibody Jak inhibitors | |
| ICI CTLA-4 blockade | Multiple irAE, including neutropenia | Regulatory T-cell inhibition and/or depletion73,74 | Corticosteroids (to suppress expanded effector T cells) G-CSF | CTLA-4 haploinsufficiency LRBA deficiency IPEX syndrome (FOXP3 deficiency) CD25 deficiency | Corticosteroids CTLA-4-Ig Rapamycin Calcineurin inhibitors |
| CAR-T | Early neutropenia | Lymphodepleting therapy and CRS75 | IL-6R antibody Corticosteroids | HLH and MAS syndromes with normal phagocytic and cytotoxic function | Corticosteroids Etoposide IFNG antibody IL-1 antibody IL-6R antibody Jak inhibitors |
| Late neutropenia | Disrupted CXCL12 bone marrow gradient inhibiting neutrophil egress46 | G-CSF | WHIM syndrome (CXCR4-GOF) Late-onset neutropenia secondary to rituximab48 | G-CSF CXCR4 inhibition |
ATG, antithymocyte globulin; GOF, gain-of-function; IFNG, interferon gamma; Ig, immunoglobulin; IPEX, immunodysregulation, polyendocrinopathy, and enteropathy, X-linked; MAS, macrophage activation syndrome.
Immune checkpoint inhibitors
Immune checkpoint inhibitors (ICIs) include agents that inhibit CTLA-4, programmed cell death 1 protein (PD-1), or programmed cell death 1 ligand 1 (PD-L1) signaling and are integral to the treatment of many cancers.26 The reported incidence of all-grade hematologic irAEs is 0.4% to 8.3% with high-grade (grade 3-5) at 0% to 1.7%.27-29 High-grade neutropenia in isolation or in concert with additional cytopenias is reported at a frequency of 0.2% to 0.94%.28,30 Neutropenia is almost always severe with ANCs near zero and can result in severe infection and death.27,28 The median time to onset is 8.5 to 10.5 weeks after ICI initiation, but neutropenia can occur at any point during ICI treatment.27,31,32
Patients with prior B-cell disorders including a history of autoimmunity31 or chronic lymphocytic leukemia may be at increased risk of irAEs.28 There is uncertainty if PD-1/PD-L1 vs CTLA-4 blockade results in higher hematologic irAEs, although the development of HLH may be more common with anti-CTLA-4.27,29,33 The development of autoreactive T cells and B cells, the increased secretion of cytokine with the subsequent infiltration of CD8+ T cells, and the immune-mediated dysfunction of HSC maturation or proliferation have all been postulated as general mechanisms of neutropenia.26 It is probable that different mechanisms exist, based on bone marrow biopsy findings demonstrating that a minority of patients have granulocytic hypoplasia with lymphocytic infiltrate (a central or hypoplastic type), while the majority of patients have normal or hyperplastic bone marrow with or without the identification of a neutrophil antibody (peripheral or hyperplastic type).31
Patients on ICIs presenting with neutropenia should undergo bone marrow evaluation to differentiate central vs peripheral neutropenia and to rule out underlying malignancy. A review of other medications and screening for infectious causes of neutropenia are also mandatory. Examination of the peripheral blood and bone marrow for large granular lymphocytes should be undertaken and immunophenotyped for large granular lymphocyte counts greater than 0.5/µL.27 The presence of high fever should alert to the possibility of HLH and pancytopenia to aplastic anemia, 2 diagnoses with poor treatment responses and outcomes.27,33 Therapy for neutropenia is not specifically addressed by the American Society of Clinical Oncology or the European Society for Medical Oncology,34,35 but general literature recommendations are to closely monitor patients with grade 1 neutropenia.31 For grade 2 toxicity, the ICI should be withheld and low-dose corticosteroids initiated if no recovery occurs in 1 week. For grade 3 to 4 toxicity, ICIs should be discontinued and high-dose corticosteroids administered with tapering over 4 to 6 weeks for responding patients.26,27 G-CSF should be prescribed to hasten recovery for all grade 2 to 4 toxicities. IVIG is likely safe and may be more effective for the peripheral destruction of neutrophils,31 but there is no consensus in the literature regarding whether it should be used as up-front therapy or as salvage for corticosteroid nonresponders. For patients with hypoplastic/central neutropenia, cyclosporine may be trialed if there is a poor response to corticosteroids,31 and antithymocyte globulin is an option for aplastic anemia.36 Most patients (approximately 75%-85%) have improvement or normalization of the neutrophil count, with resolution more likely in patients with a hyperplastic/peripheral type,31,32 but reinstitution of the ICI frequently results in neutropenia recurrence.28,31
CAR T cells
CAR T cells are genetically engineered to express a modified T-cell receptor with costimulatory domains that target common cancer antigens, such as CD19 and B-cell maturation antigen. CAR T cells have demonstrated activity against a range of B-cell malignancies, but efficacy has been limited by cytokine release syndrome (CRS) and neuroinflammation. High levels of IL-1, IL-6, interferon gamma, monocyte chemo-attractant protein-1, and granulocyte-macrophage (GM) CSF largely produced by monocytes and macrophages drive this inflammatory toxicity.37,38 Cytopenias are the most common grade 3 or greater irAE, with neutropenia being the most common deficiency.39 CAR studies have demonstrated an incidence of grade 3 to 4 neutropenia in 60% to 85% of patients and “late neutropenia” occurring 28 days or later in 3% to 53%.40-44
Early neutropenia has been attributed to immunodepleting therapy with fludarabine and cyclophosphamide preinfusion and hematopoietic suppression from CRS.45 However, late neutropenia, which can persist well after chemotherapy and resolution of CRS, is more enigmatic. Possible explanations for late neutropenia include poor bone marrow reserves and excessive inflammatory damage given the association with prior HCT/multiple lines of therapy and higher-grade CRS,45-47 respectively, in patients with prolonged hematologic toxicity. Interestingly, a subset of patients with late neutropenia show an initial recovery of neutrophils only to be followed by a second trough without any intervening therapy.45,46 One group evaluated serum CXCL12 levels and reported a correlation in patients with late neutropenia, hypothesizing that the consumption of CXCL12 from an expanding precursor B-cell population may disrupt the normal CXCL12 bone marrow gradient required for normal neutrophil egress, resulting in a recurrence of peripheral neutropenia in some CD19 CAR T-cell recipients.46 A similar mechanism has been proposed for late-onset neutropenia following rituximab therapy.48
Guidance for the evaluation and treatment of neutropenia following CAR T cells is currently lacking, but many suggest a bone marrow biopsy for neutropenia persisting 1 month following infusion.45 Both hypocellularity and normal cellularity have been reported, perhaps indicating differing mechanisms of neutropenia.45,46 G-CSF may hasten neutrophil recovery but should be avoided early (within 3 weeks) of infusion given the potential for heightened CRS. Data to support this concern include higher levels of murine G-CSF in mice correlating with CRS severity,49 elevated levels of G-CSF and GM-CSF in patients with neurotoxicity,50 and 1 small study demonstrating that G-CSF use proximal to CAR T-cell infusion was associated with higher CRS severity.37 Eltrombopag has been used with success in patients with aplasia, and autologous stem cell infusion should be considered for very late neutropenia (greater than 3 months) if cryopreserved cells are available.45
CLINICAL CASE 3
A 65-year-old woman with metastatic melanoma is treated with single-agent ipilimumab (anti-CTLA-4). Two weeks following her third cycle of therapy, she presents with fever and an ANC of 80/µL. A bone marrow biopsy demonstrates increased myeloid cells without a lymphocytic infiltrate or maturation block. Additional cycles of ipilimumab are temporarily discontinued, and she is administered corticosteroids, G-CSF, and IVIG, with a rising ANC within 4 days of treatment initiation. Her steroids are tapered over 4 weeks with a continued normal ANC. Her oncologist trials another cycle of ipilimumab, but her neutropenia recurs.
Author commentary: Neutropenia as an irAE is a rare but serious complication of ICIs. The loss of CTLA-4 through iatrogenic blockade or genetic haploinsufficiency results in the loss of regulatory T-cell suppressive activity and immune dysregulation. Subsequent neutropenia can develop through several proposed mechanisms, including T-cell mediated attack on the bone marrow, autoantibody formation, hypercytokinemia, and immune-mediated HSC dysfunction. In this case, myeloid hyperplasia without CD8+ lymphocytosis in the bone marrow would favor peripheral destruction by antineutrophil antibodies, a mechanism more likely to respond overall and to IVIG treatment. Although most patients with immune-related neutropenia recover with treatment, recurrence with reinitiation of the ICI occurs in the majority of patients and should only be trialed if deemed essential for oncology care and with careful complete blood counts with platelets and differential monitoring.
Conclusion
As an integrated component of the immune system, the neutrophil functions as a strong companion with other immune cells to protect the body under normal physiology. However, with disruption of tolerogenic pathways through genetic alteration or iatrogenic inhibitors, the neutrophil can quickly turn from friend to foe to unrestrained immune cells. Early and correct identification of immune dysregulation presenting with or complicated by neutropenia is critical to improving patient outcomes related to selecting vastly expanding targeted therapeutic options, minimizing infections through the early initiation of antimicrobial prophylaxis and vaccination strategies, monitoring for malignancies, and limiting end organ damage. Establishing an IEI diagnosis is also necessary for future pregnancy counseling and HSCT donor evaluations. While many of the mechanisms of neutropenia in IEI remain incompletely understood, the common themes of disrupted neutrophil production resulting in decreased marrow reserves, hampered mobilization from the marrow, and excessive destruction are apparent. Leveraging further mechanistic evaluation into IEI-related neutropenia may also illuminate the pathophysiology of neutropenia as a consequence of malignancy immunotherapy. Through such research, the neutrophil can hopefully be protected from the consequences of induced immune activation without sacrificing the T-cell onslaught on malignant cells.
Conflict-of-interest disclosure
Kelly Walkovich: consultancy: Swedish Orphan Biovitrum AB; advisory board member: Horizon Therapeutics, AstraZeneca, Pharming; local principal investigator: mavorixafor clinical trial sponsored by X4 Pharmaceuticals.
James A. Connelly: advisory board member: X4 Pharmaceuticals, Horizon Therapeutics.
Off-label drug use
Kelly Walkovich: therapy with rituximab, rapamycin, CXCR4 inhibition, cyclosporine, ATG, MMG, JAK inhibitors, IL-1 antibodies, IL-6R antibodies, and CTLA-4-Ig are briefly discussed as options for treating immunodysregulation from IEI or iatrogenic etiologies of immune dysregulation. While commonly utilized in these rare diseases, these conditions are off-label uses from the formal FDA label.
James A. Connelly: therapy with rituximab, rapamycin, CXCR4 inhibition, cyclosporine, ATG, MMG, JAK inhibitors, IL-1 antibodies, IL-6R antibodies, and CTLA-4-Ig are briefly discussed as options for treating immunodysregulation from IEI or iatrogenic etiologies of immune dysregulation. While commonly utilized in these rare diseases, these conditions are off-label uses from the formal FDA label.