Abstract
The identification of chronic pain and neuropathic pain as common contributors to the overall pain experience of patients with sickle cell disease (SCD) has altered the way we should evaluate difficult-to-treat pain. The recognition of these 2 entities is not generally routine among various medical specialties and provider levels that treat SCD. Due to the relative recency with which neuropathic pain was first described in SCD, validated assessment tools and evidence-based treatments remain lacking. Although clinical assessment and judgment must continue to inform all decision making in this understudied area of SCD pain management, a number of validated neuropathic pain assessment tools exist that can make possible a standardized evaluation process. Similarly, investigation of available neuropathic pain treatments for the uniquely complex pain phenotypes of SCD has only just begun and is better established in pain conditions other than SCD. The aim of this review is to briefly summarize the proposed basic pathophysiology, assessment, and treatment of neuropathic pain in patients with SCD. Furthermore, the aim of this review is to encourage an expanded framework for the assessment and treatment of SCD pain that appreciates the hidden complexities of this common complication of SCD.
Learning Objectives
Recognize the complex nature of sickle cell pain
Understand the proposed basic pathophysiology of neuropathic pain in sickle cell disease
Recognize that although robust, disease-specific evidence does not exist for all neuropathic pain assessment tools and treatments, a nuanced approach to pain management can be considered
Clinical case
A 25-year-old woman with hemoglobin SS disease presents to the emergency department with low back and leg pain refractory to repeated dosing of her home oxycodone. She rates her pain as 10/10 and is very fatigued and anxious due to its persistence. She states she has missed work for 2 separate weeklong hospital admissions recently and may lose her job. She is given IV doses of morphine and ketorolac, and after minimal relief, she is started on a morphine patient-controlled anesthesia pumps and admitted to the hospital. Over the next 8 days, there is little improvement, and she continues to have frequent demands on the patient-controlled anesthesia despite 3 escalations in the continuous morphine rate. The findings of magnetic resonance imaging of her spine, hips, and upper legs are unremarkable.
The evolution of sickle cell disease pain
An outdated model
Until relatively recently, the pain of sickle cell disease (SCD) was classified as only acute in nature and was subdivided into vaso-occlusive crisis (VOC) or noncrisis pain on the basis of severity and health care use.1 This oversimplification was in large part due to the information bias inevitable when providers only see patients whose symptoms are intolerable enough to necessitate urgent or emergent care in a health care setting. In addition, in the not so distant past, the lifespan of these patients was so greatly reduced relative to the general population that there was not sufficient time to develop chronic complications, a reality that has improved with modern preventive care. We now have evidence that almost one-third of adults with SCD experience pain on 95% of the days of their lives, despite seeking treatment in the hospital on <5% of the days.2 Thus, it is very evident that SCD pain encompasses both acute and chronic components.
Old complexities, newly defined
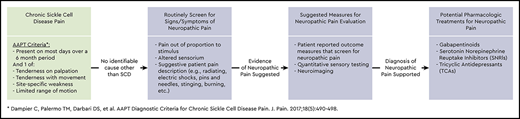
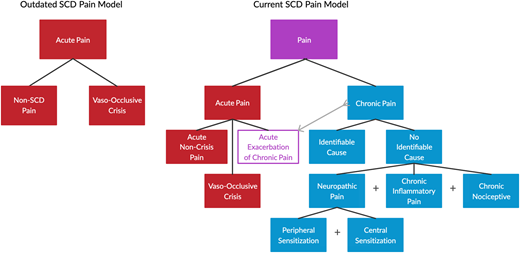
The accepted sickle cell pain model has grown significantly in complexity in the last decade and now includes hematological, neurological, immunological, and psychological contributions.3,4 In an effort to improve comparisons between studies targeting these various facets, the ACTTION-APS Pain Taxonomy and ACTTION-APS-AAPM Pain Taxonomy initiatives published definitions of acute and chronic SCD pain that take into consideration both the lack of previous evidence-based consensus and the factors that have guided definitions for other pain syndromes.5,6 The consensus classification is illustrated in Figure 1 on the basis of those publications, with emphasis on an understudied and clinically undertreated subcategory: neuropathic pain (NP). The remainder of this review focuses on the measurement and management of the neuropathic component of SCD pain.
Taxonomy of pain in SCD. The previously simple classification of pain in SCD assumed all pain was episodic and acute in nature. The current model includes the chronic pain that most patients with SCD are now known to experience, with potential contributions from multiple etiologies to varying degrees. Superimposed acute pain episodes can result from the classic vaso-occlusion or from exacerbations of chronic pain. Any one category of pain shown can likely indirectly contribute to the development of any other category, introducing even further complications.
Taxonomy of pain in SCD. The previously simple classification of pain in SCD assumed all pain was episodic and acute in nature. The current model includes the chronic pain that most patients with SCD are now known to experience, with potential contributions from multiple etiologies to varying degrees. Superimposed acute pain episodes can result from the classic vaso-occlusion or from exacerbations of chronic pain. Any one category of pain shown can likely indirectly contribute to the development of any other category, introducing even further complications.
Clinical case continued
On day 9 of hospitalization, the patient requests another increase in the continuous morphine. She reports 10/10 pain in her legs. When the resident asks her to describe it, she says, “It just hurts” and starts to cry, but she later describes it as burning and shooting down her leg. The resident notes that she recoils at the slightest touch and decides to try a dose of ketorolac because it is due soon, then consults psychiatry and social work to investigate for secondary gain.
NP in SCD
The clinical case presented illustrates the complex nature of pain assessment and treatment. The resident’s assessment is likely influenced by the negative connotations surrounding pain and opioids, as well as by a lack of consideration of NP as the etiology of the examination findings. Although the contribution of NP to SCD pain has generally become established among most hematologists specializing in SCD, it is undoubtedly less recognized among other care providers who may not be familiar with the nuances of SCD pain management. In fact, general reviews of NP published in the past 3 years do not mention SCD as an etiology of NP.7,8 The slow recognition of this entity may be due in part to the lack of a clearly demonstrated “lesion or disease of the somatosensory nervous system,” as required to meet the strict definition put forth by the International Association for the Study of Pain.9 The lack of discrete nerve injury or lesion (eg, compression, degeneration, infarction) in the case of SCD does not necessarily preclude a diagnosis of NP, however, as much as it suggests a broader interpretation of which neurologic changes represent a “lesion or disease.” As discussed below, the available evidence in SCD includes demonstrated pathologic changes to the nervous system (eg, altered cortical processing networks, altered biochemical structure and function of pain-sensing neurons), as well as patient-reported pain descriptors widely associated with NP, the combination of which seems to meet the aforementioned criteria.10-15 Whatever the reason, due to its lack of broad recognition and the significant heterogeneity among patients, NP is often overlooked and is potentially an important factor in the labeling of difficult-to-treat patients as drug seeking. In this case, the exaggerated response to light touch suggests nervous system sensitization and should prompt specific questions regarding NP descriptors. NP can contribute to both acute and chronic pain. As Figure 1 illustrates, the complex interplay between acute and chronic pain is such that various acute pain types can contribute to chronic pain development. However, acute pain can also result from exacerbations of chronic pain, making it difficult to parse the causative role of NP in a given patient’s pain phenotype. Using quantitative sensory testing (QST), patients have been shown to have global hypersensitivity to mechanical stimuli (assessed at nonpainful reference sites) both at baseline and during hospitalization for acute pain.15,16 Thus, NP should be considered in all settings (eg, acute or chronic pain).
General mechanisms
NP represents a maladaptive response of the somatosensory nervous system to injury, disease, or medications, among other etiologies, whereby a “ramp-up” in sensitivity occurs that results in either pain out of proportion to painful stimuli (hyperalgesia) or pain in response to innocuous stimuli (allodynia).7 The broadest classification divides NP according to etiology as secondary to either central nervous system (CNS) or peripheral nervous system (PNS) insult. PNS and/or CNS injuries can result in sensitization of either the CNS or PNS, which can in turn cause positive (gain of uncomfortable sensation) or negative (loss of normal function) symptoms.7,17 There is evidence of both central and peripheral sensitization in patients with SCD.13,18-20
In SCD, a likely inciting insult to peripheral nerves is chronic inflammatory and oxidative stress that intensifies during recurrent VOCs.17 Recurrent stimulation/damage of nociceptive (pain-sensing) neurons results in increased sensitivity and density of various membrane channels (eg, TRPV1) responsible for the initial transduction of pain signals into action potentials. Altered nociceptors fire at lower stimulation thresholds and from ectopic depolarizations along their axons.7,17,21 Aberrant signaling is then transmitted to dorsal root ganglia (DRG) of the spinal cord, responsible for the synthesis of signals from many localized nociceptors with modulatory inputs from descending pathways. The second-order interneurons originating there, which can be similarly hypersensitized, transmit the resulting signal to the thalamus for further processing and modulation. The importance of DRG hypersensitization is evidenced by studies showing that peripheral amyloid-β fibers normally responsible for light touch signaling will actually trigger the transmission of pain signals to the brain via sensitized DRG interneurons.21 N-methyl-D-aspartate receptors responsible for long-term potentiation of pain signals in the DRG are also believed to be very important to durable central sensitization.22 Cytokines released by overactive glial and mast cells of the CNS cause neuroinflammation that further contributes to abnormal activity of descending inhibitory pathways and subcortical pain-processing networks, resulting in activation rather than inhibition of interneurons of the DRG.17,23,24 The contributions of these alterations to SCD pain are just beginning to be understood.
Evidence of NP in SCD
Some of these pathologic changes have been identified in murine studies of SCD, with their clinical effects confirmed in patients with SCD.17 Clinical evidence for NP in SCD has been found using patient-reported outcome (PRO) measures developed for other NP states, QST, and functional magnetic resonance imaging (fMRI). The reported prevalence of NP in adult patients with SCD ranges from 25% to 40% based on studies using PRO questionnaires, and its presence is significantly associated with older age.15,25 The association with older age supports the mechanistic explanation centered on the cumulative effects of chronic SCD pathobiology.15,25,26 Psychophysical testing results have provided further support for the presence of both central and peripheral sensitization, because on the basis of QST, approximately two-thirds of patients with SCD studied were found to have reduced thresholds for pain in response to thermal stimuli, and approximately one-third in response to mechanical stimuli, compared with healthy control subjects.20,25 One study of patients with SCD compared those with high central sensitization with those with low central sensitization based on QST and found worse pain in the higher-sensitization group, as evidenced by increased VOC frequency, opioid intake, catastrophizing, negative mood, and poorer sleep continuity.27 Additional evidence for central sensitization has been found via fMRI and electroencephalogram studies demonstrating increased activity in pain-processing networks with associated increased pain frequency.11,19,24 Although they have not been validated as true diagnostic biomarkers, elevations in markers of neuroinflammation known to contribute to nervous system sensitization are present in SCD, including substance P at baseline and with further increases during acute pain.24,28,29
Clinical case continued
The patient is eventually discharged with a pain score of 4/10 after noticing slight improvement and after a slow wean of her oxycodone to a slightly higher home dose than she had received previously. After discharge, the shooting pain again worsens, but she continues to take oxycodone most days in order to avoid another hospitalization. She is seen 3 weeks later in the clinic, asking for a better pain management strategy after losing her job.
NP evaluation
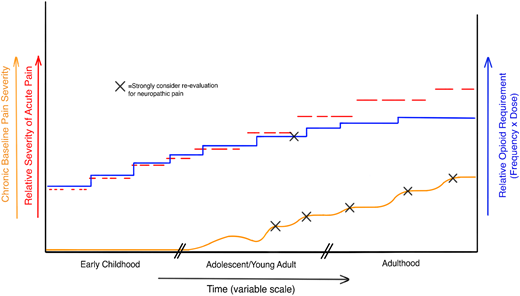
Despite extensive research into its pathobiology, there persists for NP a lack of objective measures or specific treatments. This is especially true with regard to SCD, for although the core nervous system changes may be the same as in other conditions, NP is not an entirely new symptom for patients long experiencing recurrent vaso-occlusive pain. Because of this gradual transition in phenotype from nociceptive to neuropathic/mixed-type pain, these patients may have a harder time recognizing and describing unique NP symptoms. The identification of NP therefore becomes even more heavily dependent on active investigation by the provider in response to subtle clues from the history and physical examination. Evidence that <5% of patients take an NP drug despite its much higher demonstrated prevalence suggests this active investigation is not routine.15,30 Currently, no standardized protocols exist regarding evaluation timing, but we suggest reassessment when there is a clear worsening of pain frequency or an increased opioid dose needed for effective analgesia (Figure 2).
Hypothetical model of SCD pain. Red dashed lines represent acute pain episodes worsening in severity and duration over the lifespan. Blue line represents the required opioid dose to achieve analgesia, which becomes harder and harder to achieve as chronic pain and NP develop over time. Chronic pain can be a result of acute pain episodes and their cumulative damage as well as a cause, because exacerbations of chronic pain/NP may be a trigger for acute pain. Because current assessment tools cannot definitively isolate the contributions of inflammatory pain, nociceptive pain, and NP to overall chronic pain, we suggest formally evaluating for NP when chronic pain shows clear worsening, as well as when pain seems to become refractory to escalating doses of opioid.
Hypothetical model of SCD pain. Red dashed lines represent acute pain episodes worsening in severity and duration over the lifespan. Blue line represents the required opioid dose to achieve analgesia, which becomes harder and harder to achieve as chronic pain and NP develop over time. Chronic pain can be a result of acute pain episodes and their cumulative damage as well as a cause, because exacerbations of chronic pain/NP may be a trigger for acute pain. Because current assessment tools cannot definitively isolate the contributions of inflammatory pain, nociceptive pain, and NP to overall chronic pain, we suggest formally evaluating for NP when chronic pain shows clear worsening, as well as when pain seems to become refractory to escalating doses of opioid.
Acute pain encounters such as the one described represent another important opportunity to inquire about NP symptoms. Descriptors such as burning, electric shocks, pins and needles, pricking, shooting, radiating, allodynia (as evident in the presented clinical case), and pain exacerbated by temperature changes should trigger investigation for NP.26,31,32 The best way to conduct this evaluation is unclear, and currently, no one tool can reliably guide management or measure treatment response. Nonetheless, the measures described in the next subsections can be used to support the diagnosis in concert with clinical indicators and sound judgment based on currently available evidence.
PROs
PRO measures (assessing subjective elements such as pain intensity, character/quality, and interference with daily life) represent the gold standard for pain assessment and cannot ethically be replaced by any single objective measure at this time. As such, new and more specific PROs should be used in conjunction with known biomarkers, imaging, and psychophysical testing to assess and phenotype pain.33 Several PROs for NP evaluation exist, but none have been developed specifically for NP in SCD. The National Institutes of Health has funded the development of 3 PROs applicable to SCD pain assessment: the Patient-Reported Outcome Measurement Information System, the Adult Sickle Cell Quality of Life Measurement Information System, and the Pediatric Quality of Life Inventory Sickle Cell Disease Module. These PROs evaluate and track changes in pain, function, and quality of life.33,34 Only the Patient-Reported Outcome Measurement Information System has tools specifically geared for NP, but the performance of these tools in SCD and their ability to differentiate pain states and track NP changes over time requires further evidence.34 Other PROs that have been used to investigate NP in SCD are briefly summarized in Table 1, but because these measures have thus far been used primarily on a research basis, no algorithms exist to guide timing of their implementation as far as age, frequency, relationship to pain status, or as outcomes evaluating treatment response. Although NP has been demonstrated in pediatric patients, the typical age of onset and risk factors for its development remain unknown.15,35 There is a clear need to further validate the ability of PROs, both alone and in combination with other assessment tools, to confirm the presence of NP and guide treatment. Furthermore, cutoff scores on NP-specific PROs need to be established for SCD that would trigger more time-/resource-intensive comprehensive testing. Given all of these unknowns, a specific recommendation regarding a universally “best” tool for assessing SCD-related NP cannot be made. On a research basis, all questionnaires listed in Table 1 offer unique benefits. For clinicians choosing from among those PROs previously studied in SCD, however, consideration should be given to the intent of the assessment, cost, test length, comprehensive vs “quick” screen, sensitivity/specificity, and the age of study participants.
Comparison of NP assessment tools
| Modality . | Tool . | Description . | Utility . | Limitations . |
|---|---|---|---|---|
| Patient-reported outcome (PRO) (gold standard) | Douleur neuropathique 4 questions |
| • Validated for NP as a self-report tool |
|
| Identification Pain questionnaire |
|
|
| |
| Leeds Assessment of Neuropathic Symptoms and Signs |
|
| • Not formally validated in SCD | |
| Neuropathic Pain Questionnaire |
|
|
| |
| PainDETECT |
|
|
| |
| PAINReportIt |
|
|
| |
| PROMIS |
|
|
| |
| QST | Manual von Frey filaments for mechanical QST, various digital instruments available for mechanical and thermal QST | • Assesses patient response to different stimuli to determine detection and pain thresholds |
|
|
| Imaging |
| • Established chronic pain patterns for each (on a research basis) | • Potential as a more objective measure than others |
|
| Modality . | Tool . | Description . | Utility . | Limitations . |
|---|---|---|---|---|
| Patient-reported outcome (PRO) (gold standard) | Douleur neuropathique 4 questions |
| • Validated for NP as a self-report tool |
|
| Identification Pain questionnaire |
|
|
| |
| Leeds Assessment of Neuropathic Symptoms and Signs |
|
| • Not formally validated in SCD | |
| Neuropathic Pain Questionnaire |
|
|
| |
| PainDETECT |
|
|
| |
| PAINReportIt |
|
|
| |
| PROMIS |
|
|
| |
| QST | Manual von Frey filaments for mechanical QST, various digital instruments available for mechanical and thermal QST | • Assesses patient response to different stimuli to determine detection and pain thresholds |
|
|
| Imaging |
| • Established chronic pain patterns for each (on a research basis) | • Potential as a more objective measure than others |
|
Tools used in published studies of SCD are in bold. Sensitivity/specificity numbers are reported on the basis of performance relative to expert clinical diagnosis. None have been formally validated against expert clinical examination in the SCD population.
Tools incorporating physical examination findings have higher reported sensitivity and specificity than interview-only tools.36
QST
QST represents a more direct method of assessing altered pain sensation to multiple types of stimuli, including mechanical (eg, pressure, pinprick, tactile) and thermal (ie, heat, cold), and has been used in attempts to differentiate peripheral from central sensitization. As more treatments for NP become available, it is possible that they will be divided between those targeting central sensitization and those targeting peripheral sensitization. With continued research correlating QST findings with both imaging and PROs, QST could represent a means of tailoring treatment of individual phenotypes and assessing treatment response. This research has begun in patients with SCD, but continued investigation is needed to better differentiate these states with confidence.25,27,36,37 The psychophysical nature of QST as dependent on patient responses lends to a possible role in supporting PROs as a link between underlying pathophysiology and patient experience. Currently only used on a research basis, QST is time intensive and costly to conduct and is not widely available.
Imaging
Magnetic resonance imaging, fMRI, electroencephalogram, and positron emission tomography have been investigated in the field of NP, but due to the underlying complexity of discovered and yet to be discovered neural networks, a pathognomonic imaging pattern for chronic pain and NP has yet to be described.26 The abnormalities typical of chronic pain have been identified in patients with SCD,11,14,19 but although these studies support the existence of altered central pain-processing mechanisms, similarly to QST, they cannot currently replace patient reports of NP symptoms in the diagnostic process. The majority of centers treating SCD do not have regional access to teams with experience in the identification of pain signatures based on fMRI, but that may change in the years to come as NP becomes more synonymous with SCD and further evidence is uncovered surrounding this modality.
Treatment of NP in SCD
Even in the broader NP literature, there is a lack of strong evidence for any one treatment resulting in significant and consistent improvement. What is widely recommended (and is inconsistent with general practice for SCD), however, is the downgrading of opioids to second- or third-line treatment of NP. The reasons for this recommendation include the likelihood that opioids ineffectively treat NP and the risks of tolerance and significant side effects in the face of escalating doses for chronic pain.24,38-40 The unique parallel (but not independent) development of NP and nociceptive chronic pain in SCD, wherein NP contributes to chronic pain and chronic nociceptive/inflammatory pain contributes to nervous system sensitization, make completely eliminating opioids from a pain regimen not feasible (Figure 2). Similar to other aspects of NP in SCD, there is a paucity of evidence regarding the utility of other therapies. Thus, the recent American Society of Hematology guidelines for chronic SCD pain without an identifiable cause were informed using indirect evidence from patients with fibromyalgia, deemed the disease state most comparable to SCD chronic pain without an identifiable cause.41 These recommendations are in line with those for general NP management with some caveats (in SCD, most medications have not been in widespread use, so no longitudinal data exist).38,42 These medications are listed in Table 2.26,43 Medication choices must be individualized and influenced by side effect profiles in relation to SCD-related comorbidities. Consultation with psychology/psychiatry should also be considered because chronic pain and NP have been shown to negatively affect neurocognitive/psychosocial functioning and quality of life, respectively.10,15
Pharmacologic options for NP treatment
| Therapy . | 2015 Lancet Neurology meta-analysis of treatments for NP39,* . | 2020 ASH guidelines for SCD chronic pain without identifiable cause . | Interventional studies of NP in SCD? . | Considerations . |
|---|---|---|---|---|
| Gabapentinoids (gabapentin, pregabalin) |
|
|
|
|
| Serotonin and norepinephrine reuptake inhibitors |
|
| • None | • Risk of suicidal ideation in children |
| Tricyclic antidepressants |
|
| • None |
|
| Topical patches (lidocaine 5%, capsaicin 8%) |
| • Not addressed | • Phase 2 single-arm pediatric study (lidocaine for acute pain): well tolerated, evidence for clinical efficacy | |
| Tramadol |
| • Not addressed | • None | |
| Strong opioids |
| • Current standard, no robust comparison studies found between chronic opioid and nonopioid treatment | • None for neuropathic pain | • Difficult to replace due to unique interplay between acute and chronic pain, nociceptive pain and NP |
| Ketamine | • Inconclusive evidence |
| • Studied for VOC/acute pain treatment with demonstrated benefit | • Not mentioned by either resource but used for acute SCD pain and for NP in separate studies |
| Trifluoperazine | • Not addressed | • Not addressed | • Phase 1 open label study: safe, evidence of efficacy |
| Therapy . | 2015 Lancet Neurology meta-analysis of treatments for NP39,* . | 2020 ASH guidelines for SCD chronic pain without identifiable cause . | Interventional studies of NP in SCD? . | Considerations . |
|---|---|---|---|---|
| Gabapentinoids (gabapentin, pregabalin) |
|
|
|
|
| Serotonin and norepinephrine reuptake inhibitors |
|
| • None | • Risk of suicidal ideation in children |
| Tricyclic antidepressants |
|
| • None |
|
| Topical patches (lidocaine 5%, capsaicin 8%) |
| • Not addressed | • Phase 2 single-arm pediatric study (lidocaine for acute pain): well tolerated, evidence for clinical efficacy | |
| Tramadol |
| • Not addressed | • None | |
| Strong opioids |
| • Current standard, no robust comparison studies found between chronic opioid and nonopioid treatment | • None for neuropathic pain | • Difficult to replace due to unique interplay between acute and chronic pain, nociceptive pain and NP |
| Ketamine | • Inconclusive evidence |
| • Studied for VOC/acute pain treatment with demonstrated benefit | • Not mentioned by either resource but used for acute SCD pain and for NP in separate studies |
| Trifluoperazine | • Not addressed | • Not addressed | • Phase 1 open label study: safe, evidence of efficacy |
NNT, number needed to treat.
The Lancet Neurology meta-analysis was selected for simplicity as being representative of broader NP recommendations from the pain literature (which do not include SCD). American Society of Hematology guidelines for chronic pain are based on indirect evidence from patients with fibromyalgia.
Conclusion
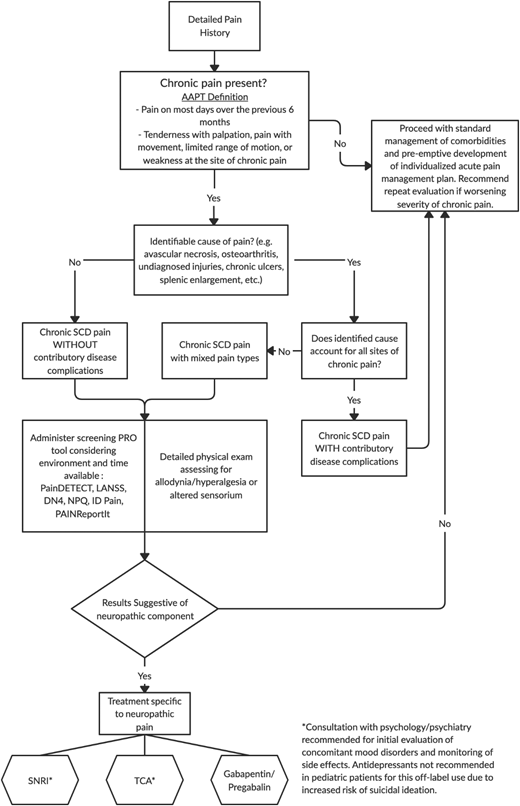
Given the lack of validated assessment tools or well-studied treatments for SCD-related NP, detailed, evidence-based recommendations cannot be made at this time. Currently available evidence of its prevalence and treatment of related conditions, however, are at least sufficient to prevent the passive allowance of suffering in affected patients. To that end, we offer a potential algorithm for evaluation and management (Figure 3) that, with ongoing research, may continue to expand in the near future.
Suggested assessment/management algorithm for NP in SCD based on the best available evidence.
Suggested assessment/management algorithm for NP in SCD based on the best available evidence.
Correspondence
Alexander Glaros, MD, Children’s Hospital of Michigan, 3rd Floor Carls Building, 3901 Beaubien Blvd, Detroit, MI 48201; e-mail: aglaros@dmc.org.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.