Abstract
Anthracycline chemotherapy remains an integral component of modern pediatric acute myeloid leukemia (AML) regimens and is often delivered at high doses to maximize cancer survival. Unfortunately, high-dose anthracyclines are associated with a significant risk of cardiotoxicity, which may result in early and/or long-term left ventricular systolic dysfunction and heart failure. Moreover, the development of cardiotoxicity during pediatric AML therapy is associated with lower event-free and overall survival, which may be partially attributable to incomplete anthracycline delivery. A combined strategy of primary cardioprotection and close cardiac monitoring can maximize chemotherapy delivery while reducing the toxicity of intensive AML therapy. Primary cardioprotection using dexrazoxane reduces short-term cardiotoxicity without compromising cancer survival. Liposomal anthracycline formulations, which are under active investigation, have the potential to mitigate cardiotoxicity while also improving antitumor efficacy. Primary cardioprotective strategies may reduce but not eliminate the risk of cardiotoxicity; therefore, close cardiac monitoring is also needed. Standard cardiac monitoring consists of serial echocardiographic assessments for left ventricular ejection fraction decline. Global longitudinal strain has prognostic utility in cancer therapy-related cardiotoxicity and may be used as an adjunct assessment. Additional cardioprotective measures should be considered in response to significant cardiotoxicity; these include cardiac remodeling medications to support cardiac recovery and anthracycline dose interruption and/or regimen modifications. However, the withholding of anthracyclines should be limited to avoid compromising cancer survival. A careful approach to cardioprotection during AML therapy is critical to maximize the efficacy of leukemia treatment while minimizing the short- and long-term risks of cardiotoxicity.
Learning Objectives
Recognize the impact of anthracycline-associated cardiotoxicity on cardiac and leukemia outcomes in the treatment of pediatric acute myeloid leukemia
Understand the role of primary cardioprotection during anthracycline-containing chemotherapy for pediatric acute myeloid leukemia
Understand the strengths and limitations of cardiac imaging modalities and the role of echocardiography during pediatric acute myeloid leukemia therapy
CLINICAL CASE
A 15-year-old girl was diagnosed with acute myeloid leukemia (AML) after presenting with fatigue, menorrhagia, and fever. Cytogenetic analyses demonstrated a t(9;11) KMT2A-MLLT3 fusion. The patient commenced induction therapy with cytarabine, daunorubicin with dexrazoxane, and gemtuzumab ozogamicin. After induction, there was no evidence of residual leukemia, and she was stratified to low-risk AML treatment per the standard arm of the Children's Oncology Group (COG) trial AAML1831 (NCT04293562) with planned receipt of 5 chemotherapy cycles including 300 mg/m2 daunorubicin and 48 mg/m2 mitoxantrone. On day 14 of intensification 1 (following 300 mg/m2 daunorubicin), she was admitted to the intensive care unit with klebsiella septic shock. After pressor initiation for fluid-refractory hypotension, echocardiography demonstrated a significant decline in left ventricular ejection fraction (LVEF) from 60% at baseline to 35%.
Introduction
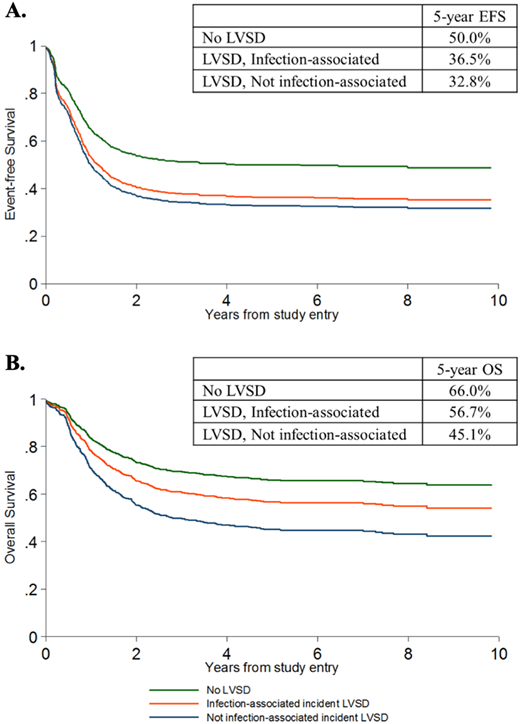
While clinical outcomes of pediatric AML have improved over time with intensification of therapy, the risk of leukemia recurrence and therapy-related morbidity and mortality remains high.1 Anthracyclines are critical for optimal survival,2 and delivered doses in AML subsets treated without consolidative transplant may exceed 600 mg/m2 of doxorubicin equivalents after accounting for the recently recognized 10:1 cardiotoxic dose equivalence of mitoxantrone in comparison with doxorubicin.3 As a result, anthracycline-associated cardiotoxicity is common, with late cardiomyopathy rates of up to 27%.4 Early LV systolic dysfunction (LVSD) during AML therapy is also common and has important implications for cardiovascular outcomes and survival. The incidence and impact of early LVSD were evaluated in 2 recent randomized phase 3 COG trials in de novo pediatric AML.5,6 In AAML0531, 12% of children with non-FLT3-mutant AML developed grade 2 + LVSD during or following therapy (n = 1022; 5-year follow-up).6 In AAML1031, with more comprehensive ascertainment due to mandated site reporting of cardiac functional parameters, the rate of grade 2 + LVSD was 21% (n = 1014; median 3.5-year follow-up).5 In further analysis of AAML0531, those who developed LVSD on therapy were much more likely to have persistent or recurrent LVSD during off-protocol follow-up (hazard ratio [HR], 12.1; 95% CI, 4.2-34.8). Moreover, on-therapy grade 2 + LVSD was associated with a >15% reduction in 5-year event-free and overall survival (Figure 1).6 Given that this protocol required discontinuation of anthracyclines after the development of LVSD, inferior survival was likely driven in part by incomplete anthracycline delivery compromising chemotherapeutic efficacy. Thus, developing an effective strategy to reduce the cardiotoxicity of AML therapy while maintaining leukemia-free survival is critical. A combined approach of primary cardioprotection and close cardiac monitoring to guide toxicity-responsive cardioprotective measures during chemotherapy can help balance the somewhat competing goals of maximal therapy delivery and cardiotoxicity reduction to optimize both leukemia and cardiovascular outcomes.
Event-free (A) and Overall (B) survival according to the occurrence of Grade 2 + LVSD, in the presence or absence of associated bloodstream infection, on COG trial AAML0531. Grade 2 + LVSD was defined as LVEF <50% or LVFS <24% based on Common Terminology Criteria for Adverse Events V3.0. Adapted with permission from Getz et al.6
Event-free (A) and Overall (B) survival according to the occurrence of Grade 2 + LVSD, in the presence or absence of associated bloodstream infection, on COG trial AAML0531. Grade 2 + LVSD was defined as LVEF <50% or LVFS <24% based on Common Terminology Criteria for Adverse Events V3.0. Adapted with permission from Getz et al.6
Primary cardioprotection
Primary cardioprotection involves the concurrent use of cardioprotective medications during anthracycline therapy or the use of less cardiotoxic agents to reduce cardiotoxicity risk. Two important primary cardioprotective strategies pertinent to pediatric AML therapy are dexrazoxane and liposomal anthracyclines.
Dexrazoxane
Dexrazoxane is the only US Food and Drug Administration-approved drug for preventing anthracycline-induced cardiotoxicity. Its approval is restricted to metastatic breast cancer patients receiving >300 mg/m2 of doxorubicin based on data across multiple randomized phase 3 trials demonstrating lower rates of cardiac events and/or heart failure with concurrent dexrazoxane.7 In 2014 dexrazoxane was designated as an orphan drug for the “prevention of cardiomyopathy for children and adolescents 0 through 16 years of age treated with anthracyclines,” allowing for its use in pediatric AML. Dexrazoxane mitigates anthracycline-induced cardiomyocyte injury by stimulating the selective degradation of topoisomerase IIβ, a molecular target of anthracyclines, thereby reducing DNA damage and oxidative stress.8 Dexrazoxane is administered as a bolus infusion over 15 minutes immediately prior to anthracycline administration at a dose ratio of 10:1 with doxorubicin.9 Dosing with alternative anthracycline agents has historically been based on their cardiotoxicity dose equivalence ratio to doxorubicin, wherein dexrazoxane is dosed at 5-10:1 with daunorubicin, 40:1 with mitoxantrone, and 50:1 with idarubicin.10 However, given evolving dose equivalence ratios as new data sets emerge,3 dexrazoxane dosing should also take into consideration demonstrated pharmacokinetics, safety, and efficacy. Studies of dexrazoxane in combination with agents commonly used in AML (ie, daunorubicin, mitoxantrone, idarubicin) have shown safety and/or efficacy in dose ranges/ratios similar to those proposed above.7,10
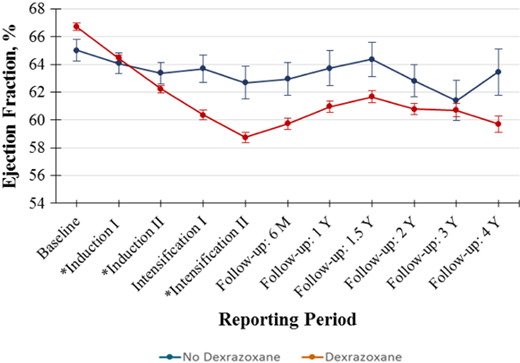
Dexrazoxane has been shown to significantly reduce short-term LVSD in anthracycline-treated children with acute lymphoblastic leukemia, non-Hodgkin lymphoma, and Ewing sarcoma.11-13 Evaluations of dexrazoxane use in pediatric AML, though promising, have historically been limited to small single-institution studies.14,15 More recently, the short-term cardioprotective effects of dexrazoxane were analyzed in the COG trial for de novo AML, AAML1031.5 Dexrazoxane use was nonrandomized and determined by institutional practice; 96 (9.5%) patients received dexrazoxane and 918 (90.5%) did not. The 4-year risk for grade 2 + LVSD was 45% lower with dexrazoxane than without (26.5% vs 42.2%; HR = 0.55; 95% CI, 0.36-0.86; P = .009), with greater reductions in risk for higher-grade cardiotoxicity. While dexrazoxane did not provide complete protection against LVSD, its use was associated with smaller declines in LVEF (Figure 2), less worsening over time, and a trend toward greater likelihood of LVSD resolution. Although labeling warns that dexrazoxane may increase the myelosuppressive effects of chemotherapy, durations of neutropenia, intensive care unit requirements, and rates of mucositis and bloodstream infection did not differ with dexrazoxane exposure. Additionally, 5-year event-free (49.0% vs 45.1%; P = .534) and overall survival estimates (65.0% vs 61.9%; P = .613) were comparable between those who did and did not receive dexrazoxane. However, significantly lower treatment-related mortality with dexrazoxane (4.6% vs 12.6%; P = .024) suggests that it may prevent acute cardiac events that contribute to early patient deaths.
Temporal trends in LVEF by dexrazoxane exposure. Standard errors of the mean LVEF measurements are presented as vertical bars. The (*) denotes treatment courses that include an anthracycline. Adapted with permission from Getz et al.5
Temporal trends in LVEF by dexrazoxane exposure. Standard errors of the mean LVEF measurements are presented as vertical bars. The (*) denotes treatment courses that include an anthracycline. Adapted with permission from Getz et al.5
Despite compelling evidence of an early cardioprotective benefit, <10% of AML patients receive dexrazoxane, likely due to early concerns for increased secondary malignancy risk.5 This association was initially suggested in an analysis of two Hodgkin lymphoma trials that included the randomized administration of dexrazoxane in the context of moderate-dose anthracycline, etoposide, and alkylator therapy, P9425 (n = 216) and P9426 (n = 255).16 However, in subsequent analyses of these trials and the COG T-cell acute lymphoblastic leukemia/lymphoma trial P9404 (n = 537) with longer follow-up (median, 12.6 years),17 dexrazoxane did not significantly increase the 10-year relapse risk (16.1% with dexrazoxane vs 19.1% without; HR = 0.81; 95% CI, 0.60-1.08), overall mortality (12.8% vs 12.2%; HR = 1.03; 95% CI, 0.73-1.45), or deaths due to secondary cancers (2.0% vs 1.6%; HR = 1.24; 95% CI, 0.49-3.15; Figure 3). Moreover, these findings remained similar in long-term analyses of the individual Hodgkin lymphoma trials. Likewise, secondary malignancies were rare over the follow-up on AAML1031 (n = 4), with only one occurring after dexrazoxane therapy.5
Cumulative incidence of relapse and overall mortality in the combined COG randomized trials of DRZ. Cumulative incidences at 10 years were not significantly different by DRZ status for either outcome. DRZ, dexrazoxane; DRZ+, exposed to DRZ; DRZ−, not exposed to DRZ. Adapted with permission from Chow et al.17
Cumulative incidence of relapse and overall mortality in the combined COG randomized trials of DRZ. Cumulative incidences at 10 years were not significantly different by DRZ status for either outcome. DRZ, dexrazoxane; DRZ+, exposed to DRZ; DRZ−, not exposed to DRZ. Adapted with permission from Chow et al.17
Overall, sufficient data exist to support the use of dexrazoxane in children with AML to mitigate short-term LVSD and improve the potential for target anthracycline dose delivery. It should be noted that dexrazoxane reduces but does not eliminate short-term cardiotoxicity. In addition, further research is needed to assess noncardiac toxicities such as typhlitis rates, which were qualitatively higher in Hodgkin lymphoma patients receiving dexrazoxane in P9425 (8.4% vs 2.8%) and to determine the long-term effects of dexrazoxane on cardiovascular morbidity and mortality.18
Liposomal anthracyclines
Liposomal delivery systems for anthracyclines are a promising strategy to mitigate cardiotoxicity while enhancing antitumor efficacy.19-21 Liposomal drug encapsulation reduces its penetrance into the tight capillary junctions of the heart while allowing sustained systemic circulation and preferential accumulation in the tumor.22 Meta-analyses of adult trials comparing liposomal doxorubicin to free drug demonstrate a >50% reduction in clinical and subclinical cardiac dysfunction with liposomal formulations.23,24 Intensification of chemotherapy with liposomal encapsulated daunorubicin, DaunoXome® (DNX), has proven effective and tolerable in the context of pediatric AML studies conducted by the International Berlin-Frankfurt-Münster Study Group. In the relapse setting, DNX plus fludarabine and cytarabine (FLAG) demonstrated enhanced leukemic efficacy and comparable cardiotoxicity compared to FLAG alone.25 Children with de novo AML experienced similar rates of cardiotoxicity and less overall treatment-related toxicity with DNX, given at a higher than equivalent dose vs idarubicin, during induction.26 DNX is no longer being manufactured and thus is not available for use in AML.
CPX-351 is a liposomal formulation of daunorubicin plus cytarabine recently approved by the US Food and Drug Administration in adults and children with therapy-related AML or AML/myelodysplastic syndrome, given its favorable efficacy and tolerable safety profile.27,28 The cardioprotective effects of CPX-351 are not established; however, clinical cardiotoxicity has been described in early trials. A randomized study of CPX-351 vs daunorubicin and cytarabine in 309 older adults with secondary AML demonstrated similar rates of cardiotoxicity despite nearly double the rate of postremission consolidative anthracycline delivery on the CPX-351 arm.28 In 38 heavily anthracycline pretreated children with relapsed AML who received CPX-351 followed by FLAG on the COG phase 1/2 study, grade 2 + LVSD occurred in 7 (18%).27 There are a paucity of data to inform how this compares with traditional anthracycline or nonanthracycline salvage regimens. CPX-351 is currently being studied in the up-front setting by COG in a phase 3 randomized study in de novo AML (NCT04293562). If found to mitigate cardiotoxicity while enhancing or even maintaining leukemia-free survival, liposomal anthracyclines could substantially improve therapeutic options for AML.
Cardiac monitoring and toxicity-responsive cardioprotection
While primary cardioprotection may reduce the risk for cardiotoxicity, the above data demonstrate that these approaches have not eliminated cardiac risk. Thus, close monitoring of cardiac function during and after AML therapy remains critical to clinical management and is used to guide toxicity-responsive cardioprotective interventions.
Cardiac monitoring
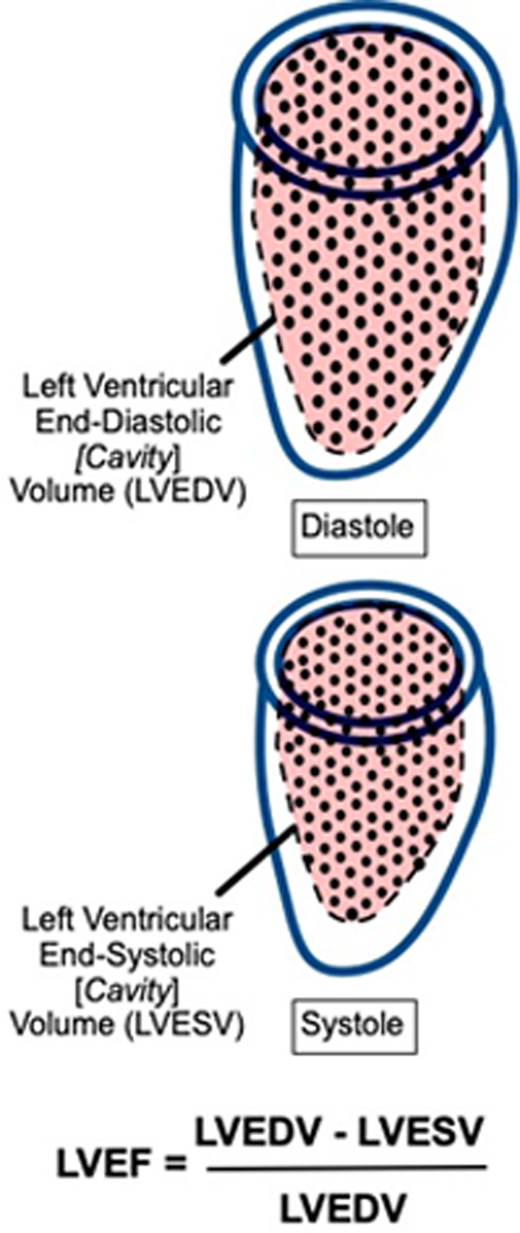
Cardiac monitoring during pediatric AML therapy typically consists of serial precycle echocardiography to assess for LVSD, manifested as declines in LV fractional shortening (LVFS), LVEF, and/or global longitudinal strain (GLS). Each of these methods has strengths and limitations that are relevant to clinical management (Table 1).
Indices used to assess for LVSD
| Measure . | Strengths . | Limitations . | Threshold for significant LVSD . | Recommendation for use . | |
|---|---|---|---|---|---|
| LVFS | |||||
| • Long historical precedent • Easy to acquire and measure | • Highly angle dependent • Poor reliability (~7% error when in the normal range)30 | <24%a | Adjunct measure only except in the setting of inadequate windows for alternate measures | ||
| LVEF | |||||
| Teichholz formula (from linear dimensions) | • Easy to acquire and measure | • Significant geometric assumptions • Inaccurate | <50%a | Not recommended | |
| 2D echo (5/6 area length or biplane Simpson's method) | • Standard across most laboratories • Established utility in heart failure | • Geometric assumptions • Measurement error • Only ~50% sensitivity to detect LVSD in comparison with CMRI (in survivor data) | May be used as primary screening | ||
| 3D echo | • Better reproducibility • 53%–68% sensitivity to detect LVSD in comparison with CMRI (in survivor data) | • Requires specialized training and equipment • Limited data in younger children | Preferred for primary screening in adolescents and young adults, when available | ||
| CMRI | • Optimal LVEF reproducibility and accuracy • Can measure other potentially important cardiac features (eg, precise measurement of mass; edema, fibrosis) | • Requires specialized training and equipment • Less availableb • Compliance challenges in younger childrenb • An improvement in outcomes has not been demonstrated in adult cardio-oncology populations • Limited pediatric cardio-oncology data | Secondary evaluation as indicatedb | ||
| GLS | |||||
| • More sensitive measure of cardiac function • Prognostic of subsequent LVEF declines37 | • An improvement in outcomes has not been demonstrated in adult cardio-oncology populations39 • Limited pediatric cardio-oncology data | >−16% (approximate)42 or ≥15% worsening from baseline valuec | Adjunct measure only (further study needed) | ||
| Measure . | Strengths . | Limitations . | Threshold for significant LVSD . | Recommendation for use . | |
|---|---|---|---|---|---|
| LVFS | |||||
| • Long historical precedent • Easy to acquire and measure | • Highly angle dependent • Poor reliability (~7% error when in the normal range)30 | <24%a | Adjunct measure only except in the setting of inadequate windows for alternate measures | ||
| LVEF | |||||
| Teichholz formula (from linear dimensions) | • Easy to acquire and measure | • Significant geometric assumptions • Inaccurate | <50%a | Not recommended | |
| 2D echo (5/6 area length or biplane Simpson's method) | • Standard across most laboratories • Established utility in heart failure | • Geometric assumptions • Measurement error • Only ~50% sensitivity to detect LVSD in comparison with CMRI (in survivor data) | May be used as primary screening | ||
| 3D echo | • Better reproducibility • 53%–68% sensitivity to detect LVSD in comparison with CMRI (in survivor data) | • Requires specialized training and equipment • Limited data in younger children | Preferred for primary screening in adolescents and young adults, when available | ||
| CMRI | • Optimal LVEF reproducibility and accuracy • Can measure other potentially important cardiac features (eg, precise measurement of mass; edema, fibrosis) | • Requires specialized training and equipment • Less availableb • Compliance challenges in younger childrenb • An improvement in outcomes has not been demonstrated in adult cardio-oncology populations • Limited pediatric cardio-oncology data | Secondary evaluation as indicatedb | ||
| GLS | |||||
| • More sensitive measure of cardiac function • Prognostic of subsequent LVEF declines37 | • An improvement in outcomes has not been demonstrated in adult cardio-oncology populations39 • Limited pediatric cardio-oncology data | >−16% (approximate)42 or ≥15% worsening from baseline valuec | Adjunct measure only (further study needed) | ||
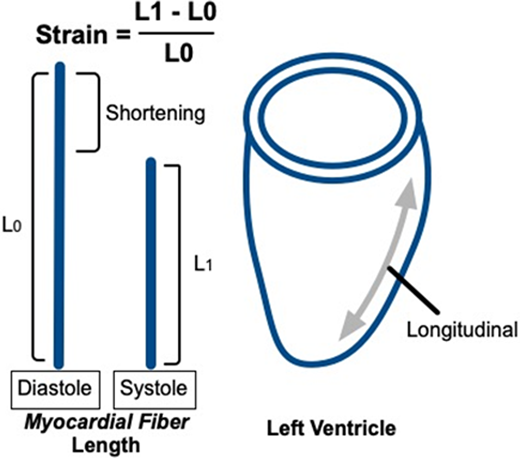
The table depicts the derivations of imaging-based measures of left ventricular systolic function. LVFS is the fractional change in cavity diameter during the cardiac cycle. LVEF is the fractional change in cavity volume during the cardiac cycle. Strain is the fractional change in myocardial fiber length, or deformation, during the cardiac cycle, typically derived from speckle-tracking analysis of 2D echocardiographic images. GLS is the average deformation in the longitudinal dimension. 2D or 3D echocardiographic derivations of LVEF should be the primary screening measure used to assess for LVSD during pediatric AML therapy. Strengths, limitations, and recommendations for use are based on general cardiovascular-imaging quantification guidelines and adult cardio-oncology guidelines, with additional supportive references listed within the table and footnotes.29,32,34,40
LVFS and LVEF cutoffs are based on grade 2 severity events for the terms “left ventricular systolic dysfunction” and “ejection fraction decreased” in the National Cancer Institute Common Terminology Criteria for Adverse Events (Versions 3.0 and 5.0, respectively). Precise cutoffs may vary among treatment protocols, and clinicians should consult the specific protocol for further guidance.
Some indications for CMRI in secondary evaluation are poor echocardiographic windows resulting in inadequate assessment of function, borderline/indeterminate echocardiographic results in which a more precise LVEF estimate would change clinical management, or baseline or significant LVSD to evaluate for underlying cardiomyopathy.31,32 CMRI does not employ ionizing radiation, and assessment of LV volumes, mass, and LVEF does not require intravenous contrast. However, younger children require sedation/anesthesia to complete CMRI, which is an important consideration.43 Increasing availability coupled with ongoing technological improvements that reduce motion artifacts/patient compliance demands, scanning time, and postprocessing time may result in increased CMRI use in pediatric cancer populations in the future.
GLS values are typically expressed as a negative percentage; less negative values reflect worse systolic function (eg, −16% is worse than −18%). The limit of normal has not been precisely established in pediatrics; the reported limit of −16% is an imprecise estimate based on prior pediatric studies.42 The worsening of 15% from the baseline value is based on adult cardio-oncology guidelines and refers to the percent worsening from the baseline value,32 not the absolute worsening in GLS (eg, a change from −20% to −17% is a 15% worsening). LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume.
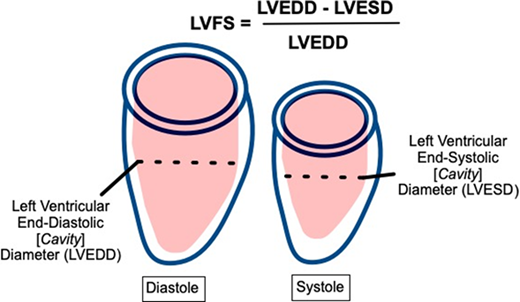
LVFS is the fractional cavity diameter change between diastole and systole. It is assessed with two-dimensional (2D) or M-mode echocardiography via a linear interrogation through the center of the LV. LVFS has a long historical precedent and is easy to acquire and measure.29 However, it is suboptimal in screening given its significant angle dependence and poor reliability between acquisitions.30
LVEF is the fractional cavity volume change between diastole and systole. Cardiac magnetic resonance imaging (CMRI) is the optimal modality for LVEF derivation given its high-resolution, direct LV measurement in 3 dimensions without geometric assumptions.31 However, CMRI is currently reserved for secondary evaluation, primarily related to its lesser availability.32 Echocardiography, the standard primary screening modality, can be used to derive LVEF using multiple methods. The Teichholz formula can be used to calculate LVEF from linear dimensions; however, this method is not recommended due to its significant geometric assumptions and resulting inaccuracy.33,34 Alternate 2D LVEF derivations include the five/sixths area-length (ie, “bullet”) method and the biplane Simpson's method; preference among these is generally laboratory dependent. Although superior to Teichholz, these methods also have limitations due to challenges with angle dependence, measurement reproducibility, and geometric assumptions such that their sensitivity to detect abnormal LVEF compared to CMRI in childhood cancer survivor populations is only approximately 50%.33,35
Three-dimensional (3D) echocardiography allows complete LV visualization, yielding a more reproducible and accurate LVEF measurement in comparison with 2D methods; the sensitivity to detect LVSD in comparison with MRI is 53% to 68% in childhood cancer survivor populations.33,35 However, limitations include the need for specialized equipment, software, and training and a paucity of data supporting its use in younger children with cancer. When available, 3D LVEF is the preferred screening method in adolescents and young adults.32
Although valid and important heart failure screening measures, LVEF and LVFS declines may be late findings in the progression of anthracycline-related cardiomyopathy.36 Strain, specifically GLS, has been proposed as an alternative measure to facilitate earlier, more sensitive cardiotoxicity detection. Strain is the fractional change in myocardial fiber length, or deformation, during the cardiac cycle. GLS represents strain along the LV long axis, averaged across the LV. GLS declines precede and are prognostic of LVEF declines in adults during and after anthracycline therapy.37 GLS abnormalities are also prevalent in childhood cancer survivors in the presence of normal LVEF and are associated with established cardiovascular risk factors, suggesting that they are clinically relevant.38 In recent years GLS has been incorporated in adult cardio-oncology imaging guidelines, and its use is becoming more widespread.32 However, 1-year results from the only randomized trial comparing the efficacy of a GLS- vs LVEF-guided approach to cardioprotective therapy initiation in adults receiving anthracyclines failed to demonstrate a benefit of GLS.39 In addition, pediatric data attesting to the utility of GLS during anthracycline therapy are limited. Based on the current evidence, GLS may serve as an adjunct measure, but AML treatment decisions should not be altered based on GLS alone.40
Toxicity-responsive cardioprotection during chemotherapy
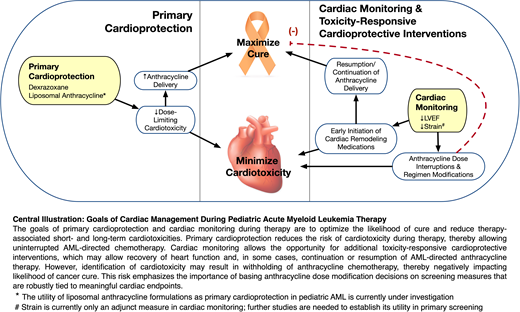
Cardiac monitoring provides the opportunity for additional cardioprotective interventions in response to cardiotoxicity detection, with the goal of reducing further cardiac injury and supporting cardiac recovery. The two main strategies of cardioprotection in response to LVSD during chemotherapy include withholding anthracyclines (ideally temporarily) and using cardiac remodeling medications. There are limited data to guide clinicians regarding when to institute these interventions; acknowledging this paucity, our proposed management strategy is depicted in Figure 4. Dose interruptions or reductions in anthracycline delivery should be minimized given the potential to reduce chemotherapeutic efficacy. Brief delays may be considered in the setting of LVSD, with the goal of functional recovery and resumption of anthracycline delivery. Withholding anthracycline should be considered in cases of significant dysfunction; in these settings we suggest replacing it with an intensive non-anthracycline-containing chemotherapy block such as high-dose cytarabine and asparaginase (Capizzi II). The presence of persistent and/or significant LVSD should prompt cardiology consultation, further cardiac testing, and consideration of cardiac remodeling medications such as angiotensin converting enzyme inhibitor or beta-blocker therapy; further pediatric data are needed to determine the benefit of these medications in this setting. Recovery of function after these toxicity-responsive interventions may allow for the resumption or continuation of anthracycline delivery.
Proposed management of cardiotoxicity during pediatric AML therapy. This figure depicts our proposed algorithm to manage cardiotoxicity during AML therapy, with the goal of balancing cardiac safety with effective delivery of AML-directed chemotherapy. LVEF cutoffs are based on National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) V3.0 for LVSD: grade 2 LVSD is defined as asymptomatic LVEF decline of 40% to 49%. Grade 3 LVSD is defined as LVEF decline of 20% to 39%, or symptomatic heart failure. These LVEF thresholds are consistent with grade 2/3 “ejection fraction decreased” in CTCAE V5.0. *Additional testing may include biomarkers (eg, N-terminal-pro B-type natriuretic peptide), CMRI, or alternate testing as indicated. Biomarkers currently have an established role in secondary/further evaluation but are under investigation as a primary screening modality. ^Cardiac remodeling medications may include angiotensin converting enzyme inhibitors (eg, enalapril) and beta-blockers (eg, carvedilol). #When omission of anthracyclines is necessary due to persistent LVSD, efforts should be made to maintain the intensity of chemotherapy, if appropriate. Thus, we suggest replacing with an intensive non-anthracycline-containing chemotherapy block, such as high-dose cytarabine and asparaginase (Capizzi II).
Proposed management of cardiotoxicity during pediatric AML therapy. This figure depicts our proposed algorithm to manage cardiotoxicity during AML therapy, with the goal of balancing cardiac safety with effective delivery of AML-directed chemotherapy. LVEF cutoffs are based on National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) V3.0 for LVSD: grade 2 LVSD is defined as asymptomatic LVEF decline of 40% to 49%. Grade 3 LVSD is defined as LVEF decline of 20% to 39%, or symptomatic heart failure. These LVEF thresholds are consistent with grade 2/3 “ejection fraction decreased” in CTCAE V5.0. *Additional testing may include biomarkers (eg, N-terminal-pro B-type natriuretic peptide), CMRI, or alternate testing as indicated. Biomarkers currently have an established role in secondary/further evaluation but are under investigation as a primary screening modality. ^Cardiac remodeling medications may include angiotensin converting enzyme inhibitors (eg, enalapril) and beta-blockers (eg, carvedilol). #When omission of anthracyclines is necessary due to persistent LVSD, efforts should be made to maintain the intensity of chemotherapy, if appropriate. Thus, we suggest replacing with an intensive non-anthracycline-containing chemotherapy block, such as high-dose cytarabine and asparaginase (Capizzi II).
Summary
The goals of care during pediatric AML therapy are to maximize cure while minimizing short- and long-term cardiotoxicity. To promote the safety and efficacy of chemotherapy, treatment regimens should include primary cardioprotection in the form of concurrent dexrazoxane and cardiac monitoring with additional toxicity-responsive cardioprotective interventions as indicated. Given the delicate balance needed to manage competing oncologic and cardiovascular risks, a close collaboration between oncologists and cardiologists is critical to optimizing patient care. This collaboration should begin during chemotherapy and continue in survivorship, with long-term monitoring and the potential implementation of secondary and tertiary heart failure prevention measures.41 As oncologic therapies continue to rapidly evolve, the development of dedicated, interdisciplinary pediatric cardio-oncology programs may serve to meet this important need.
CLINICAL CASE (continued)
The patient was diagnosed with grade 3 LVSD in the setting of sepsis. With intensive supportive care, the patient recovered and was discharged on cycle day 34. LVEF improved to 48% on echocardiography at discharge, with further recovery to 55% 1.5 weeks later. The patient was admitted for intensification 2, including full-dose mitoxantrone with dexrazoxane, and her chemotherapy course was completed without further complications. The patient is currently 1 year following therapy and is being monitored with annual echocardiograms due to high-dose anthracycline exposure and a history of on-therapy LVSD.
Acknowledgments
Hari K. Narayan is supported by Padres Pedal the Cause/RADY no. #PTC2020 and UC San Diego Moore Cancer Center, Specialized Cancer Control Support Grant NIH/NCI P30CA023100. Kelly Getz is supported by an National Heart, Lung, and Blood Institute Career Development Award (1K01HL143153-01).
Conflict-of-interest disclosure
Hari K. Narayan: nothing to disclose.
Kelly Getz: nothing to disclose.
Kasey J. Leger: research funding: Abbott Diagnostics; advisory board: Jazz Pharmaceuticals.
Off-label drug use
Hari K. Narayan: Off-label use of several drugs is discussed: angiotensin converting enzyme inhibitors (ex. enalapril); beta blockers (ex. Carvedilol); asparaginase dexrazoxane (given under FDA orphan drug designation for pediatric cancers, but not technically a labeled indication).
Kelly Getz: Off-label use of several drugs is discussed: angiotensin converting enzyme inhibitors (ex. enalapril); beta blockers (ex. Carvedilol); asparaginase dexrazoxane (given under FDA orphan drug designation for pediatric cancers, but not technically a labeled indication).
Kasey J. Leger: Off-label use of several drugs is discussed: angiotensin converting enzyme inhibitors (ex. enalapril); beta blockers (ex. Carvedilol); asparaginase dexrazoxane (given under FDA orphan drug designation for pediatric cancers, but not technically a labeled indication).