Abstract
Platelets express ABO antigens and are collected in plasma, which contains ABO antibodies as would be consistent with the donor ABO group. Platelet ABO antigens that are incompatible with recipient ABO antibodies may have accelerated clearance from circulation and result in lower count increments. ABO antibodies that are passively transferred from donor plasma may result in hemolysis of recipient red blood cells. Although platelets do not express Rh antigens, they contain small numbers of intact red blood cells or fragments, which can lead to alloimmunization in the recipient. Alloimmunization to the RhD antigen may occur when platelets obtained from RhD-positive donors are transfused to RhD-negative recipients. All of these compatibility considerations must be balanced against the available supply, which may be limited due to the 5- to 7-day shelf life of platelets. This articles describes considerations for platelet ABO and RhD selection for platelet transfusions, including the impact of major ABO incompatibility on count increments, the risks of hemolysis associated with minor ABO incompatibility, and the risk of RhD alloimmunization when RhD-negative patients receive platelets obtained from RhD-positive donors.
Learning Objectives
Describe the role of ABO matching in platelet transfusions, including the impact of major ABO incompatibility on count increments and the risks for hemolysis associated with minor ABO incompatibility
Recognize the risk of RhD alloimmunization when RhD-negative patients receive platelets obtained from RhD-positive donors and describe how Rh immune globulin is used to mitigate this risk
Clinical case
A 60-year-old woman with newly diagnosed acute myeloid leukemia is admitted for induction therapy. She eventually develops chemotherapy-associated thrombocytopenia. The clinical team requests platelet transfusion when her platelet count drops below 10 × 103 per microliter. The patient is A negative. The blood bank has a limited supply of apheresis platelets, including a “low-titer” O-positive unit expiring today at midnight, a B-negative unit expiring in 2 days, and an A-positive unit expiring in 3 days.
What are the risks and benefits associated with each of the platelet options for this patient, and which unit should the blood bank provide?
Platelet compatibility
When considering a platelet transfusion, one must consider the ABO compatibility of the platelets themselves, as well as the accompanying plasma. Platelets, like red blood cells (RBCs), express ABO antigens, although expression is variable and strongly expressed in only 4% to 7% of individuals.1 Platelets are also suspended in roughly 1 unit of donor plasma, which contains the ABO antibodies predicted by the donor blood type (Table 1). Platelets do not express Rh antigens but contain small numbers of RBCs or fragments, which can cause alloimmunization to red cell antigens, including the RhD antigen when platelets obtained from RhD-positive donors are transfused to RhD-negative recipients.
Platelet antigens and plasma antibodies based on donor ABO group
| Platelet ABO group . | AB . | A . | B . | O . |
|---|---|---|---|---|
| Antigens expressed on platelet surface | A and B | A | B | None |
| Antibodies present in plasma | None | Anti-B | Anti-A | Anti-A and anti-B |
| Platelet ABO group . | AB . | A . | B . | O . |
|---|---|---|---|---|
| Antigens expressed on platelet surface | A and B | A | B | None |
| Antibodies present in plasma | None | Anti-B | Anti-A | Anti-A and anti-B |
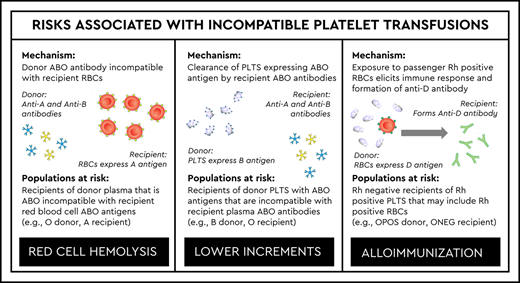
Unlike RBCs, platelets are not at risk for intravascular hemolysis when transfused out of group. However, the accompanying plasma may cause hemolysis when ABO incompatible with recipient RBCs. Because of this risk, AABB standards require transfusion services to have policies concerning transfusion of components that contain significant amounts of incompatible ABO antibodies.2 Additionally, because of the risk for alloimmunization to the RhD antigen, AABB standards also require transfusion services to have policies for the use of RhD-positive RBC‐containing components in RhD-negative recipients.2
In terms of ABO compatibility, platelets may be identical, major incompatible, minor incompatible, or bidirectional incompatible (Table 2). In major incompatibility, donor ABO antigens are incompatible with recipient ABO antibodies. In minor incompatibility, donor ABO antibodies are incompatible with recipient ABO antigens. Both types of incompatibility are present in bidirectional incompatible transfusions.
Platelet ABO compatibility
| ABO . | Platelet recipient ABO group . | ||||
|---|---|---|---|---|---|
| AB . | A . | B . | O . | ||
| Platelet donor ABO group | AB | Identical | Major | Major | Major |
| A | Minor | Identical | Bidirectional | Major | |
| B | Minor | Bidirectional | Identical | Major | |
| O | Minor | Minor | Minor | Identical | |
| ABO . | Platelet recipient ABO group . | ||||
|---|---|---|---|---|---|
| AB . | A . | B . | O . | ||
| Platelet donor ABO group | AB | Identical | Major | Major | Major |
| A | Minor | Identical | Bidirectional | Major | |
| B | Minor | Bidirectional | Identical | Major | |
| O | Minor | Minor | Minor | Identical | |
| RhD . | Platelet recipient RhD type . | ||||
|---|---|---|---|---|---|
| Positive . | Negative . | ||||
| Platelet donor RhD type | Positive | Identical | Mismatch | ||
| At risk for RhD alloimmunization | |||||
| Negative | Mismatch | Identical | |||
| No risk for RhD alloimmunization | |||||
| RhD . | Platelet recipient RhD type . | ||||
|---|---|---|---|---|---|
| Positive . | Negative . | ||||
| Platelet donor RhD type | Positive | Identical | Mismatch | ||
| At risk for RhD alloimmunization | |||||
| Negative | Mismatch | Identical | |||
| No risk for RhD alloimmunization | |||||
ABO identical: donor and recipient have the same ABO antigens and antibodies. Major ABO mismatch: donor ABO antigens are incompatible with recipient ABO antibodies. Minor ABO mismatch: donor ABO antibodies are incompatible with recipient's ABO antigens. ABO bidirectional mismatch: donor ABO antibodies are incompatible with recipient ABO antigens and donor ABO antigens are incompatible with recipient ABO antibodies. Rh mismatch: Rh type of the recipient is different from the Rh type of the donor. RhD-negative recipients of platelets obtained from RhD-positive donors are the only ones at risk for RhD alloimmunization.
RhD compatibility is more nuanced (Table 2). The formation of antibodies against the RhD antigen (ie, anti-D) requires exposure to red cell antigens, such as through pregnancy, transfusion, or transplantation. Thus, transfusion recipients with anti-D antibodies are much less common than those with the anti-A and/or anti-B antibodies that are predictably present based on ABO group. This is because ABO antibodies are formed without red cell exposure (ie, “naturally occurring”).
Platelet transfusions from RhD-positive donors to recipients with anti-D antibodies do not result in hemolysis, because they contain very few RBCs. Only products containing >2 mL of incompatible RBCs require a serologic crossmatch per AABB standards.2 In addition, all blood component donors undergo antibody screening to ensure that plasma-containing components, such as platelets, do not contain non-ABO antibodies (eg, anti-D). Therefore, RhD-positive patients may receive platelets obtained from RhD-positive or RhD-negative donors without risk. Only RhD-negative recipients of platelets obtained from RhD-positive donors are at risk for RhD alloimmunization.
ABO identical platelets
In an ideal world with unlimited resources, platelet transfusions would be ABO identical to the recipient and obtained from RhD-negative donors when the recipient is RhD negative. However, the short shelf life (5-7 days) and supply limitations make this challenging for most transfusion services.3 In addition, the ABO composition of the platelet donor pool may not reflect the ABO distribution of the patient population.4 In an effort to conserve resources, most transfusion services use a first in/first out platelet-transfusion strategy.
Not surprisingly, use of ABO identical platelets has been associated with increased outdate rates.5 Further, provision of ABO identical platelet transfusions has not been shown to provide any clear benefit in terms of clinical outcomes.6 One study in autologous hematopoietic stem cell transplant recipients did not find any impact on morbidity or survival among recipients of ABO nonidentical platelets.7
Major ABO incompatible platelets
Although the name implies increased patient risk, major incompatible platelet transfusions are common and typically uneventful. One study observed higher transfusion reaction rates among recipients of major incompatible platelet transfusions,8 but other studies have not shown an association between ABO compatibility and transfusion reaction rates.9
Unlike major incompatible RBC transfusions, there is no risk for hemolysis. Recent data indicate that ∼31% of platelet transfusions in the United States are major incompatible.10 In this scenario, the recipient has antibody directed against platelet ABO antigens, as would be predicted based on ABO group, which may result in accelerated platelet clearance. Major compatible platelet transfusions are associated with higher count increments. However, this difference is small and not clinically significant in terms of bleeding risk.11 Because major incompatible platelet transfusions result in slightly lower increments, a decreased platelet transfusion interval has also been observed.11 When I investigate patients who appear to be refractory to platelet transfusions, major ABO incompatibility is always in the differential. Patients who are found to have high-titer anti-A or anti-B antibodies may respond better to major compatible platelet transfusions. I typically support these patients with ABO compatible platelet transfusions, when possible.
Minor ABO incompatible platelets
Currently, ∼19% of platelet transfusions in the United States are plasma incompatible.10 Although the name implies negligible risk, minor incompatible transfusions can result in hemolysis of recipient RBCs, which may even be fatal. Although this complication is rare, there have been 6 fatalities reported to the US Food and Drug Administration from 2007 to 2017 that were due to hemolysis from minor ABO incompatible platelet transfusions (Table 3).12 Clinically detectable reactions that are not fatal are also reported in the literature.13 Most case reports of hemolysis following minor ABO incompatible platelets involve transfusion of group O apheresis platelets to group A or AB recipients.14 This is due to that fact that a subset of group O donors produce high-titer anti-A antibodies.15 However, there are case reports associated with non-O donors.16
Fatalities associated with incompatible platelet transfusions reported to the US Food and Drug Administration from 2007 to 2017
| Fiscal year . | Patient ABO . | Platelet ABO . | Antibody titer . |
|---|---|---|---|
| 2015 | B | A | Anti-B (1:2048) |
| 2014 | A | O | Anti-A (1:2048) |
| 2014 | AB | O* | Anti-A and anti-B (1:128)Anti-A and anti-B (1:128) |
| O* | |||
| 2012 | A | O† | “High titer”Data not provided |
| O† | |||
| 2011 | A‡ | O | Anti-A 1:512 for IgM and 1:2048 for IgG25 |
| 2008 | B‡ | O | “High titer”Data not provided. |
| Fiscal year . | Patient ABO . | Platelet ABO . | Antibody titer . |
|---|---|---|---|
| 2015 | B | A | Anti-B (1:2048) |
| 2014 | A | O | Anti-A (1:2048) |
| 2014 | AB | O* | Anti-A and anti-B (1:128)Anti-A and anti-B (1:128) |
| O* | |||
| 2012 | A | O† | “High titer”Data not provided |
| O† | |||
| 2011 | A‡ | O | Anti-A 1:512 for IgM and 1:2048 for IgG25 |
| 2008 | B‡ | O | “High titer”Data not provided. |
IgG, immunoglobulin G; IgM, immunoglobulin M.
Recipient received 2 units of group O platelets from the same donor.
Recipient received 2 units of group O platelets from 2 different donors.
Recipient’s blood group recently changed following ABO-mismatched hematopoietic stem cell transplant.
Hemolytic reactions occur most commonly if the antibody titer exceeds 100 in saline or 400 with antiglobulin reagent.14 Some centers attempt to mitigate this risk by performing screening titers.17 In the United States, there is no standardized titer method and no agreement on the definition of a high-titer unit.18-20 One center, which investigated using a saline titer cutoff of 250, found that 25% of group O platelets would be identified as high titer and, therefore, limited to group O recipients.21
Interestingly, units retrospectively identified as high titer are not always predictive of hemolysis.22,23 This suggests that risk may be impacted by other recipient factors. Protective mechanisms include the presence of ABO antigen on endothelial cells and the presence of soluble A and/or B substance in the plasma of secretors, both of which may decrease the interaction of incompatible antibodies with recipient RBCs.24
Other risk-mitigation approaches have been used to address the volume of ABO incompatible plasma (Table 4). Some centers limit the allowable volume of incompatible plasma over defined periods to decrease the risk of a cumulative effect of transfused incompatible antibody.17,25 Pooled whole blood–derived platelets may dilute the plasma from any high-titer donors, but case reports exist of hemolysis following pooled platelet transfusions.26
Summary of platelet minor ABO incompatibility risk-mitigation strategies
| Mitigation strategy . | Drawbacks . |
|---|---|
| Issue only ABO identical or compatible units | Identical or compatible units may not always be available in inventoryPotential for increased outdate rates |
| Limit volume of incompatible plasma | Compatible units may not always be available in inventoryPotential for increased outdate rates |
| Provide pooled whole blood–derived platelets | Pooled platelets are not widely available in the United StatesHemolytic transfusion reactions have been reported in pooled platelet units |
| Provide units suspended in PAS | PAS platelets are not widely available in the United StatesHemolytic transfusion reactions have been reported in PAS units |
| Provide low-titer units | Titer method is not standardizedNo agreed upon high-titer cutoffSubstantial percentage of group O units may be high titer, depending on cutoffSaline titer methods may miss units with high-titer IgG antibodiesHemolytic transfusion reactions have been reported in low-titer units |
| Volume reduction | Shortens shelf lifeDecreases platelet contentIncreases the time required for unit preparation and blood bank workloadNot feasible for emergency transfusions |
| Washing | Shortens shelf lifeDecreases platelet contentIncreases the time required for unit preparation and blood bank workloadNot feasible for emergency transfusions |
| Mitigation strategy . | Drawbacks . |
|---|---|
| Issue only ABO identical or compatible units | Identical or compatible units may not always be available in inventoryPotential for increased outdate rates |
| Limit volume of incompatible plasma | Compatible units may not always be available in inventoryPotential for increased outdate rates |
| Provide pooled whole blood–derived platelets | Pooled platelets are not widely available in the United StatesHemolytic transfusion reactions have been reported in pooled platelet units |
| Provide units suspended in PAS | PAS platelets are not widely available in the United StatesHemolytic transfusion reactions have been reported in PAS units |
| Provide low-titer units | Titer method is not standardizedNo agreed upon high-titer cutoffSubstantial percentage of group O units may be high titer, depending on cutoffSaline titer methods may miss units with high-titer IgG antibodiesHemolytic transfusion reactions have been reported in low-titer units |
| Volume reduction | Shortens shelf lifeDecreases platelet contentIncreases the time required for unit preparation and blood bank workloadNot feasible for emergency transfusions |
| Washing | Shortens shelf lifeDecreases platelet contentIncreases the time required for unit preparation and blood bank workloadNot feasible for emergency transfusions |
IgG, immunoglobulin G; PAS, platelet additive solution.
Another dilution strategy is the use of platelets suspended in platelet additive solution (PAS), a manufacturing modification that replaces 65% of the donor plasma.27 Studies have shown that PAS can reduce ABO antibody titers by 50%, which would reduce the number of high-titer group O units and expand the potential for out-of-group transfusions.28 However, only 8.5% of surveyed facilities in the United States were using PAS platelets in 2017.29 Case reports also exist of hemolysis following PAS platelet transfusions.30
Volume reduction and washing are component-modification strategies that also reduce risk from ABO incompatible plasma. Both of these component modifications increase the time required for component preparation and reduce the product shelf life to 4 hours. One center that implemented volume reduction for all minor ABO incompatible platelet transfusions estimated that the blood bank workload increased by 0.34 full-time equivalent.25
RhD compatibility
Platelets do not express Rh antigens, but platelet components contain residual intact RBCs or fragments that can result in alloimmunization to RBC antigens, including RhD.3 The volume of contaminating RBCs varies by component-manufacturing method. Whole blood platelet production in the United States utilizes the platelet-rich plasma method, whereas Europe and Canada use the buffy coat method.32 However, use of whole blood–derived platelets in the United States is limited, and 91% of platelet doses in 2017 were apheresis platelets.33 Whole blood–derived pooled platelets may contain up to 0.3 mL of residual RBCs, whereas apheresis platelets contain <0.001 mL.34 This is likely why the risk of alloimmunization appears to be higher for whole blood–derived platelets compared with apheresis platelets.35
Although there have been reports of RhD alloimmunization following platelet transfusions, this appears to be an uncommon complication.36 One center that attempts to provide platelets obtained from RhD-negative donors to RhD-negative patients has observed that this practice results in fewer of these patients receiving ABO identical transfusions.4
In a recent study, only 39% of platelet transfusions to RhD-negative patients were obtained from RhD-negative donors.10 Current clinical practice guidelines recommend prophylaxis with Rh immune globulin (RhIg) when RhD-negative girls or women of childbearing potential receive platelets obtained from RhD-positive donors.37 A standard 300-μg dose is sufficient for prevention of alloimmunization.38 RhIg has a half-life of 3 weeks, and the volume of RBCs in each platelet dose is quite small. Therefore, a 300-μg dose should provide prophylaxis for multiple transfusions of platelets obtained from RhD-positive donors over a 2- to 4-week period. Given the low risk, some centers advocate the use of platelets without consideration of RhD status and without RhIg prophylaxis.39,40
Clinical case
Returning to the 60-year-old woman with newly diagnosed acute myeloid leukemia who requires platelet transfusion for chemotherapy-induced thrombocytopenia, in the example provided, the patient is A negative and the blood bank has a “low-titer” O-positive unit expiring today at midnight, a B-negative unit expiring in 2 days, and an A-positive unit expiring in 3 days.
The oldest platelet, and the one that should be used if inventory management is the only consideration, is the “low-titer” O-positive unit expiring today at midnight. The fact that it is “low titer” indicates that the blood bank routinely performs titer assessment of group O platelets and that this unit has anti-A and anti-B titers below the critical cutoff defined by this laboratory. Therefore, the risk for hemolysis is low if the unit is selected for this group A patient. This platelet is from an RhD-positive donor and poses a theoretical risk for RhD alloimmunization if given to this RhD-negative patient. However, given her age, there is no concern for a future pregnancy complicated by HDFN.
The second option is the B-negative platelet expiring in 2 days. Use of this unit may result in outdate of the O-positive unit that is expiring sooner. This unit is also minor ABO and major ABO incompatible (ie, bidirectional). Because it is not designated a “low-titer” unit, it suggests that titer of ABO antibodies has not been assessed. However, this unit is from an RhD-negative donor, which negates any risk for RhD alloimmunization in this patient.
The final option is the A-positive unit expiring in 3 days. As above, use of this unit may result in outdate of the O-positive and the B-negative units, which both expire sooner. This unit is ABO identical but is obtained from an RhD-positive donor.
Given these options, my hospital blood bank would most likely issue the O-positive platelet. If the group O unit were high titer, however, we would select the A-positive unit over the B-negative unit without anti-A titer. In either case, I would not offer prophylaxis with RhIg to this patient.
Summary
Management of the platelet inventory requires careful consideration, given the short shelf life of this product. The supply does not always allow for provision of ABO identical and/or platelets obtained from RhD-negative donors for RhD-negative patients. Risks of giving non-ABO identical transfusions include decreased count increments and shorter transfusion intervals for major ABO incompatible transfusions and hemolysis of recipient RBCs for minor ABO incompatible transfusions. Provision of platelets obtained from RhD-positive donors for RhD-negative patients may result in alloimmunization to the RhD antigen. This risk appears to be quite low but can be mitigated by the use of RhIg in at-risk populations.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.
CorrespondenceNancy Dunbar, Dartmouth-Hitchcock Medical Center, One Medical Center Dr, Lebanon, NH 03756; e-mail: nancy.m.dunbar@hitchcock.org.