Abstract
Several chemotherapeutic agents and novel immunotherapies provide excellent control of systemic and central nervous system (CNS) leukemia but can be highly neurotoxic. The manifestations of subacute methotrexate neurotoxicity are diverse and require vigilant management; nonetheless, symptoms are transient in almost all patients. As methotrexate is a crucial drug to prevent CNS relapse, it is important to aim to resume it after full neurologic recovery. Most children tolerate methotrexate rechallenge without significant delays or prophylactic medications. Neurotoxicity is more frequent with newer immunotherapies such as CD19– chimeric antigen receptor T (CAR T) cells and blinatumomab. A uniform grading system for immune effector cell–associated neurotoxicity syndrome (ICANS) and algorithms for management based on severity have been developed. Low-grade ICANS usually resolves within a few days with supportive measures, but severe ICANS requires multispecialty care in the intensive care unit for life-threatening seizures and cerebral edema. Pharmacologic interventions include anticonvulsants for seizure control and glucocorticoids to reduce neuroinflammation. Anticytokine therapies targeted to the pathophysiology of ICANS are in development. By using illustrative patient cases, we discuss the management of neurotoxicity from methotrexate, CAR T cells, and blinatumomab in this review.
Learning Objectives
Discuss management of methotrexate neurotoxicity and rechallenge strategy
Review assessment and management of ICANS associated with CAR T-cell therapy and blinatumomab
Introduction
Optimization of chemotherapeutic regimens has enabled substitution of cranial irradiation with systemic and intrathecal agents for prophylaxis of central nervous system (CNS) disease in childhood acute lymphoblastic leukemia (ALL).1 However, even radiation-free contemporary regimens can cause severe neurotoxicity, leading to morbidity and mortality, therapy delays, and fear in patients and families. The incidence of symptomatic neurotoxicity during frontline ALL therapy is approximately 10% to 12%, and its manifestations are varied, as are the inciting agents.2,3 Methotrexate-related stroke-like syndrome (SLS), posterior reversible encephalopathy syndrome, cerebral sinus venous thrombosis, and seizures are the common CNS events that result from standard ALL chemotherapeutic agents.4 With increasing use of immunotherapies for patients with ALL, neurotoxicity is being reported more frequently, with incidence of up to 50% in patients treated with CD19– chimeric antigen receptor T (CAR T)–cell therapy.5 Multiple studies are investigating predictors and mediators of immune effector cell–associated neurotoxicity syndrome (ICANS), with the goal of identifying those at high risk and developing interventions to mitigate its severe manifestations. In this review, we discuss the management of neurotoxicity associated with methotrexate, CAR T-cell therapy, and blinatumomab in children with ALL.
CLINICAL CASE 1
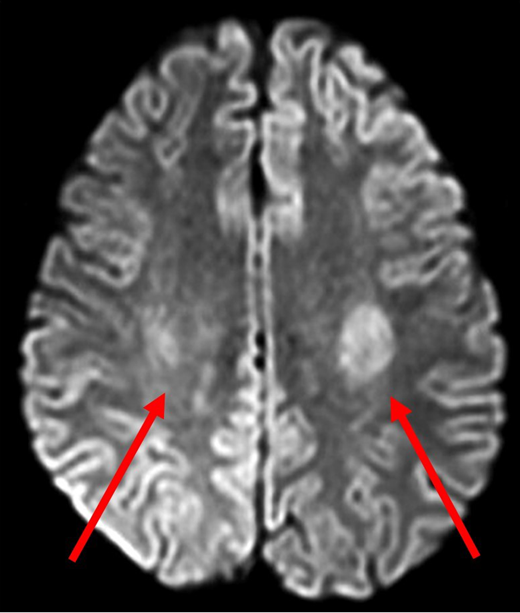
A 14-year-old Hispanic girl with high-risk B-ALL without CNS involvement was receiving standard-of-care chemotherapy according to the Children's Oncology Group protocol AALL0232. She had tolerated chemotherapy relatively well, including 6 doses of intrathecal methotrexate in the induction and consolidation phases. The first interim maintenance phase was initiated with 15 mg of intrathecal methotrexate followed by 5 g/m2 of intravenous methotrexate over 24 hours. Methotrexate cleared appropriately, and she received 3 doses of leucovorin 15 mg/m2 at hours 42, 48, and 54. Nine days later, she presented to the emergency department with acute-onset right-sided leg weakness, which progressed to the right arm and face over a 2-hour period. In addition to right paraparesis and facial droop, she was noted to have aphasia and dysarthria. Magnetic resonance imaging (MRI) performed 4 hours after the onset of symptoms showed restricted diffusion bilaterally in the centrum semiovale, greater on the left side (Figure 1). She was admitted to the intensive care unit for close observation. During the next 24 hours, her speech worsened, and she became more confused. There was moderate improvement in right-sided weakness, but she developed weakness in the left arm and leg. With continued supportive care, beginning day 3 of hospitalization, her symptoms gradually improved, facial asymmetry resolved, her speech became clearer, and she was able to swallow. She required physical therapy for 2 more days to regain her baseline strength and ambulate independently.
Diffusion-weighted MRI of patient 1 four hours after onset of symptoms. DWI demonstrated abnormal diffusion in bilateral centrum semiovale (red arrows), greater on the left side. These findings indicate increased diffusion of water in the affected regions suggestive of cytotoxic edema.
Diffusion-weighted MRI of patient 1 four hours after onset of symptoms. DWI demonstrated abnormal diffusion in bilateral centrum semiovale (red arrows), greater on the left side. These findings indicate increased diffusion of water in the affected regions suggestive of cytotoxic edema.
Subacute methotrexate neurotoxicity
Approximately 3% to 7% of children treated with contemporary frontline ALL regimens develop subacute methotrexate neurotoxicity.6-8 Seizures, encephalopathy, aphasia, and SLS are common presentations.7-9 Symptoms typically begin 3 to 11 days after intrathecal or intravenous methotrexate, can wax and wane over several hours or days, and are characteristically transient and resolve in 1 to 4 days. Most patients make a full recovery, although recovery may require several months of intensive rehabilitation in a small subset of patients with severe SLS.9 The defining characteristics of methotrexate-SLS are listed in Table 1.10 In brief, methotrexate-SLS is defined as neurotoxicity that occurs within 21 days of intravenous or intrathecal methotrexate with specified clinical and radiographic features, while excluding other identifiable causes. Risk factors for methotrexate neurotoxicity identified in multivariable analyses are age >10 years, Hispanic ethnicity, and elevated serum aspartate transaminase during induction/consolidation.8,11 Coadministration of intrathecal methotrexate in systemic cytarabine- and cyclophosphamide-containing treatment blocks has been noted as a period of increased risk.8,9
Defining characteristics of methotrexate-SLS (reproduced with permission10 )
| Neurotoxicity occurring within 21 days of intravenous or intrathecal methotrexate with 3 characteristics that all need to be fulfilled: |
| 1. New onset of one or more of paresis or paralysis; movement disorder or bilateral weakness; aphasia or dysarthria; altered mental status including consciousness (e.g., somnolence, confusion, disorientation, and emotional lability); and/or seizures with at least one of the other symptoms. |
| 2. Either characteristic, but often transient, white matter changes indicating leukoencephalopathy on MRI or a characteristic clinical course with waxing and waning symptoms usually leading to complete (sometimes partial) resolution within a week. |
| 3. No other identifiable cause. |
| Characteristic oval-shaped lesions of the subcortical white matter (mostly frontal or parietal) on MRI are best seen on diffusion-weighted (hyperintense) or apparent diffusion coefficient (hypointense) images. Can be graded 1-5 according to CTCAEv4.03 for encephalopathy. |
| Neurotoxicity occurring within 21 days of intravenous or intrathecal methotrexate with 3 characteristics that all need to be fulfilled: |
| 1. New onset of one or more of paresis or paralysis; movement disorder or bilateral weakness; aphasia or dysarthria; altered mental status including consciousness (e.g., somnolence, confusion, disorientation, and emotional lability); and/or seizures with at least one of the other symptoms. |
| 2. Either characteristic, but often transient, white matter changes indicating leukoencephalopathy on MRI or a characteristic clinical course with waxing and waning symptoms usually leading to complete (sometimes partial) resolution within a week. |
| 3. No other identifiable cause. |
| Characteristic oval-shaped lesions of the subcortical white matter (mostly frontal or parietal) on MRI are best seen on diffusion-weighted (hyperintense) or apparent diffusion coefficient (hypointense) images. Can be graded 1-5 according to CTCAEv4.03 for encephalopathy. |
This consensus definition was developed by the Ponte de Legno international childhood ALL group to assist clinicians in differentiating methotrexate -SLS from similar neurotoxicities (eg, posterior reversible encephalopathy syndrome) and to enable reliable comparisons of incidence of methotrexate -SLS between different trials.
Leukoencephalopathy or subcortical white matter changes are the characteristic MRI findings of methotrexate neurotoxicity. Diffusion-weighted imaging (DWI) is the most sensitive modality during the acute phase and shows abnormally high diffusion of water in brain areas that correspond with clinically apparent neurologic deficits.12 Because changes noted on DWI can be transient, a negative MRI does not exclude a diagnosis of methotrexate neurotoxicity. On the other hand, white matter hyperintensities in T2-weighted and fluid-attenuated inversion recovery MRIs can persist and evolve over time; therefore, awaiting complete normalization of MRI findings prior to rechallenge with methotrexate is not practical.
The pathophysiology of subacute methotrexate neurotoxicity is not completely understood. Accumulation of homocysteine and its metabolites in plasma and cerebrospinal fluid (CSF) is toxic to vascular endothelium. Release of adenosine and neuronal injury are also proposed mechanisms.13
Acute management of methotrexate neurotoxicity
Supportive care and close monitoring are the mainstays of management during the acute episode. A single, brief seizure will not require pharmacologic intervention, but anticonvulsants are administered for prolonged or repeated seizures. Symptoms can wax and wane; therefore, frequent neurologic examinations guide subsequent management. Oral feeds should be held until swallowing is deemed safe. Because aphasia and dysarthria are distressing to patients and families, psychosocial support, reassurance, and providing alternate methods of communication may lessen anxiety. Computerized tomography (CT) is not generally helpful but may be needed if symptoms are suggestive of CNS bleed. CSF examination is only indicated if the patient is febrile and there are concerns for CNS infection. As detailed above, MRI with DWI can support the diagnosis of methotrexate neurotoxicity, but its utility in cases of mild, transient, and classical symptoms is limited. Input from a neurologist is valuable in most cases, particularly with prolonged symptoms. Severe SLS is best managed in a critical care setting, as patients may require intubation with mechanical ventilation for airway protection.
Based on the presumed mechanisms of methotrexate neurotoxicity and evidence of homocysteine causing excitatory effects on N-methyl-D-aspartate receptors, the use of the N-methyl-D-aspartate antagonist dextromethorphan has been reported in the acute setting and for secondary prophylaxis.14 To combat vasodilatation from adenosine, aminophylline use has also been described, as it displaces adenosine from its receptor on endothelial cells.15 However, supporting such approaches is difficult due to a lack of controls, particularly in the setting of the transient natural course of methotrexate neurotoxicity and rapid resolution of symptoms without pharmaceutical intervention in most patients. In addition, dextromethorphan itself can cause CNS effects such as dizziness and restlessness, whereas aminophylline can cause tachycardia, anxiety, headache, and seizures.16 Current gaps in knowledge regarding the utility of dextromethorphan and aminophylline for the treatment and prophylaxis of methotrexate neurotoxicity preclude specific recommendations.
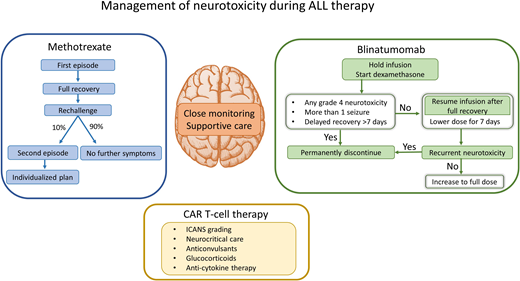
Rechallenge with methotrexate after the first episode of neurotoxicity
Multiple studies have validated the safety of rechallenge with methotrexate after the first episode of neurotoxicity, as 82% to 92% of patients did not develop recurrent symptoms.6-9 Importantly, omission of methotrexate for CNS-directed therapy after a neurotoxic episode increased the risk of CNS relapse, as demonstrated in a large Australian cohort of 1251 pediatric patients with ALL, including 95 who experienced methotrexate neurotoxicity.8 The 5-year CNS relapse-free survival was 89% when intrathecal methotrexate was permanently discontinued and replaced with alternate intrathecal therapy after a symptomatic neurotoxicity episode, compared with 95% if patients continued to receive protocol-prescribed doses of intrathecal methotrexate (P = .047). These data highlight the importance for the continuation of optimal therapy to maximize cure.
Rechallenge with methotrexate should be delayed until full neurologic recovery, which is typically less than 7 days. For some patients, this may result in the omission or delay of methotrexate therapy by 1 to 2 weeks. Successful resumption of standard therapy is possible in most patients, without the need for excessive delays, substitutions, or prophylactic medications. Substitution of intrathecal methotrexate by cytarabine with or without hydrocortisone can be considered temporarily for 1 or 2 doses, with the resumption of intrathecal methotrexate as soon as possible. Leucovorin rescue of 2 systemic doses (5 mg/m2/dose) at hours 24 and 30 after intrathecal methotrexate rechallenge is a reasonable strategy to attempt to minimize recurrent episodes. For systemic methotrexate rechallenge, early leucovorin rescue at hour 30 or 36 after the start of the 24-hour infusion of high-dose methotrexate could be considered. Although there are no randomized studies for this secondary prophylaxis, leucovorin is the only drug with strong clinical evidence for reducing the incidence of methotrexate neurotoxicity.17-19 The theoretical concern of “rescue” of intrathecal methotrexate by leucovorin and consequent reduction of its antileukemic effect is offset by the ability to continue administration of this important drug to reduce risk of relapse. Additional leucovorin rescue may not be needed indefinitely, especially if the patient tolerates the subsequent rechallenge well.
CLINICAL CASE 1 (continued)
This patient made a full recovery 5 days after the onset of symptoms. Her second course of intravenous high-dose methotrexate was delayed by 2 weeks, during which she continued to receive mercaptopurine. Leucovorin rescue was initiated early at hour 30 and given every 6 hours for 5 doses during courses 2 and 3. As she did not develop recurrent neurotoxicity, the last course of high-dose methotrexate was administered with the standard leucovorin rescue. She completed all planned therapy for ALL without further complications.
Recurrence of methotrexate neurotoxicity
Due to the rarity of recurrences, there are no available guidelines for a second rechallenge. Patients with recurrent brief seizures may tolerate subsequent methotrexate under the cover of appropriate anticonvulsants for the remainder of therapy. Very limited data are available to assess outcomes of second rechallenge after recurrent SLS. In the UKALL 2003 report, 4 patients with a second episode of SLS continued to receive methotrexate after complete neurologic recovery.9 Of these, 3 patients tolerated methotrexate well, and 1 developed persistent neurologic deficits, suggesting that a subset of patients may continue to receive methotrexate safely even after 2 episodes of neurotoxicity. However, caution is warranted as there are case reports of patients with poor neurologic outcomes and rare fatalities after repeated episodes of methotrexate neurotoxicity.9,20 In these uncommon scenarios, particularly when patients do not make a full recovery in the expected time frame or have recurrent severe methotrexate neurotoxicity, completion of CNS-directed therapy with alternate agents would require an individualized approach based on the patient's risk of CNS relapse.
CLINICAL CASE 2
The patient is a 16-year-old Caucasian male with B-ALL in second relapse with high bone marrow leukemia burden. He received levetiracetam for seizure prophylaxis and fludarabine and cyclophosphamide for lymphodepletion prior to infusion of autologous anti-CD19 CAR T cells. On postinfusion day 3, he developed grade 2 cytokine release syndrome (CRS) manifested by fevers, hypoxia, and hypotension.21 Initial management included oxygen supplementation, intravenous fluid bolus, tocilizumab, and methylprednisolone. After transient improvement, grade 2 CRS recurred with worsening hypotension on day 5, requiring dopamine infusion. A second dose of tocilizumab was administered for CRS. On day 6, he was lethargic, with expressive dysphasia and an immune effector cell–associated encephalopathy (ICE) score of 5, meeting criteria for grade 2 ICANS.21 Dexamethasone was initiated for ICANS, and CT of the head was performed, which was normal. However, his neurologic status rapidly declined throughout the day, and he developed a generalized tonic-clonic seizure that abated after 2 doses of lorazepam. He was given a loading dose of levetiracetam and transferred to the intensive care unit. He was stuporous on examination and required intubation for apneic episodes. ICANS had progressed to grade 4. Aggressive neuroprotective measures were instituted with elevation of head of bed and supportive interventions to maintain normotension and normothermia. Anakinra and high-dose methylprednisolone were initiated.
ICANS
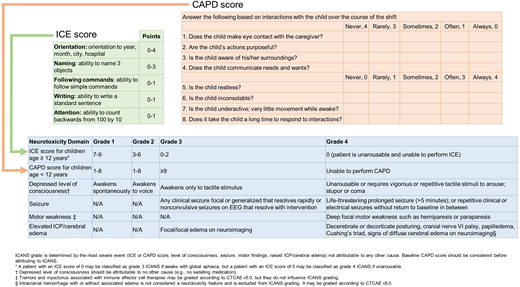
Accurate assessments of the incidence and severity of neurotoxicity were limited by differing definitions and grading criteria used in earlier CAR T studies. Therefore, the American Society of Transplantation and Cellular Therapy (ASTCT) published consensus definitions and grading in 2019 for uniformity across studies (Figure 2).21 ICANS is defined as “a disorder characterized by a pathologic process involving the CNS following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”21 With CD19 CAR T therapy, neurotoxicity of all grades has been reported in 28% to 64% of patients and grades ≥3 in 13% to 50%.5 Fatal ICANS was reported in approximately 3% of adult patients.22 ICANS begins in the first week postinfusion (median 4-6 days) in 90% of patients, soon after the onset of CRS or as CRS symptoms subside. Cases of ICANS without CRS are uncommon and have less severe manifestations. Typically, neurologic symptoms are transient and resolve in 5 to 11 days but can be noted for up to 8 weeks.5,23 Delayed-onset ICANS at 1 month after infusion has also been described.24 Early signs of ICANS include expressive dysphasia, tremor, and dysgraphia and can progress to seizures, depressed level of consciousness, encephalopathy, and coma. Cerebral edema is a dreaded consequence of ICANS. Baseline risk factors for ICANS include high bone marrow disease burden and preexisting neurologic deficits, whereas postinfusion risk factors include higher-grade CRS, inflammatory markers such as fever and increased ferritin, and high serum levels of specific cytokines (eg, interleukin [IL]–2, IL-6, IL-10, IL-15).22,23,25 Although mechanisms leading to ICANS are unclear, experimental models suggest cytokine-induced neuroinflammation and endothelial activation, with subsequent disruption of the blood-brain barrier and direct neuronal injury.22,25
ASTCT consensus grading for ICANS. Adapted from Lee et al21 and Traube et al44 with permission. ICANS grading (blue box) has 5 elements. The first element assesses encephalopathy and uses a scoring tool: ICE (green box) for patients ≥12 years and CAPD (orange box) for patients <12 years of age. CAPD, Cornell Assessment of Pediatric Delirium; ICE, immune effector cell–associated encephalopathy; ICP, increased intracranial pressure.
ASTCT consensus grading for ICANS. Adapted from Lee et al21 and Traube et al44 with permission. ICANS grading (blue box) has 5 elements. The first element assesses encephalopathy and uses a scoring tool: ICE (green box) for patients ≥12 years and CAPD (orange box) for patients <12 years of age. CAPD, Cornell Assessment of Pediatric Delirium; ICE, immune effector cell–associated encephalopathy; ICP, increased intracranial pressure.
Acute management of ICANS
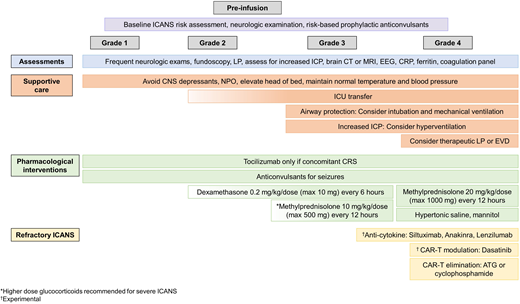
Commercially available CAR T-cell products in the United States are governed under the Food and Drug Administration Risk Evaluation and Mitigation Strategy drug safety program to ensure that health care providers and relevant staff are trained in the recognition and management of toxicities associated with CAR T therapy, including ICANS. With broader experience gained in the management of ICANS, several clinical management guidelines have been published in the past 4 years.26-28 General recommendations are summarized in Figure 3.
General guidelines for management of ICANS. Frequent neurologic assessments, multidisciplinary supportive care, and glucocorticoids form the backbone of ICANS management. In addition, several novel strategies are being studied for refractory ICANS, as described in the text. ATG, anti–thymocyte globulin; CRP, C-reactive protein; EVD, external ventricular drain; ICU, intensive care unit; LP, lumbar puncture; NPO, nothing by mouth.
General guidelines for management of ICANS. Frequent neurologic assessments, multidisciplinary supportive care, and glucocorticoids form the backbone of ICANS management. In addition, several novel strategies are being studied for refractory ICANS, as described in the text. ATG, anti–thymocyte globulin; CRP, C-reactive protein; EVD, external ventricular drain; ICU, intensive care unit; LP, lumbar puncture; NPO, nothing by mouth.
Although prophylactic anticonvulsants are not used uniformly due to limited evidence for prevention of ICANS-related seizures, they are recommended in patients with a history of seizures or other neurologic comorbidities. Close clinical monitoring and attentive supportive care are often sufficient for grade 1 ICANS. Tocilizumab (anti–IL-6 receptor antibody) is only indicated for concurrent CRS, not for treatment of ICANS. Due to its large size (molecular weight 149 kDA), tocilizumab does not cross the blood-brain barrier and may also facilitate a paradoxical shift of IL-6 from the blood to the brain.22 Neuroimaging preferably with MRI, electroencephalogram (EEG), and lumbar puncture with opening pressure can support the diagnosis of ICANS and rule out other causes of neurologic symptoms such as infection, CNS leukemia. or hemorrhage. MRI changes of focal or diffuse cerebral edema guide ICANS grading and management. Leptomeningeal enhancement and multifocal microhemorrhages have also been observed with severe ICANS.22 In an study of adult patients, EEG showed diffuse slowing in 76% of patients during acute ICANS and was also helpful in detecting subclinical seizures.22 Characteristic CSF findings for ICANS are moderate leukocytosis with lymphocyte predominance and markedly elevated protein.22
For grade 2 ICANS, glucocorticoids (dexamethasone 0.2 mg/kg/dose, maximum 10 mg every 6 hours, or methylprednisolone equivalents) should be strongly considered as brief courses do not negatively affect the efficacy of the CAR T cells and may shorten the duration of symptoms.29 For higher-grade ICANS 3 or 4, along with continued supportive care in collaboration with intensivists, neurologists, and neurosurgeons, the dose of glucocorticoids should be increased (methylprednisolone 10 or 20 mg/kg/dose, maximum 500 or 1000 mg every 12 hours) until improvement, followed by rapid taper. In addition, multiple anticonvulsants for optimal seizure control, airway protection by intubation and mechanical ventilation, and decreasing intracranial pressure with hyperosmolar therapy or neurosurgical intervention may be warranted.
There are very limited clinical data on other pharmacologic interventions targeted to the pathophysiology of ICANS. Siltuximab binds to IL-6 (unlike tocilizumab that binds to the IL-6 receptor) and facilitates removal of IL-6 from the circulation, leaving less IL-6 to enter the brain.27 Anakinra, the IL-1 receptor antagonist, may also be of benefit as monocyte-derived IL-1 can precede and induce IL-6 secretion.30,31 The molecular weight of anakinra is 17.3 kDA, and therapeutic concentrations can be achieved in the CSF.32 Another potential approach to reduce neuroinflammatory cytokine production by monocytes is the use of lenzilumab, the anti–granulocyte-macrophage colony-stimulating factor monoclonal antibody. Lenzilumab reduced neuroinflammation after CAR T-cells in preclinical models.33 Clinical trials of these agents as prophylactic strategies for ICANS are ongoing (NCT04359784, NCT04150913, NCT04432506, NCT04148430, NCT04205838, NCT04314843). Dasatinib, a tyrosine kinase inhibitor, reduces CAR T-cell signaling reversibly by interfering with lymphocyte-specific protein tyrosine kinase.34 In a murine model, the dose of dasatinib was titrated to achieve partial or complete inhibition of CAR T-cell function.34 This strategy of modulation of CAR T-cell function with dasatinib is being studied as an intervention for CRS and ICANS in an ongoing clinical trial (NCT04603872). The use of intrathecal hydrocortisone, methotrexate, and cytarabine in steroid-refractory ICANS has been described in case reports.35,36 Additional studies are needed to investigate if such attempts to reduce neuroinflammation by intrathecal therapy and eliminate immune effector cells in the CSF with cytotoxic agents will be beneficial in the management of ICANS without negatively affecting CNS leukemia control by CAR T cells. However, in severe life-threatening ICANS, elimination of the CAR T cells is deliberate, for example, with anti– thymocyte globulin36 or cyclophosphamide or by activating CAR T-cell suicide approaches designed into specific constructs.37
CLINICAL CASE 2 (continued)
With continued aggressive neurocritical care and multimodal therapy, his clinical condition began to improve on day 9, evidenced by spontaneous movements and withdrawal to pain. No further seizure activity was noted on EEG. Methylprednisolone was tapered day 10 onward, and he was extubated on day 11. He continued to have residual aphasia that gradually recovered to baseline by day 15.
CLINICAL CASE 3
A 9-year old Hispanic girl with B-ALL in first relapse had minimal residual disease 0.2% after reinduction chemotherapy. Blinatumomab continuous infusion 15 µg/kg/d was initiated, and she was monitored inpatient. On day 4, she complained of headache that improved with acetaminophen. Three hours later, she developed acute-onset confusion and delirium. She was agitated and moaning, she could not follow commands, and her speech was incoherent. She was assessed to have grade 2 encephalopathy. Blinatumomab infusion was immediately stopped, and dexamethasone was administered. Within an hour of these interventions, her symptoms began to improve, and 6 hours later, her neurologic status was back to normal. Because symptoms did not recur, blinatumomab infusion was restarted after 3 days at a lower dose of 5 µg/kg/d, along with dexamethasone premedication. The dose of blinatumomab was escalated to 15 µg/kg/d a week later, and she tolerated the rest of the course well.
Neurotoxicity after blinatumomab
Similar principles of grading and management of ICANS from CAR T apply to neurotoxicity from blinatumomab. All clinical trials to date have reported grades of individual neurotoxic events based on the Common Terminology Criteria for Adverse Events (CTCAE), but future studies will likely follow the ASTCT ICANS guidelines. The incidence of blinatumomab-associated neurotoxicity was 54% in adult patients with relapsed/refractory ALL38 and 24% in a similar pediatric cohort.39 Of all neurologic events, 10% to 17% were seizures. In adult patients, symptoms began at a median of 9 days and also included tremor, somnolence, dizziness, confusion, and encephalopathy.38 Median time to onset of neurotoxicity may be earlier in pediatric patients.39
Blinatumomab has a very short elimination half-life (1.25 hours), and most toxicities resolve after interrupting the infusion and initiating dexamethasone.38,39 Blinatumomab infusion should be stopped for any seizure and for grade 3 or 4 nonseizure neurotoxicity.40 Prompt initiation of dexamethasone (0.2-0.4 mg/kg/d, maximum 24 mg, in 3 divided doses for 1-3 days) is recommended for any severe neurotoxicity. Blinatumomab is permanently discontinued if the patient develops more than 1 seizure, any grade 4 neurotoxicity, or if neurologic recovery is delayed > 7 days. For all other patients, blinatumomab can be restarted at one-third of the dose (ie, 5 µg/kg, maximum 9 µg) with dexamethasone premedication (5 mg/m2, maximum 20 mg) after improvement to grade ≤ 1 and a minimum of 3 days. If neurotoxicity does not recur, blinatumomab can be escalated to full dose after 7 days. Secondary seizure prophylaxis with anticonvulsants may be considered for the remainder of the cycle and for subsequent cycles. Rechallenge of blinatumomab is not recommended after recurrence of the initial neurotoxic event.
Conclusions
Table 2 summarizes the manifestations and management of neurotoxicity from methotrexate, CAR T cells, and blinatumomab. A detailed understanding of the mechanisms of these neurotoxicities will lead to the development of targeted approaches for prevention and treatment. Until then, close monitoring and attentive, multimodal supportive care form the backbone of managing clinical manifestations. In addition, glucocorticoids and anticytokine therapies dampen the neuroinflammatory cascade in ICANS. Neurologic symptoms are usually transient, and most patients appear to make a full recovery. However, screening MRIs detect leukoencephalopathy in 20% of children receiving methotrexate during frontline ALL therapy,7 and even asymptomatic patients with leukoencephalopathy are at high risk for long-term adverse neurobehavioral problems.41 Survivors of childhood ALL treated with chemotherapy alone continue to have inferior neurocognitive outcomes, particularly in domains of executive function, attention, and memory.42 Similar pediatric data are not available after immunotherapy, but a recent study noted clinically significant neuropsychiatric problems in 50% of adult patients 1 to 5 years after CD19 CAR T-cell therapy.43 Therefore, in addition to developing strategies to decrease the frequency and morbidity of acute neurotoxicity from ALL therapy, it is also imperative to devise approaches to reduce its consequences on long-term outcomes.
Summary of manifestations and management of neurotoxicity from methotrexate, CAR T cells, and blinatumomab
| Characteristic . | Methotrexate . | CAR T cells . | Blinatumomab . |
|---|---|---|---|
| Frequency of neurotoxicity | 3%-7% | 28%-64% | 24%-54% |
| Risk factors | Older age, Hispanic ethnicity, history of elevated transaminases | High disease burden, high-grade CRS, preexisting neurologic deficits | Older age, nonwhite race, prior neurologic events, >2 prior salvage therapies |
| Time of onset from therapy initiation | 3-11 days | 4-6 days | Median 9 days in adult patients, may be earlier in pediatric patients |
| Common signs/symptoms | Seizures, encephalopathy, SLS | Seizures, dysphasia, encephalopathy, cerebral edema | Seizures, tremor, somnolence, encephalopathy |
| Average time to resolution | 1-4 days | 5-11 days | 1-5 days |
| MRI findings | Leukoencephalopathy | Cerebral edema, leptomeningeal enhancement, multifocal microhemorrhages | Insufficient data available |
| Management | Supportive care | Neurocritical care, steroids, anticonvulsants, anticytokine therapies | Steroids, interruption of blinatumomab infusion |
| Characteristic . | Methotrexate . | CAR T cells . | Blinatumomab . |
|---|---|---|---|
| Frequency of neurotoxicity | 3%-7% | 28%-64% | 24%-54% |
| Risk factors | Older age, Hispanic ethnicity, history of elevated transaminases | High disease burden, high-grade CRS, preexisting neurologic deficits | Older age, nonwhite race, prior neurologic events, >2 prior salvage therapies |
| Time of onset from therapy initiation | 3-11 days | 4-6 days | Median 9 days in adult patients, may be earlier in pediatric patients |
| Common signs/symptoms | Seizures, encephalopathy, SLS | Seizures, dysphasia, encephalopathy, cerebral edema | Seizures, tremor, somnolence, encephalopathy |
| Average time to resolution | 1-4 days | 5-11 days | 1-5 days |
| MRI findings | Leukoencephalopathy | Cerebral edema, leptomeningeal enhancement, multifocal microhemorrhages | Insufficient data available |
| Management | Supportive care | Neurocritical care, steroids, anticonvulsants, anticytokine therapies | Steroids, interruption of blinatumomab infusion |
Conflict-of-interest disclosure
Deepa Bhojwani: no competing financial interests to declare.
Ravi Bansal: no competing financial interests to declare.
Alan S. Wayne: receives research funding from Kite Pharma (a Gilead company) and Institut de Recherches Internationales Servier.
Off-label drug use
Deepa Bhojwani: multiple off-label drugs are discussed in the article: dextromethorphan, aminophylline, siltuximab, anakinra, lenzilumab, and dasatinib.
Ravi Bansal: multiple off-label drugs are discussed in the article: dextromethorphan, aminophylline, siltuximab, anakinra, lenzilumab, and dasatinib.
Alan S. Wayne: multiple off-label drugs are discussed in the article: dextromethorphan, aminophylline, siltuximab, anakinra, lenzilumab, and dasatinib.