Abstract
Determining the cause of a low neutrophil count in a pediatric or adult patient is essential for the hematologist's clinical decision-making. Fundamental to this diagnostic process is establishing the presence or lack of a mature neutrophil storage pool, as absence places the patient at higher risk for infection and the need for supportive care measures. Many diagnostic tests, eg, a peripheral blood smear and bone marrow biopsy, remain important tools, but greater understanding of the diversity of neutropenic disorders has added new emphasis on evaluating for immune disorders and genetic testing. In this article, a structure is provided to assess patients based on the mechanism of neutropenia and to prioritize testing based on patient age and hypothesized pathophysiology. Common medical quandaries including fever management, need for growth factor support, risk of malignant transformation, and curative options in congenital neutropenia are reviewed to guide medical decision-making in neutropenic patients.
Learning Objectives
Recognize the different pathophysiologic mechanisms of neutropenia
Evaluate the cause of neutropenia
Assess the infectious and malignancy risk in neutropenia patients
Provide directed therapy and supportive care to neutropenia patients
Introduction
Neutropenia, defined as an absolute neutrophil count (ANC) <1500/µL after the first year of life, is a common abnormality confronting the hematologist. Establishing the correct diagnosis and differentiating benign vs severe pathologic mechanisms of neutropenia are critical to inform medical decision-making regarding infectious risk, the utility of supportive care measures, the possibility of malignant transformation, and curative approaches. This article provides a diagnostic algorithm for evaluation of the pediatric and adult neutropenic patient followed by evidence-based approaches, where available, or expert consensus recommendations on the management of medical complications. Additionally, the complexity inherent to diagnosing and managing neutropenia is illustrated via a patient case.
CLINICAL CASE
A 16-year-old generally healthy African American adolescent boy is referred for an ANC of 1200/µL incidentally identified on labs obtained as part of a sports-related physical. He has no history of recent, recurrent, or unusual infections and no complaints concerning for an underlying rheumatologic condition or malignancy. He takes no medications and denies recreational drug use. His mother denies any family history of malignancy, bone marrow failure (BMF) syndromes, or immunodeficiency from the maternal side of the family. His height is in the 10th percentile; weight is in the 10th percentile. The physical exam is unremarkable, including a detailed oral, skeletal, and skin assessment. A review of his peripheral smear is consistent with mild neutropenia; neutrophils are normal in morphologic appearance. Hemoglobin level, platelet counts, mean corpuscular volume, and the remainder of differential are unremarkable. A repeat complete blood count (CBC) obtained within 1 month of the referral redemonstrates an ANC of 1100/µL. The patient remains in excellent health and as such is counseled that his ANC result is most likely secondary to the absence of the Duffy antigen, a phenotype most often seen in individuals of African, Caribbean, Middle Eastern, and/or West Indian descent.1 Return instructions are provided.
Author commentary: This adolescent is healthy, with an incidental finding of neutropenia during a well-child examination. There are no other CBC abnormalities, and the ANC is stable on repeat examination. There are no concerning findings on history or physical exam to suspect a congenital disorder or underlying malignancy that would impair neutrophil production during this initial visit. Individuals with Duffy-null (Fy[a−b−]) status have a lower neutrophil count but are at no increased risk of infections and should not be labeled as neutropenic. Providing education for concerning signs and symptoms of an evolving marrow process with return instructions is an appropriate action.
Neutropenia classification by pathophysiology
Classically, the degree of neutropenia is subdivided into mild (ANC of 1000-1499/µL), moderate (ANC of 500-999/µL), or severe (ANC <500/µL), with the term “agranulocytosis” reserved for patients with an ANC <200/µL and an absence of neutrophil precursors on bone marrow examination. This classification system is applied to noninfants only because neonates have an elevated ANC during the first 2 weeks of life followed by a lower ANC limit of 1000/µL until 1 year of age. Although this organization is instructive for individuals with a decreased capacity for neutrophil production, in which the risk for infection increases as the ANC falls below 1000/µL and the duration of neutropenia lengthens, it is not as valuable for predicting infectious risk for other mechanisms of neutropenia. It also fails to recognize the ethnic differences in the ANC secondary to Duffy-null status that frequently result in an ANC <1500/µL.1
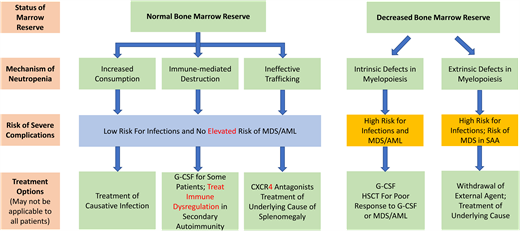
An alternative approach is to classify neutropenia by the pathophysiology driving the decreased peripheral blood ANC.2 The advantage of this classification system is that it provides a framework to interpret the peripheral blood ANC in the context of diagnostic investigations such as a bone marrow examination and provides insights into management. Central to this approach is appreciating whether the neutropenic patient has a decreased or normal bone marrow reserve because circulating neutrophils account for only 3% to 5% of the total body neutrophil count and therefore may not reflect the ability to mobilize myeloid cells in response to external threats.
Patients may have hindered proliferation and/or maturation of their neutrophils from intrinsic defects or extrinsic factors resulting in decreased marrow reserves. Intrinsic defects in myelopoiesis stem from germline pathogenic variants, eg, ELANE-related neutropenia, or somatic genetic alterations, eg, acquired myelodysplastic syndrome (MDS), that have a negative impact on the efficiency or development of myeloid progenitors into functional polymorphonuclear neutrophils. These patients are at high risk for infection without supportive and/or definitive care and may have a risk of progression to MDS and acute myeloid leukemia (AML).
In contrast, patients with extrinsic limitations on myelopoiesis have external pressures that inhibit an otherwise normal bone marrow from adequately producing neutrophils either through injury to or physical obscuration of the marrow niche, eg, neuroblastoma or fibrosis or nutritional deficiencies. These patients are also at increased risk of infection; however, many of the external pressures are treatable with prompt recognition.
Patients with a normal marrow reserve can have neutropenia through 3 different mechanisms: ineffective trafficking, immune-mediated destruction, or increased consumption. For patients with ineffective trafficking, neutrophils mature appropriately but are either unable to egress from the marrow, eg, due to defects in the CXCL12/CXCR4 axis, as in warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome, or are stored inefficiently, as seen in inappropriate sequestration of neutrophils within the marginated splenic pool in hypersplenism.3 Immune-mediated destruction is common after infections or drug insults, can be driven by T- and B-cell autoimmunity (eg, primary autoimmune neutropenia) as well as innate immune cell inflammatory cytokine production (eg, chronic idiopathic neutropenia), or may manifest secondary to broader immune syndromes (eg, secondary autoimmune neutropenia).4 Increased consumption is typically driven by heightened demand during infections.
Differential diagnosis and evaluation of neutropenia
Most patients presenting for evaluation of neutropenia will have benign causes. The most likely etiologies are primarily driven by age (Table 1), highlighting the importance of an age-focused history. For neonates, it is important to assess the maternal health with regard to any maternal hypertension/preeclampsia associated with restricted placental blood flow and subsequent reduced neonatal bone marrow production as well as known autoimmunity,5 eg, systemic lupus erythematosus or prior pregnancies complicated by known antineutrophil immunoglobulin G (IgG) antibodies that may cross the placenta and result in alloimmune or isoimmune neutropenia. Significant complications at delivery, eg, the prolonged rupture of membranes with chorioamnionitis or neonatal sepsis, can exhaust neutrophil supplies, resulting in prolonged neutropenia. Additionally, premature infants are more likely to have neutropenia as the production of neutrophils occurs primarily during the third trimester.6
Etiologies of neutropenia by mechanism and age
| . | Neonates . | Infants/children . | Adolescents and young adults . | Adults . |
|---|---|---|---|---|
| Decreased bone marrow reserve | ||||
| Intrinsic defects in myelopoiesis | - Inborn errors of myelopoiesis (eg, ELANE-related neutropenia) | - Inborn errors of myelopoiesis (eg, SDS, ELANE-related neutropenia, GATA2 haploinsufficiency) | - Inborn errors of myelopoiesis (eg, GATA2 haploinsufficiency, BMF syndromes) | - Acquired MDS - Inborn errors of myelopoiesis (eg, GATA2 haploinsufficiency) |
| Extrinsic limitations on myelopoiesis | - Maternal hypertension - Prematurity - Viral infection - Infiltration of the marrow space (eg, neuroblastoma, osteopetrosis) | - Viral infection - Nutritional deficiencies - Medications - Infiltration of the marrow space (eg, acute leukemia) | - Viral infection - Medications - Idiopathic aplastic anemia - Nutritional deficiencies (eg, anorexia nervosa) - Drug abuse - Alcoholism | - Medications - Viral infection - Nutritional deficiencies (eg, vitamin B12 deficiency, copper deficiency) - Idiopathic aplastic anemia - Infiltration of the marrow space (eg, T-LGL, fibrosis, myeloma) - Thymoma with pure WBC aplasia - Drug abuse - Alcoholism |
| Normal bone marrow reserve | ||||
| Ineffective trafficking | - Inborn errors of immunity (eg, WHIM syndrome) | - Inborn errors of immunity (eg, WHIM syndrome) | - Inborn errors of immunity (eg, WHIM syndrome) - Hypersplenism | - Hypersplenism |
| Immune-mediated destruction | - Postinfectious antibody-mediated neutropenia - Isoimmune neutropenia - Alloimmune neutropenia | - Postinfectious antibody-mediated neutropenia - Autoimmune neutropenia of infancy - Drug-related antibody-mediated neutropenia | - Postinfectious antibody- mediated neutropenia - Drug-related antibody- mediated neutropenia - Primary autoimmune neutropenia - Autoimmune neutropenia associated with inborn errors of immunity | - Drug-related antibody-mediated neutropenia - Postinfectious antibody-mediated neutropenia - Autoimmune neutropenia associated with autoimmune disorders - Primary autoimmune neutropenia - Cytokine-induced apoptosis (eg, chronic idiopathic neutropenia) |
| Increased consumption | - Neonatal sepsis | - Bacterial infection | - Bacterial infection | - Bacterial infection |
| . | Neonates . | Infants/children . | Adolescents and young adults . | Adults . |
|---|---|---|---|---|
| Decreased bone marrow reserve | ||||
| Intrinsic defects in myelopoiesis | - Inborn errors of myelopoiesis (eg, ELANE-related neutropenia) | - Inborn errors of myelopoiesis (eg, SDS, ELANE-related neutropenia, GATA2 haploinsufficiency) | - Inborn errors of myelopoiesis (eg, GATA2 haploinsufficiency, BMF syndromes) | - Acquired MDS - Inborn errors of myelopoiesis (eg, GATA2 haploinsufficiency) |
| Extrinsic limitations on myelopoiesis | - Maternal hypertension - Prematurity - Viral infection - Infiltration of the marrow space (eg, neuroblastoma, osteopetrosis) | - Viral infection - Nutritional deficiencies - Medications - Infiltration of the marrow space (eg, acute leukemia) | - Viral infection - Medications - Idiopathic aplastic anemia - Nutritional deficiencies (eg, anorexia nervosa) - Drug abuse - Alcoholism | - Medications - Viral infection - Nutritional deficiencies (eg, vitamin B12 deficiency, copper deficiency) - Idiopathic aplastic anemia - Infiltration of the marrow space (eg, T-LGL, fibrosis, myeloma) - Thymoma with pure WBC aplasia - Drug abuse - Alcoholism |
| Normal bone marrow reserve | ||||
| Ineffective trafficking | - Inborn errors of immunity (eg, WHIM syndrome) | - Inborn errors of immunity (eg, WHIM syndrome) | - Inborn errors of immunity (eg, WHIM syndrome) - Hypersplenism | - Hypersplenism |
| Immune-mediated destruction | - Postinfectious antibody-mediated neutropenia - Isoimmune neutropenia - Alloimmune neutropenia | - Postinfectious antibody-mediated neutropenia - Autoimmune neutropenia of infancy - Drug-related antibody-mediated neutropenia | - Postinfectious antibody- mediated neutropenia - Drug-related antibody- mediated neutropenia - Primary autoimmune neutropenia - Autoimmune neutropenia associated with inborn errors of immunity | - Drug-related antibody-mediated neutropenia - Postinfectious antibody-mediated neutropenia - Autoimmune neutropenia associated with autoimmune disorders - Primary autoimmune neutropenia - Cytokine-induced apoptosis (eg, chronic idiopathic neutropenia) |
| Increased consumption | - Neonatal sepsis | - Bacterial infection | - Bacterial infection | - Bacterial infection |
WBC, white blood cell.
For infants and children with benign etiologies, including primary autoimmune neutropenia due to the development of antineutrophil autoantibodies, symptoms are usually lacking, and the discovery of neutropenia is incidental. Additional questions to screen for more concerning or reversible etiologies of neutropenia include detailing the presence or absence of recurrent fevers, oral ulcers, gingivitis, dental infections, recurrent or deep-seated pyogenic infections, colitis, and nutritional deficiencies secondary to restrictive diets (eg, veganism or extended total parenteral nutrition use) or malabsorption. Care should be taken to assess for recent infection as transient postinfectious neutropenia is common. The medication history should be reviewed for commonly offending agents used in children, eg, antiepileptics and antibiotics, as withdrawal, close monitoring, or transition to an alternate agent may be indicated.
Further features elevating concern for an underlying inborn error of immunity,7 such as a personal or family history of pancreatic insufficiency, malignancy (especially myeloid and lymphoid), early edentulism or abnormal teeth, early graying, liver or lung fibrosis, mycobacterial infections, warts, lymphedema, nonmalignant lymphoproliferation, vasculitis/stroke, autoimmunity, or childhood death from infection should be solicited because many congenital neutropenias or syndromes inclusive of neutropenia present in the first years of life but may have an incomplete spectrum of symptoms.
For adolescents and young adults, a history similar to infants and children should be obtained with additional screening questions related to rheumatologic disorders since the incidence of secondary autoimmune neutropenia increases with age. Additional history questions aimed at assessing risk for eating disorders, eg, anorexia nervosa, or recreational drug use should be completed. Neutropenia secondary to the use of cocaine adulterated with levamisole is documented,8 as are cases of aplastic anemia secondary to ecstasy (3,4-methylenedioxymethamphetamine) and inhaling glue.9,10 Alcoholism can also result in neutropenia from impaired granulopoiesis.11 Many of the BMF syndromes present with clinically significant cytopenias in the second and third decades of life, so like younger children, a full history should be obtained to screen for underlying etiologies that may have been previously quiescent.
For adults, acquired etiologies, particularly drug exposures, remain the most likely etiology, and thus a careful review of the patient's medication exposures is critical. Nutritional deficiencies are also common, eg, copper deficiency secondary to gastric bypass.12 Relative to pediatric patients, both autoimmunity and malignancy are more likely in adults, so focused questions regarding arthralgias, arthritis, rashes, fatigue, unexplained fevers, bone pain, weight loss, and night sweats should be included in the history.
The physical exam evaluation for all patients should focus on the oral cavity to assess for aphthous ulcers, gingivitis, and dental abnormalities signaling clinically significant neutropenia as well as on the assessment of height, skeletal abnormalities, skin changes (eg, albinism, malar rash, vitiligo, cutaneous vasculitis, folliculitis, warts, eczema), nail anomalies, lymphadenopathy, and hepatosplenomegaly, which are potential clues to an underlying etiology.
Diagnostically, all patients benefit from a CBC and differential, a measurement of liver and renal function, and a direct review of the peripheral blood smear to ensure a lack of pseudoneutropenia due to in vitro leukocyte aggregation (due to ethylenediaminetetraacetic acid or cold agglutinins). The remainder of the diagnostic evaluation should be tailored to both the age and presenting clinical features of the patient (Table 2). An important factor in determining risk of infection in patients with a low peripheral ANC is the presence or absence of a bone marrow reserve pool of mature myeloid cells. Although a bone marrow biopsy can provide this information, it usually is not indicated in the initial evaluation if the history and physical are nonconcerning for bone marrow dysfunction. Alternative methods to assess for a bone marrow reserve include documenting a rise in the ANC either during an infectious episode, although failure to increase the ANC may be a false-negative in the setting of a viral infection, or through a granulocyte colony-stimulating factor (G-CSF) stimulation test in which an ANC is measured before and 4 to 6 hours after a single dose.
Laboratory evaluation of neutropenia by age
| Neonates . | Infants/children . | Adolescents and young adults . | Adults . |
|---|---|---|---|
| Recommended for all patients | |||
| CBC with differential Liver and renal function Peripheral blood smear | CBC with differential Liver and renal function Peripheral blood smear | CBC with differential Liver and renal function Peripheral blood smear | CBC with differential Liver and renal function Peripheral blood smear HIV antibody screening T-LGL flow cytometry |
| To be considered based on patient-specific history | |||
| Maternal antineutrophil antibodies | Antineutrophil antibodies Vitamin B12, folate, copper Amylase isoenzymes Fecal elastase Telomere length Chromosomal breakage ADA2 enzyme activity IgG/A/M/E T/B/NK subsets | Vitamin B12, folate, copper HIV Urine drug screen Amylase isoenzymes Fecal elastase Telomere length Chromosomal breakage ADA2 enzyme activity ANA, ESR, CRP IgG/A/M/E T/B/NK subsets | Vitamin B12, folate, copper Urine drug screen ANA, rheumatoid factor ESR, CRP Amylase isoenzymes Fecal elastase Telomere length Chromosomal breakage ADA2 enzyme activity IgG/A/M/E T/B/NK subsets |
| Neonates . | Infants/children . | Adolescents and young adults . | Adults . |
|---|---|---|---|
| Recommended for all patients | |||
| CBC with differential Liver and renal function Peripheral blood smear | CBC with differential Liver and renal function Peripheral blood smear | CBC with differential Liver and renal function Peripheral blood smear | CBC with differential Liver and renal function Peripheral blood smear HIV antibody screening T-LGL flow cytometry |
| To be considered based on patient-specific history | |||
| Maternal antineutrophil antibodies | Antineutrophil antibodies Vitamin B12, folate, copper Amylase isoenzymes Fecal elastase Telomere length Chromosomal breakage ADA2 enzyme activity IgG/A/M/E T/B/NK subsets | Vitamin B12, folate, copper HIV Urine drug screen Amylase isoenzymes Fecal elastase Telomere length Chromosomal breakage ADA2 enzyme activity ANA, ESR, CRP IgG/A/M/E T/B/NK subsets | Vitamin B12, folate, copper Urine drug screen ANA, rheumatoid factor ESR, CRP Amylase isoenzymes Fecal elastase Telomere length Chromosomal breakage ADA2 enzyme activity IgG/A/M/E T/B/NK subsets |
T/B/NK, T cell/B cell/natural killer cell.
For neonates, screening for maternal IgG antineutrophil antibodies may assist in securing a diagnosis of alloimmune or isoimmune neutropenia. For infants and children, antineutrophil antibodies can be assessed to lend support to a suspected diagnosis of autoimmune neutropenia of infancy; however, the absence of such antibodies does not preclude the diagnosis. If an antibody is not detected, though, the neutropenia is often referred to as chronic idiopathic neutropenia of childhood.13 Additionally, positive antineutrophil antibodies do not guarantee the absence of other etiologies, including severe congenital neutropenia (SCN).14 Secondary to concerns regarding the poor sensitivity and specificity of available assays, the measurement of antineutrophil antibodies is not uniformly practiced by hematologists. Test performance can be improved by including both the granulocyte immunofluorescence test and the granulocyte agglutination test and by repeating the assay.15
Depending on the solicited history, screening for pancreatic insufficiency with isoamylase, fecal elastase, and a pancreatic ultrasound and/or skeletal imaging may be useful because many patients with Shwachman-Diamond syndrome (SDS) have clinically subtle pancreatic or skeletal defects.16 Other screening tests for BMF, eg, telomere length (short telomere syndromes), chromosomal breakage (Fanconi anemia), or ADA2 enzyme activity (a deficiency of ADA2), may be appropriate. Similarly, screening for underlying immune deficiency with quantitative Igs and enumeration of lymphocyte subsets and function or for autoimmune disease with an antinuclear antibody (ANA) may be of value. Evaluation with folate, vitamin B12, and copper is recommended in all older adult patients with suspected nutritional deficiencies.
For adults, ANA, rheumatoid factor, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) tests are valuable to screen for autoimmune disease. Screening for HIV and for T-cell large granular lymphocyte leukemia (T-LGL) is recommended even in patients without lymphocytosis or a history of autoimmune disease.17 Depending on the history, additional tests such as a serum protein electrophoresis or imaging may be warranted.
Regardless of age, if a satisfactory etiology of the neutropenia is not determined or other key physical anomalies or history elements prompt concern for an underlying disorder, then a bone marrow aspirate and biopsy to assess myelopoiesis and evidence of clonal evolution is warranted. Additionally, given the increased recognition of neutropenia with inborn errors of hematopoiesis and immunity and limitations in the clinical classification of disease,18 genetic sequencing is increasingly being utilized to secure a molecular diagnosis.19 Confirmation of a genetic diagnosis is valuable because it facilitates biologically rational and personally tailored prognostication, supportive care, and therapy. For patients with neutropenia, it can offer specific insights into the utility of G-CSF and the risk of transformation to malignancies and inform decisions related to donor selection and conditioning regimens for hematopoietic stem cell transplant (HSCT). In some cases, particularly in patients with neutropenia as part of an inborn error of immunity, the genetic diagnosis may identify an exploitable molecular pathway that is amenable to targeted therapy. For further information on the intersection between neutropenia and inborn errors of immunity, the readers are directed to the accompanying article “Understanding Neutropenia Secondary to Intrinsic or Iatrogenic Immune Dysregulation” by Dr. Walkovich. Patients with suspected benign causes of neutropenia should have repeat CBCs every few months to monitor for resolution of neutropenia. If the neutropenia does not resolve in an expected time frame based on the suspected mechanism, reassessment is warranted. This may include repeat genetic testing if the initial testing was conducted more than 2 years earlier to account for newly discovered inborn errors of hematopoiesis and immunity.
CLINICAL CASE (continued)
Four years later at age 20, the patient returns prior to a planned surgical procedure to remove his wisdom teeth and is concerned his neutropenia may place him at risk of infection. He is clinically doing well, but a CBC is notable for an ANC of 1000/µL and a platelet count of 90 000/µL. Given the new thrombocytopenia, a bone marrow biopsy is performed that demonstrates hypocellularity (50%) and mild myeloid and megakaryocyte dysplasia. Karyotyping reveals a small del(20q) clone but no abnormal blast population by flow cytometry. The comprehensive marrow report mentions the possibility of SDS, but there is no history of diarrhea, and an inherited neutropenia next-generation sequencing (NGS) panel is negative for pathogenic variants. Given the lack of morphological evidence of MDS or AML, it is recommended that the patient be followed closely with monthly CBCs and a repeat bone marrow in 3 months.
Author commentary: Unlike his first visit, there are several features during this evaluation concerning for a congenital neutropenia disorder. His marrow aspiration demonstrated hypocellularity and cellular dyscrasias, and his cytogenetics identified a common and likely benign clonal hematopoiesis seen in SDS. The physician recognized the association of del(20q) with SDS but assumed that a lack of steatorrhea and a negative NGS panel was sufficient to rule out SDS. However, the classic presentation of diarrhea and neutropenia is only seen in approximately 50% of SDS patients.15 It is also important to review the genes present in NGS panels to ascertain whether they incorporate suspected genes of interest. SBDS may not be included on some NGS panels secondary to the presence of a pseudogene (SBDSP1), requiring high-quality mapping and informatics or other approaches, such as Sanger sequencing, for differentiation. Additional genes (EFL1, DNAJC21, SRP54) have recently been discovered to be associated with SDS and may not yet have been added to some panels. A high suspicion of SDS should prompt other screens to detect pancreatic exocrine insufficiency, including serum pancreatic isoamylase level and fecal elastase tests, and an abdominal ultrasound to evaluate for pancreatic atrophy/fatty replacement. The physician should have ordered these tests to help determine a clinical diagnosis of SDS. The patient's marrow findings are common for SDS, but as the diagnosis is still unknown in this patient, close follow-up as prescribed by the physician is appropriate.
Therapeutic decision-making for the neutropenic patient
Following identification of the neutropenic patient and evaluation for an underlying cause, challenges arise regarding treatment decisions, monitoring for infection and/or MDS and AML, and, for some disorders, consideration of curative options, eg, HSCT. Unfortunately, for many neutropenic disorders and complications the evidence to support medical decision-making is lacking or reliant on experience gained in oncologic neutropenic patients. The development of neutropenia registries such as the Severe Chronic Neutropenia International Registry (SCNIR) and the Shwachman-Diamond Syndrome Registry has provided impactful data to close this information gap, but much guidance on clinical decision-making still depends on expert and consensus opinion. Knowing this limitation in the field, the following discussion on treating the neutropenic patient is based on evidence where available but also observes standard treatment practices in which expert consensus has been achieved and areas of ongoing management debate among hematologists.
Management of fever
Historically, fevers have been treated aggressively, even in well- looking patients, over concerns that signs of inflammation may be diminished without infiltrating neutrophils and that the risk of not treating an occult bacteremia will lead to poor outcomes. However, frequent hospital admissions and antibiotic use may lead to harmful consequences, including allergic reactions, an increase in bacterial resistance to antibiotics, hospital-acquired infections, large costs to the health care system, and a decreased quality of life for patients with chronic neutropenia. This careful balance must be weighed for each neutropenic patient who presents with fever, and the approach will differ if the patient is a known neutropenic patient or presents with newly identified neutropenia in the context of fever. Additional factors such as the possibility of neutrophil dysfunction, the duration of the neutropenia, the status of vaccinations, the presence of a central venous catheter, and any concurrent immune defects related to the underlying disorder or immunosuppressive medications must also be considered in determining the risk of bacteremia in the neutropenic patient.
Fever management in the newly diagnosed neutropenic patient
The identification of isolated neutropenia in a previously healthy patient presenting with fever is challenging, as the ability to mount neutrophil immunity to lower the risk of bacteremia is likely unknown. Several studies have examined the risk of severe bacterial infection (SBI) in previously healthy children who present with fever and neutropenia, and the majority have demonstrated these patients to be at low risk, with a similar prevalence of bacterial blood and urinary tract infections (UTIs) as nonneutropenic patients presenting with fever.20 The largest study, conducted at the Boston Children's Emergency Department, US, evaluated the outcomes of 1888 pediatric patients who presented for evaluation of unsuspected and isolated neutropenia.21 For febrile infants <3 months of age, the SBI rate was 6.2%, which was similar to the SBI rate of 7% reported in a prior study of nonneutropenic infants.22 SBI was very rare in children over 3 months of age (2.0%; 1 bacteremia, 15 UTIs, 0 meningitis), with the single case of bacteremia presenting with obvious clinical signs of septic shock in a 9-month-old with Streptococcus pneumoniae. A study in Barcelona, Spain, evaluated 190 well-appearing febrile and unsuspected neutropenia patients and demonstrated an SBI in 4 patients (2.1%; 2 UTIs, 2 pneumonias), but 101 patients (53.2%) were admitted, and 48 (47.5%) received broad-spectrum antibiotics.23 A case control study compared pediatric patients >3 months of age who presented with fever and unsuspected neutropenia with an age-matched control group without neutropenia. SBIs were identified at a higher rate in the control group (19 vs 6 patients), which may be explained by the association of viral infections with fever and neutropenia (Assaf Harofeh Medical Center, Israel).24 Two additional studies demonstrated similar low SBI rates in only 1 of 52 (1.9%) patients aged 1.5 to 36 months (Children's Hospital of Wisconsin, US) and in 0 of 51 well-appearing children but in 5 of 17 (30%) who appeared ill (Dana-Dwek Children Hospital, Israel).25,26 Higher rates of bacterial infections have been reported in studies focused on hospitalized neutropenic patients (12.7%-14.9%, with 1 study demonstrating 0%),27 which may have resulted from the admission of sicker patients and the infectious environment of the population, as brucellosis and rickettsia, bacterial infections known to be associated with neutropenia, were reported etiologies in 3 out of 4 of these inpatient-only studies.28-31
The conclusion from these studies is that the risk of bacterial infection in the outpatient setting for the well-appearing pediatric patient with isolated and unsuspected neutropenia is low and similar to patients with nonneutropenic fever. Screening for bacterial infection, including a blood culture, a urinalysis with urine culture, and, for symptomatic patients, a chest x-ray, is suggested, but aggressive management including admission and empiric antibiotics may not be necessary for all patients. Patients who are unwell, have additional cytopenias that raise concern for malignancy or BMF, have other constitutional features, have a history of infections, or have a family history of hematologic malignancies or cytopenias should enact more aggressive measures including admission for empiric antibiotics that include Pseudomonas spp. and gram-negative coverage. Similarly, adults who present with severe neutropenia (ANC <500/µL) are more likely to have serious disorders such as underlying malignancy and should be admitted for evaluation for SBIs.17 Medications associated with the suppression of myelopoiesis that are not of vital importance should also be discontinued because drug-induced agranulocytosis can lead to fatality with continued exposure.32
Fever management in the established neutropenic patient
The approach to the patient with known neutropenia is often less challenging, as the etiology of the neutropenia and the history of infectious complications may guide management. Disorders such as primary autoimmune neutropenia and chronic idiopathic neutropenia typically have adequate bone marrow storage pools of neutrophils to respond to infection and therefore present a low risk for SBIs. In a study of 43 pediatric patients with primary autoimmune or chronic idiopathic neutropenia, there were 133 emergency room encounters for fever with no patients identified as having a true bacterial infection (Texas Children's Cancer and Hematology Centers, US).33 In the Italian Neutropenia Registry, over the course of 9.5 years they identified only 1 bloodstream infection in 38 patients with autoimmune neutropenia and 2 bloodstream infections in 23 idiopathic neutropenia patients.34 For the pediatric patient with autoimmune or chronic idiopathic neutropenia, if a rise in the neutrophil count can be demonstrated during times of infection and there is no history of severe infections, evaluation by the child's primary care physician should be sufficient, with emergency room visits limited to when the patient appears unwell.35
Adults with primary autoimmune and chronic idiopathic neutropenia usually do not develop severe infections, although they may suffer from recurrent stomatitis and respiratory infections.35 This was demonstrated in a French Severe Chronic Neutropenia Registry report in which adult patients with primary autoimmune and chronic idiopathic neutropenia had low rates of SBIs at 3.85 per 100 patient-years.36 Secondary autoimmune neutropenia in both children and adults is often associated with additional cytopenias, immune defects, or immunosuppressive medications and carries a higher risk of infection. The Italian Neutropenia Registry reported SBIs in 10 of 26 (40%) patients with secondary autoimmune neutropenia, which is significantly higher than in the 11.8% of patients with primary autoimmune neutropenia, over the course of 15 years.37 Extra caution should therefore be taken in patients with secondary autoimmune neutropenia who present with fever, including consideration of fungal and other atypical infections related to additional T-cell immunodeficiency.
Disorders of impaired myelopoiesis, including SCN, will lead to an impaired ability to mount neutrophil immunity in the setting of infection. For patients with a poor response to G-CSF, an emergent evaluation for any fever is necessary secondary to the risk of bacterial sepsis. However, patients who have consistent ANCs above 1000/µL are at low risk of infection,13 and management of fever may be based on the clinical appearance of the patient, with unwell patients evaluated in the emergency room and well patients evaluated in outpatient clinics.
Indications for G-CSF
The use of G-CSF is mainly employed in patients with defective myelopoiesis and has proven to be a lifesaving intervention in patients with SCN. The SCNIR recommends a starting dose of 5 µg/kg/d for patients with SCN with escalation to 10 µg/kg/d and then an increase by increments of 10 µg/kg/d at 14-day intervals while the ANC remains below 1000/µL.38 The maintenance dose of G-CSF for SCN is variable depending on the genetic disorder as well as the specific pathogenic variant within the gene, with a median dose of 7.3 #x00B5;g/kg/d needed to achieve an ANC between 1000 and 1500/µL.39 Higher ANCs are not necessary in most patients, and maintaining the smallest effective dose avoids the consequences of higher dosing, including bone pain, splenomegaly, and theoretical higher selective pressure for hematopoietic clones more responsive to G-CSF.40 For cyclic neutropenia, the dose is much lower, typically 1 to 3 µg/kg/d given every 1 to 3 days, which shortens the duration of the neutropenic period and effectively eliminates sepsis and death from sepsis based on outcome data from the SCNIR.13 Granulocyte-macrophage-CSF treatment is ineffective in most forms of congenital neutropenia, as, unlike G-CSF, it is unable to induce nicotinamide phosphoribosyl transferase-dependent granulopoiesis.41
G-CSF use in acquired causes of neutropenia is typically guided by the patient history and is likely safe, as no definitive association with G-CSF use and cancer development has been established in this patient population.35 G-CSF treatment for primary autoimmune and chronic idiopathic neutropenia should not be determined based on the detection of antineutrophil antibodies but rather is recommended for patients with severe neutropenia with recurrent infections or stomatitis.42 As myeloid production is relatively preserved in these conditions, small doses of 0.5 to 3 µg/kg/d of G-CSF given every 1 to 3 days can be used to increase the ANC to a target maintenance of 1000 to 1500/uL.43 In the French Severe Chronic Neutropenia Registry, the use of G-CSF provided a clinical benefit in adult patients with primary autoimmune or chronic idiopathic neutropenia in 23 of 24 and 20 of 26 routinely scheduled and sporadic G-CSF cohorts, respectively.36 Intermittent dosing of G-CSF may be required in patients who present with severe infection or in the setting of surgical procedures at high risk for infection or poor wound healing.44 G-CSF is almost always given in patients with suspected drug-induced agranulocytosis, although evidence supporting this recommendation is lacking,35 and may also be used in patients with only moderate drug-induced suppression of myelopoiesis in whom continuation of the offending agent is necessary.
Role of immune suppression in autoimmune neutropenia
Immune suppression to treat primary autoimmune and chronic idiopathic neutropenia is not recommended for children due to poor response and low infectious risk and has limited success in adults.44 In the French Severe Congenital Neutropenia Registry, response to commonly used immunosuppressant agents (glucocorticosteroids, methotrexate, cyclosporine) was observed in half of the adult patients with primary autoimmune or chronic idiopathic neutropenia, but the response was mostly partial and was lost upon tapering or discontinuing the treatment.36 In the SCNIR, almost all of the 48 adult patients with autoimmune or chronic idiopathic neutropenia on immune modulation (glucocorticosteroids, gamma globulin, methotrexate, cyclosporine) discontinued this therapy without resumption once placed on G-CSF.42 Additional reports have also been published demonstrating the low response rate and high relapse rate with glucocorticosteroids, gamma globulin, cyclosporine, and rituximab.45 However, treatment with these agents may be considered if patients fail to respond to G-CSF.
In contrast, although rarely requiring treatment, secondary autoimmune neutropenia often responds to the immune suppression used to treat other autoimmune symptoms.17 LGL-associated neutropenia is often severe and requires treatment. G-CSF can increase the neutrophil count but is associated with the exacerbation of splenomegaly and the acute flare of joint symptoms while cyclophosphamide, methotrexate, and cyclosporine can be effective.46
Monitoring congenital neutropenia patients at risk for MDS/AML
Many congenital neutropenia disorders impose a risk of transformation to a myeloid malignancy (see Table 3). The mechanism of leukemogenesis in these disorders is still actively under investigation and is unique to each disorder. As knowledge of leukemogenesis in these disorders increases, the ability to predict and detect early malignant cells will hopefully be a natural consequence. Historic and current recommendations are to perform frequent (every 3-4 months) CBCs and annual bone marrow aspiration and biopsies to monitor for cytopenias and morphologic changes concerning for MDS/AML development in congenital neutropenias secondary to mutations in ELANE, HAX1, G6PC3, SBDS, WAS (X-linked neutropenia), and GATA2.43 However, data are needed to inform surveillance practices and their effect on outcomes for patients with congenital neutropenia.
Congenital neutropenia disorders at risk for MDS/AML
| Disease, gene, and inheritance pattern . | Risk of MDS/AML . | Common somatic alterations . | Additional information including nonhematologic features . |
|---|---|---|---|
| ELANE SCN ELANE, AD | 15%-20%51 ; increased incidence of MDS/AML in patients on high doses of G-CSF with poor neutrophil response.54 | CSF3R, RUNX1, ASXL1, SUZ12, EP300, monosomy 7, trisomy 21.75 | Acquired CSF3R mutations common (approximately 50%) in patients without MDS/AML.75 |
| Cyclic neutropenia ELANE, AD | AML rarely reported.76 | CSF3R, monosomy 7, trisomy 21 in the 1 patient with AML.76 | Acquired CSF3R mutations rarely identified in patients without MDS/AML.76 |
| HAX1-SCN HAX1, AR | MDS/AML reported, but incidence of leukemia not precisely reported.77 | Somatic alterations not published. | Acquired CSF3R mutations common (approximately 45%) in patients without MDS/AML.75 |
| G6PC3 deficiency G6PC3, AR | AML rarely reported.78 | T(8;21)(q22;q22) identified by karyotype in one patient.78 | Inflammatory bowel disease, congenital heart and urogenital defects, and endocrine disorders. Acquired CSF3R mutations identified in patients without MDS/AML.75 |
| X-linked neutropenia WAS GOF, XL | MDS/AML rarely reported.79 | Somatic alterations not published. | Acquired CSF3R mutations identified in patients without MDS/AML.75 |
| Glycogen storage disease type 1b G6PT1, AR | 4% with MDS/AML in the SCNIR.80 | Monosomy 7. Short telomeres also reported.81 | Hypoglycemia, hepatomegaly, and enterocolitis. |
| Poikiloderma with neutropenia USB1, AR | MDS/AML reported, but incidence of leukemia not precisely reported.82 Myelodysplastic changes in most patients. | Somatic alterations not published. | Poikiloderma, nail dystrophy, and palmar/plantar hyperkeratosis. |
| SDS SBDS, AR | 20%-30%83,84 ; patients also at risk of BMF.16 | Biallelic TP53, RUNX1, SETBP1, BRAF, NRAS, ETNK1, alterations in chromosomes 3, 5, 7, and 20.85 | Pancreatic exocrine insufficiency, skeletal abnormalities. Somatic alterations not associated with MDS/AML include i(7)(q10), del(20q), and EIF6 mutations. |
| GATA2 haploinsufficiency GATA2, AD | 80%86 ; occurrence typically in later adolescence and early adulthood and may be presenting feature. | Monosomy 7, trisomy 8, ASXL1, der(1;7)(q10;p10).87 | Warts, lymphedema, panniculitis, deafness, infection, and pulmonary alveolar proteinosis. |
| SAMD9/SAMD9L GOF disorder SAMD9 or SAMD9L, AD | Risk of MDS and AML unknown. Patients also at risk of BMF that may spontaneously resolve.88 | Monosomy 7, del(7q), RUNX1, SETBP1, ETV6.89 | SAMD9: adrenal hypoplasia, growth restriction, genital abnormalities, enteropathy, infections. SAMD9L: neurologic symptoms, infections. |
| Disease, gene, and inheritance pattern . | Risk of MDS/AML . | Common somatic alterations . | Additional information including nonhematologic features . |
|---|---|---|---|
| ELANE SCN ELANE, AD | 15%-20%51 ; increased incidence of MDS/AML in patients on high doses of G-CSF with poor neutrophil response.54 | CSF3R, RUNX1, ASXL1, SUZ12, EP300, monosomy 7, trisomy 21.75 | Acquired CSF3R mutations common (approximately 50%) in patients without MDS/AML.75 |
| Cyclic neutropenia ELANE, AD | AML rarely reported.76 | CSF3R, monosomy 7, trisomy 21 in the 1 patient with AML.76 | Acquired CSF3R mutations rarely identified in patients without MDS/AML.76 |
| HAX1-SCN HAX1, AR | MDS/AML reported, but incidence of leukemia not precisely reported.77 | Somatic alterations not published. | Acquired CSF3R mutations common (approximately 45%) in patients without MDS/AML.75 |
| G6PC3 deficiency G6PC3, AR | AML rarely reported.78 | T(8;21)(q22;q22) identified by karyotype in one patient.78 | Inflammatory bowel disease, congenital heart and urogenital defects, and endocrine disorders. Acquired CSF3R mutations identified in patients without MDS/AML.75 |
| X-linked neutropenia WAS GOF, XL | MDS/AML rarely reported.79 | Somatic alterations not published. | Acquired CSF3R mutations identified in patients without MDS/AML.75 |
| Glycogen storage disease type 1b G6PT1, AR | 4% with MDS/AML in the SCNIR.80 | Monosomy 7. Short telomeres also reported.81 | Hypoglycemia, hepatomegaly, and enterocolitis. |
| Poikiloderma with neutropenia USB1, AR | MDS/AML reported, but incidence of leukemia not precisely reported.82 Myelodysplastic changes in most patients. | Somatic alterations not published. | Poikiloderma, nail dystrophy, and palmar/plantar hyperkeratosis. |
| SDS SBDS, AR | 20%-30%83,84 ; patients also at risk of BMF.16 | Biallelic TP53, RUNX1, SETBP1, BRAF, NRAS, ETNK1, alterations in chromosomes 3, 5, 7, and 20.85 | Pancreatic exocrine insufficiency, skeletal abnormalities. Somatic alterations not associated with MDS/AML include i(7)(q10), del(20q), and EIF6 mutations. |
| GATA2 haploinsufficiency GATA2, AD | 80%86 ; occurrence typically in later adolescence and early adulthood and may be presenting feature. | Monosomy 7, trisomy 8, ASXL1, der(1;7)(q10;p10).87 | Warts, lymphedema, panniculitis, deafness, infection, and pulmonary alveolar proteinosis. |
| SAMD9/SAMD9L GOF disorder SAMD9 or SAMD9L, AD | Risk of MDS and AML unknown. Patients also at risk of BMF that may spontaneously resolve.88 | Monosomy 7, del(7q), RUNX1, SETBP1, ETV6.89 | SAMD9: adrenal hypoplasia, growth restriction, genital abnormalities, enteropathy, infections. SAMD9L: neurologic symptoms, infections. |
AD, autosomal dominant; AR, autosomal recessive, GOF, gain of function; XL, X-linked.
CLINICAL CASE (Continued)
The patient undergoes CBCs every 3 to 4 months and 2 surveillance bone marrow examinations over the following year. His CBCs continue to show mild neutropenia and thrombocytopenia, and his bone marrow results are consistent with findings from his first examination. At the age of 22, he undergoes another bone marrow biopsy, which demonstrates an i(7)(q10) clone on cytogenetics and a TP53 pathogenic variant in a somatic NGS panel. There continues to be no morphologic or flow cytometric evidence of increasing dysplasia or an abnormal blast count. Genetic testing is revisited, and Sanger sequencing of the SBDS locus identifies 2 pathogenic mutations independently inherited from each parent. The presence of a chromosome 7 abnormality and a new TP53 clonal hematopoiesis raises the concern for a malignancy, and the patient is referred for HSCT consultation.
Author commentary: The physician was able to diagnose SDS in this patient with genetic testing, but this case illustrates the complexity of clinical sequencing in that NGS technology has several shortcomings, including the inability to differentiate genes from pseudogenes without excellent mapping and analytics and to identify deep intronic variants. In most clinical scenarios, chromosome 7 aberrations raise the concern for MDS/AML. But similar to del(20q), i(7)(q10) is a somatic change that does not have an impact on tumor surveillance machinery. TP53 mutations increase cellular fitness in SDS, but at the expense of decreased tumor surveillance, and likely increase the risk of MDS/AML within the clone. However, TP53 clonal hematopoiesis is common in SDS without malignancy, and identification by itself is not an indication for HSCT. After discussions with experts in SDS, a decision is made for continued close monitoring that includes CBCs every 3 to 4 months and annual bone marrow monitoring only.
Curative approach with HSCT for congenital neutropenia
Absolute and possible indications to proceed to transplant depend on the underlying genetic condition, but common causes to proceed to HSCT include poor response to G-CSF, recurrent and severe infections, BMF, and transformation to a myeloid malignancy. The approach to HSCT is not uniform within or between genetic disorders, but each transplant method shares unique obstacles to success in neutropenic patients. The risk of bacterial and fungal infection is higher early in transplant due to preceding neutropenia that rapidly declines with the discontinuation of G-CSF. Evaluation for occult infection pretransplant with sinus, chest, abdomen, and pelvis imaging is employed, with more invasive measures such as bronchoscopy or biopsy indicated for areas concerning for infection. Bacterial and mold prophylaxis at the start of conditioning therapy is initiated to mitigate the very early neutropenia in these patients.
Chimerism analysis to differentiate between residual and engrafted donor hematopoiesis is important for all HSCTs but has special considerations in congenital neutropenia. The measurement of hematopoietic progenitor cell (HPC) chimerism is difficult in the clinical setting, and typically, the measurement of mature myeloid cells in the peripheral blood is used as a surrogate for HPC engraftment. However, myeloid markers, such as CD33, that are present on neutrophils will not be an accurate proxy for HPC chimerism because recipient HPCs will have a significant disadvantage compared to donor HPCs in contributing to the peripheral blood neutrophil pool. Therefore, markers weakly expressed or absent on neutrophils, such as CD14 (monocytes), CD56 (natural killer cells), CD19 (B cells), CD34 (HPCs), and CD3 (T cells), should be included in measuring peripheral blood chimerism, particulalry in disorders such as GATA2 haploinsufficiency, which affect multiple cell lineages.
The impact of partial chimerism and long-term outcomes for patients with congenital neutropenia is poorly understood, in large part because myeloablative regimens have dominated early transplant approaches, and literature reports have not routinely focused on this transplant result or relied on neutrophil-specific markers for chimerism reporting. Mixed chimerism has been shown to cure the neutropenia in congenital neutropenia patients.47 Although the percentage required to eliminate the risk of bacterial infection and G-CSF independence is uncertain, from anecdotal reports and experience it is likely 20% to 30% HPC donor chimerism.47
The more difficult question is whether the persistence of recipient hematopoiesis following exposure to chemotherapy will continue to present a risk for myeloid malignancies post transplant. Our current understanding of leukemogenesis in each disorder provides theoretical hypotheses, but prolonged follow-up in patients with mixed chimerism will be required to answer this question definitively. In patients with ELANE SCN, a marrow microenvironment with high levels of G-CSF provides a competitive setting to select out clones with enhanced response to G-CSF. However, in the posttransplant setting with ablation of the neutropenia defect by donor HPCs, the selective environment driven by high levels of G-CSF is eliminated. If this postulate is correct, the risk of malignant transformation should also be minimized, if not abolished, with donor hematopoiesis. In patients with SDS, the leukemogenesis hypothesis is less reliant on external pressures for hematopoiesis but dependent on individual cell fitness through relieving ribosomal stress through TP53 mutations. Whether the reduction of replicative and inflammatory stress through donor hematopoiesis is sufficient to prevent leukemogenesis in recipient stem cells under ribosomal stress needs further study. As leukemia may not be a common event in posttransplant patients, research evaluating clonal dynamics with a particular focus on CSF3R in SCN and TP53 in SDS will be incredibly valuable to understanding hematopoiesis dynamics in the mixed-chimera patient.
Graft rejection for both malignant and nonmalignant indications in congenital neutropenia is relatively high compared to other comparable conditions. Several hypotheses have been generated to explain this phenomenon, including competent lymphocyte immunity preceding conditioning therapy, an inflammatory microenvironment induced from recurrent infection, alterations in the bone marrow niche secondary to the underlying defect or high exposure to G-CSF,48 and prior exposure to foreign blood products in patients with BMF. Serotherapy is almost standard today for nonmalignant transplant to help address concerns for increased alloreactivity, but questions regarding the marrow niche are more difficult and will require continued research in this area, particularly if pre- and posttransplant marrow aspiration samples are available for study.
Congenital neutropenia patients who progress to malignancy present the most challenging and most controversial scenario for clinicians. First, the diagnosis of transformation is not straightforward because differentiating hematopoietic dyscrasias present in many inborn errors of hematopoiesis from dysplastic clones can be difficult even for experienced hematopathologists. This was illustrated in a study of SDS patients with MDS/AML who underwent a central pathologic review that showed a 56% discrepancy from the original pathology report.49 Clonal hematopoiesis is also common in congenital neutropenia, and some of these changes are not believed to be inducers of leukemogenic changes. Knowledge of pathologic clonal hematopoiesis is key in interpreting these findings.
Second, cytoreductive chemotherapy to reduce blast counts, as is typically done for de novo AML, can be incredibly toxic to congenital neutropenia patients who have difficulty recovering neutrophils for infection eradication and tissue repair or who have underlying organ dysfunction from the underlying genetic defect or recurrent infections.50 As such, recent approaches often eliminate cytoreductive therapy completely or have adopted regimens used in medically fragile patients to bridge the patient while awaiting identification of a suitable bone marrow donor. Driving blast production with G-CSF has been demonstrated in SCN, and judicious use following chemotherapy is warranted. The admission of all patients following chemotherapy to monitor for infection and institution of G-CSF for evidence of severe bacterial or fungal disease is a common approach to balance these risks. G-CSF is not recommended post HSCT for patients with SCN and malignancy without severe infection, but whether G-CSF use following transplant increases the risk of relapse is unknown.
HSCT outcomes for SCN
HSCT for SCN is conducted for G-CSF refractoriness or the development of MDS/AML. Patients with ELANE mutations at high risk of progression to AML, including p.G214R, p.C151Y, and p.C223ter, may be considered for preemptive HSCT, but data to inform the risks and benefits of this practice are currently lacking.51,52 Some groups also consider high-dose G-CSF (>15 µg/kg/d) as an indication to offer transplant because patients on higher G-CSF doses with decreased ANC response have shown an increased frequency of leukemia development.53,54 This approach has not been universally adopted secondary to the desire to avoid HSCT exposure to the majority of patients on high-dose G-CSF who will not develop malignancy.
Most literature on HSCT for SCN is based on case reports and small case series that have demonstrated good outcomes in patients without malignancy (overall survival [OS], approximately 90%; event-free survival [EFS], approximately 75%) despite high rates of graft failure (approximately 20%).55 Historical outcomes with malignancy have been less successful, with an OS of approximately 45% and an EFS of approximately 40%, but many cases were transplanted prior to the adoption of modern practices to avoid intensive cytoreductive therapy prior to transplant. A larger report of 136 SCN patients from the European Society for Blood and Marrow Transplantation demonstrated a 3-year OS and EFS of 87% and 77% for patients without malignancy and 79% and 64% with malignancy.56 Graft rejection was 10% for the entire cohort, but relapse was only seen in 2 of 14 (14%) patients. An American Society of Hematology abstract from the European branch of the SCNIR reported outcomes on 51 patients after the 2001 implementation of guidelines to avoid induction chemotherapy and demonstrated an OS of 77.8% and 83.3% in SCN patients treated with and without malignancy.57 Despite the fact that not all patients with SCN and secondary malignancy proceed to HSCT in remission, the reported relapse rates are much lower in comparison to de novo MDS/AML (10%-15% vs 25%-40% for MDS and 40%-50% for AML).55,56,58-60 The reason for reduced relapsed rates may be secondary to the growth restraint on malignant cells afforded by the germline defect, a feature reflected in some case reports of SCN patients with “indolent” AML.61 Although reported OS between myeloablation (MA) and reduced intensity conditioning (RIC) is similar in SCN patients, myeloablative (MA) conditioning is recommended for patients with underlying malignancy for elimination of the malignant clone.
HSCT outcomes for SDS
HSCT for SDS is conducted in patients with BMF or hematologic malignancy. Unlike some patients with SCN, the consensus is to not perform HSCT preemptively in this disorder. Early experience in HSCT demonstrated high nonrelapse transplant mortality, as shown in a recent long-term outcome study reporting that 21% of patients died from toxicity.62 This realization prompted many clinicians to use RIC for all SDS patients with BMF.63,64 In a study by the Center for International Blood and Marrow Transplant Research, early deaths were seen in 4 of 13 (31%) SDS BMF patients receiving a MA conditioning regimen compared to 4 of 26 (15%) early deaths in patients receiving RIC.65 This report showed a 5-year OS of 72% in patients with BMF and graft rejection in 3 of 39 patients (8%). The largest study to date published by the Severe Aplastic Anemia Working Party of the European Society for Blood and Marrow Transplantation on 61 patients (1988-2016) with BMF demonstrated a 5-year OS of 70.7% with a graft failure rate of 13%.62 OS with RIC and MA was identical, but the segregation of conditioning therapy by indication (BMF vs leukemia) was not provided, and the authors concluded that MA regimens should be reserved for malignant transformation only.
Transplant survival for SDS patients who develop malignancy are abysmal across literature reports. A multicenter study focused on malignant transplant outcomes demonstrated a 3-year OS of 51% and 11% for patients with MDS and AML, respectively.49 This result is in contrast to recent SCN outcomes for malignancy, indicating a higher degree of resistance to transplant, perhaps in part related to increased chemotherapy resistance from mutated TP53, as seen in other cancers.66 The importance of surveillance was suggested by an improved 3-year OS of 62% vs 28% in patients who had regular bone marrow evaluation, but regular CBCs had poor sensitivity in identifying marrow disease.49 Although early detection may provide an incremental improvement in survival, novel approaches to avoid excessive toxicity from conditioning therapy while preventing relapse are clearly needed for SDS patients who develop malignancy.
Gene therapy and other potential treatment modalities for congenital neutropenia
As our understanding of the mechanism of neutropenia and leukemia formation evolves, new therapeutic modalities have been tested in preclinical models with a primary focus on increasing neutrophil maturation and production. Investigative therapies include nicotinamide to increase the expression of G-CSF and G-CSF receptors in SCN,67,68 neutrophil elastase inhibitors to prevent the unfolded protein response from mutated ELANE,69,70 and gene editing to repair mutated ELANE or reduce translation of the ELANE gene.71-73 Nicotinamide has been used in a small clinical trial with positive results, but other modalities have only reported results in the preclinical setting. The use of small- molecule inhibitors to promote nonsense suppression in patients with termination SBDS mutations (c.183-184TA > TC) has also demonstrated promise in reducing apoptosis and increasing neutrophil maturation in bone marrow progenitor cells.74 Inhibition of EIF6, a protein removed by SBDS to advance ribosome formation, is also an attractive therapeutic target for SDS treatment but still requires preclinical investigation.
Summary
The diagnosis and management of the neutropenia patient are significantly aided by classifying disorders into the presence or absence of adequate bone marrow reserves. Patients with inefficient myelopoiesis have a lower threshold to initiate empiric treatment for fever and may require monitoring for transformation into a myeloid malignancy. Intact myelopoiesis patients with adequate marrow neutrophil reserves are at much lower risk of these complications despite a low peripheral blood ANC. G-CSF is commonly used for poor neutrophil production and may provide a benefit in some scenarios of competent neutrophil production. HSCT offers an opportunity for cure in congenital neutropenias, but questions regarding the indications, the importance of posttransplant chimerism, and the techniques to reduce toxicity and malignant relapse in SDS limit the optimization of patient selection, conditioning approaches, and outcomes. Gene editing may provide an alternative option to HSCT to cure the neutropenia, but the analogous question to HSCT chimerism of residual uncorrected hematopoietic stem and progenitor cells resulting in continued cancer risk is unknown. Continued collaborative research in clinical registries and basic science investigation are necessary to answer these questions and provide additional evidence for important clinical decisions for neutropenic patients.
Conflict-of-interest disclosure
James A. Connelly: advisory board member: X4 Pharmaceuticals, Horizon Therapeutics.
Kelly Walkovich: consultancy: Swedish Orphan Biovitrum AB; advisory board member: Horizon Therapeutics, Pharming; local principal investigator: clinical trial on mavorixafor sponsored by X4 Pharmaceuticals.
Off-label drug use
James A. Connelly: nothing to disclose.
Kelly Walkovich: nothing to disclose.