Abstract
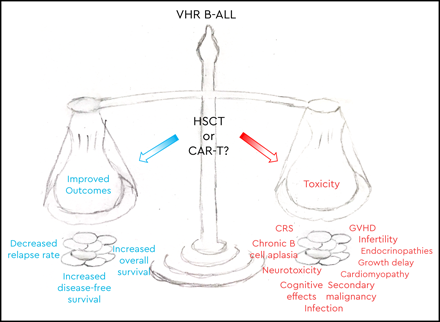
For subgroups of children with B-cell acute lymphoblastic leukemia (B-ALL) at very high risk of relapse, intensive multiagent chemotherapy has failed. Traditionally, the field has turned to allogeneic hematopoietic stem cell transplantation (HSCT) for patients with poor outcomes. While HSCT confers a survival benefit for several B-ALL populations, often HSCT becomes standard-of-care in subsets of de novo ALL with poor risk features despite limited or no data showing a survival benefit in these populations, yet the additive morbidity and mortality can be substantial. With the advent of targeted immunotherapies and the transformative impact of CD19-directed chimeric antigen receptor (CAR)–modified T cells on relapsed or refractory B-ALL, this approach is currently under investigation in frontline therapy for a subset of patients with poor-risk B-ALL: high-risk B-ALL with persistent minimal residual disease at the end of consolidation, which has been designated very high risk. Comparisons of these 2 approaches are fraught with issues, including single-arm trials, differing eligibility criteria, comparisons to historical control populations, and vastly different toxicity profiles. Nevertheless, much can be learned from available data and ongoing trials. We will review data for HSCT for pediatric B-ALL in first remission and the efficacy of CD19 CAR T-cell therapy in relapsed or refractory B-ALL, and we will discuss an ongoing international phase 2 clinical trial of CD19 CAR T cells for very-high-risk B-ALL in first remission.
Learning Objectives
Recognize the need for novel mechanistic approaches for children with poor-risk B-ALL
Identify the indications and evidence for allogeneic HSCT in first complete remission for poor-risk ALL
Understand the efficacy data for CD19 CAR T-cell therapies in refractory B-ALL and review the ongoing trial of CD19 CAR T cells in frontline therapy
Clinical case
A 15-year-old girl diagnosed with B-cell acute lymphoblastic leukemia (B-ALL) receives 4-drug induction chemotherapy according to a high-risk (HR) protocol. Minimal residual disease (MRD) at the end of induction (EOI) is detectable at 0.26%. After HR consolidation chemotherapy, MRD remains positive at 0.23%. The poor prognosis with continuation of standard HR chemotherapy regimens and alternative therapy options are discussed.
Introduction
Survival rates for children with de novo B-ALL approach 90% in the modern era, improving dramatically over the past 60 years with intensification of multiagent chemotherapy, risk stratification, and further intensification for subgroups of patients at higher risk of relapse.1 Despite these improvements in outcome for the majority of patients, subgroups of patients remain at very high risk of relapse with current intensive chemotherapy regimens. Some of these patients are identified by the underlying genomics of the leukemic blasts, but many poor-risk ALLs can be identified on the basis of a poor early response to therapy. Multiple studies have shown MRD response to be the single most important independent prognostic indicator.2-5 Data from a Children’s Oncology Group (COG) phase 3 study for HR B-ALL, AALL0232 (ClinicalTrials.gov identifier: NCT00075725), showed extremely poor 5-year disease-free survival (DFS) in patients with persistent MRD by multiparameter flow cytometry after 2 cycles of chemotherapy. Patients with MRD ≥0.01% at the end of consolidation (EOC) had a 5-year DFS of 39% compared with 79% for those with EOI MRD ≥0.1% but EOC MRD <0.01% (P < .0001).2 Persistent disease after 3 months of multiagent chemotherapy and high risk of relapse despite intensive multiagent chemotherapy suggests that these leukemias are chemotherapy refractory. These patients are in need of novel therapeutic approaches.
One approach used for poor-risk ALL that carries a high risk of relapse with standard chemotherapy regimens alone is hematopoietic stem cell transplantation (HSCT). With the advent of targeted immunotherapies such as CD19-directed chimeric antigen receptor (CAR)–modified T cells, which have demonstrated transformative outcomes in relapsed/refractory (R/R) B-ALL, this approach is being studied in 1 poor-risk ALL population, HR B-ALL with persistent MRD, which is defined as very high risk (VHR) on current COG ALL protocols. We will discuss the evidence and ongoing questions for both of these approaches.
Allogeneic transplantation for poor-risk ALL in first complete remission
Traditionally, HSCT in first complete remission (CR1) has been considered in ALL for poor-risk subgroups that have demonstrated poor outcomes with standard chemotherapy.6 Indications for HSCT in CR1 vary across cooperative trial groups and are evolving,7 and the benefit of HSCT varies by indication or is not well studied.
Indications
Common indications for transplantation for ALL in CR1 across most trial groups have included induction failure and severe hypodiploidy (Table 1).8 Several other criteria that were common in previous studies, such as a high white blood cell (WBC) count at presentation, Philadelphia chromosome positivity (Ph+), or translocation (4;11), are no longer considered strict indications for transplantation.8,9 Cooperative groups differ in their response-based indications for HSCT, with some European groups taking children to HSCT in CR1 on the basis of a poor response to a prednisone prophase in combination with other criteria: Ph+, t(4;11), WBC >×109/L, or slow response.10 Many groups also consider HSCT if MRD is positive at ≥10−3 at week 12.10 The COG used a different response-based criteria on a previous generation of trials, with HSCT considered for patients with MRD ≥1% at EOI that persists despite receiving 2 additional weeks of induction therapy.6 Because most studies combine indications, and HSCT may be of greater benefit for some children than for others, it is challenging to assess outcomes in specific subsets.
Summary of HSCT indications and outcomes for ALL in CR1 on pediatric trials
| Trial . | Years of enrollment . | Region . | Criteria for HSCT in CR1 . | N* . | Outcomes . | Comments . |
|---|---|---|---|---|---|---|
| AALL00316 | 2002-2006 | USA | Hypodiploidy, MLL rearrangement plus SER, induction failure | 30 | Hypodiploidy: 4-year DFS for chemotherapy, 50% ± 11% vs HSCT, 62% ± 14% (P = .65). Induction failure: 4-year DFS for chemotherapy, 44% ± 23%; chemotherapy vs HSCT, 75% ± 19% (P = .14) | Ph+ ALL excluded from analysis; nonrandomized study |
| Total Therapy 1312,49 | 1991-1998 | USA | Ph+ ALL, induction failure | 57 | Combined Total Therapy 13/14 trials 5-year OS: 28% (95% CI, 17%-40%) | Nonrandomized study |
| Total Therapy 144,12 | 1998-1999 | USA | Ph+ ALL, induction failure | Nonrandomized study; trial terminated early because of excess toxicity | ||
| Total Therapy 1512,50 | 2000-2007 | USA | Ph+ ALL, induction failure; MRD ≥1% after 6 weeks of induction | 37 | 5-year OS: 65% (95% CI, 46%-78%) | Nonrandomized study |
| ALL BFM-9051 | 1990-1995 | Europe | Induction failure, Ph+ ALL or PPR and T-ALL; myeloid marker BFM-RF ≥1.7 or t(4;11) | 35 | NR | Nonrandomized study |
| ALL BFM-9511 | 1995-2000 | Europe | Induction failure, Ph+ ALL, t(4;11), PPR and T-ALL or WBC ≥100 × 109/L; only patients with matched related donor underwent HSCT | 77 | 5-year DFS for chemotherapy: 40.6% (SE, 3.1%); chemotherapy vs HSCT, 56.7% (SE, 5.7%) (hazard ratio, 0.67; 95% CI, 0.46-0.99; P = .02) | Patients were randomly assigned between chemotherapy and related-donor HSCT |
| AEIOP-BFM ALL 200024 | 2000-2006 | Europe |
| 81 | 5-year DFS for chemotherapy vs HSCT: (1) 67.7% (SE=6.3) vs 83.3% (SE=10.8; p=0.31); (2) 47.2% (SE=6.6) vs 51.1% (SE=9.6; p=0.74); (3) 54.7% (SE=13.6) vs 50.5% (SE=8; p=0.79) | Ph+ ALL excluded; nonrandomized study; required matched donor (related for subgroups 1/2) for HSCT |
| NOPHO ALL-925 | 1992-2001 | Scandinavia | No uniform criteria | 57 | NR | Nonrandomized study |
| NOPHO ALL-20005 | 2002-2007 | Scandinavia | Induction failure, Ph+ ALL, WBC ≥200 × 109/L, MLL rearrangement and age older than 10 years, hypodiploidy (<34); optional: MRD ≥10−3 at 3 months | 62 | NR | Nonrandomized study |
| NOPHO ALL-200825 | 2008-2016 | Scandinavia | Induction failure, MRD ≥0.1% at day 79; optional: hypodiploidy (<44) with good response | 71 | DFS, 79.1% (95% CI, 69.8%-89.6%) at median follow-up of 5.5 years since HSCT | Ph+ ALL patients excluded; nonrandomized study |
| Trial . | Years of enrollment . | Region . | Criteria for HSCT in CR1 . | N* . | Outcomes . | Comments . |
|---|---|---|---|---|---|---|
| AALL00316 | 2002-2006 | USA | Hypodiploidy, MLL rearrangement plus SER, induction failure | 30 | Hypodiploidy: 4-year DFS for chemotherapy, 50% ± 11% vs HSCT, 62% ± 14% (P = .65). Induction failure: 4-year DFS for chemotherapy, 44% ± 23%; chemotherapy vs HSCT, 75% ± 19% (P = .14) | Ph+ ALL excluded from analysis; nonrandomized study |
| Total Therapy 1312,49 | 1991-1998 | USA | Ph+ ALL, induction failure | 57 | Combined Total Therapy 13/14 trials 5-year OS: 28% (95% CI, 17%-40%) | Nonrandomized study |
| Total Therapy 144,12 | 1998-1999 | USA | Ph+ ALL, induction failure | Nonrandomized study; trial terminated early because of excess toxicity | ||
| Total Therapy 1512,50 | 2000-2007 | USA | Ph+ ALL, induction failure; MRD ≥1% after 6 weeks of induction | 37 | 5-year OS: 65% (95% CI, 46%-78%) | Nonrandomized study |
| ALL BFM-9051 | 1990-1995 | Europe | Induction failure, Ph+ ALL or PPR and T-ALL; myeloid marker BFM-RF ≥1.7 or t(4;11) | 35 | NR | Nonrandomized study |
| ALL BFM-9511 | 1995-2000 | Europe | Induction failure, Ph+ ALL, t(4;11), PPR and T-ALL or WBC ≥100 × 109/L; only patients with matched related donor underwent HSCT | 77 | 5-year DFS for chemotherapy: 40.6% (SE, 3.1%); chemotherapy vs HSCT, 56.7% (SE, 5.7%) (hazard ratio, 0.67; 95% CI, 0.46-0.99; P = .02) | Patients were randomly assigned between chemotherapy and related-donor HSCT |
| AEIOP-BFM ALL 200024 | 2000-2006 | Europe |
| 81 | 5-year DFS for chemotherapy vs HSCT: (1) 67.7% (SE=6.3) vs 83.3% (SE=10.8; p=0.31); (2) 47.2% (SE=6.6) vs 51.1% (SE=9.6; p=0.74); (3) 54.7% (SE=13.6) vs 50.5% (SE=8; p=0.79) | Ph+ ALL excluded; nonrandomized study; required matched donor (related for subgroups 1/2) for HSCT |
| NOPHO ALL-925 | 1992-2001 | Scandinavia | No uniform criteria | 57 | NR | Nonrandomized study |
| NOPHO ALL-20005 | 2002-2007 | Scandinavia | Induction failure, Ph+ ALL, WBC ≥200 × 109/L, MLL rearrangement and age older than 10 years, hypodiploidy (<34); optional: MRD ≥10−3 at 3 months | 62 | NR | Nonrandomized study |
| NOPHO ALL-200825 | 2008-2016 | Scandinavia | Induction failure, MRD ≥0.1% at day 79; optional: hypodiploidy (<44) with good response | 71 | DFS, 79.1% (95% CI, 69.8%-89.6%) at median follow-up of 5.5 years since HSCT | Ph+ ALL patients excluded; nonrandomized study |
Berlin-Frankfurt-Muenster risk factor (BFM-RF) calculated by 0.2 × log (peripheral blood blasts per μL + 1) + 0.06 × liver size in centimeters below the costal margin + 0.04 × spleen size in centimeters below the costal margin.
NR, not reported; NOPHO, optional indication for HSCT; OS, overall survival; PGR, prednisone good; PPR, poor prednisone response; SE, standard error; SER, slow early response; WBC, white blood cell count.
Number of patients who underwent HSCT.
Outcomes
The prospective BFM-95 trial of chemotherapy vs HSCT for children with VHR ALL (defined in Table 1) demonstrated a survival benefit to HSCT.11 A retrospective analysis of patients with ALL treated on 3 consecutive St Jude trials (Total Therapy 13, -14, -15) examined HSCT for HR patients (Table 1) and showed improved survival for HSCT patients over time, regardless of donor type.12 A retrospective analysis of the consecutive Italian trials AEIOP-88, -91, -95, and -2000 examined the role of HSCT for patients with HR ALL in CR1. Indications for HSCT varied between the trials, but the overall 10-year DFS was 61%.13 In summary, several trials have demonstrated a survival benefit with HSCT for poor-risk ALL in CR1 (Table 1); however, it should be noted that these trials were not randomized and they carried the inherent bias of necessitating remission and a matched donor for HSCT; several of the studied indications would no longer be considered, with improvements in risk stratification, chemotherapy regimens, and targeted therapies.8
Ph+ ALL
Previously, Ph+ ALL was considered an absolute indication for HSCT in CR1. However, the risk-benefit ratio of transplantation has been drastically altered by the availability of the tyrosine kinase inhibitor imatinib. A retrospective analysis published in 2000, before the availability of imatinib, demonstrated a 65% event-free survival (EFS) in children with Ph+ ALL with transplant vs 25% EFS with chemotherapy alone, establishing Ph+ ALL as a clear indication for HSCT.14 The COG AALL0031 trial later demonstrated that patients with Ph+ ALL treated with imatinib had significantly improved survival relative to historical controls and that there was no survival benefit for patients treated with imatinib who underwent HSCT.15,16 On the basis of these data, an ongoing international intergroup trial reserves HSCT for patients with induction failure or persistent MRD.
Hypodiploidy
Children with hypodiploid ALL, here defined as modal chromosome number <44 chromosomes or DNA index <0.81, have an inferior prognosis with standard or intensified chemotherapy and thus have been historically considered for HSCT in CR1, but improved outcomes have not been demonstrated.17-19 A recent retrospective analysis of 131 children with hypodiploid ALL enrolled on the COG biology study AALL03B1 failed to demonstrate a survival benefit of HSCT in CR1 (5-year EFS: 57.4% with HSCT vs 47.8% without HSCT; P = .49).17 Another recent retrospective study of patients with hypodiploid ALL enrolled in several international cooperative trials also failed to demonstrate a benefit of HSCT.18 Lack of randomized direct comparisons limit these data; nevertheless, they call into question the consideration of HSCT based solely on poor prognosis.
Induction failure
Most cooperative groups consider induction failure, variably defined as M3 marrow (>25% leukemic blasts) or failure to achieve morphologic remission (<5% leukemic blasts) after 1 month of induction chemotherapy, as an indication for allogeneic transplantation in CR1.7 In a retrospective analysis of 1041 children with ALL and induction failure treated across 14 cooperative groups between 1985 and 2000, HSCT from any donor seemed to benefit children with T-cell ALL and some patients with B-ALL.20 Matched sibling transplants were of benefit to patients older than age 6 years with B-ALL, but not other types of transplants. In children younger than age 6 years with B-ALL, chemotherapy provided significantly better survival rates than HSCT.20 These results must be interpreted with caution because treatment-related mortality of HSCT has improved significantly in the intervening period since 1985.21
Persistent MRD
With the increased availability of MRD and the recognition of its prognostic utility, persistent MRD is increasingly used as an indication for transplantation.22,23 The AIEOP-BFM ALL 2000 trial stratified children with persistent MRD ≥10−3 at day 78 of therapy to HSCT, but found no statistically significant difference in DFS between HSCT and chemotherapy alone (Table 1).24 The Nordic Society of Paediatric Haematology and Oncology (NOPHO) cooperative group used MRD >5% at EOI or persistent MRD ≥10−3 after 3 months of therapy as a potential indication for transplant on the NOPHO ALL-2000 trial and allocated these patients to intensified chemotherapy and HSCT on the subsequent ALL2008 trial.5 NOPHO recently retrospectively examined HSCT outcomes in CR1 and reported a 5-year DFS of 79.1%.25 Persistent MRD was the indication for HSCT in only 35% of this cohort, and 17% received a transplant without a protocol indication. The population studied differed from the COG AALL0232 population referenced above in several potentially relevant features; however, these and other promising results in CR1 led many to consider HSCT for persistent MRD, for example, the current prospective examination on the European trial AEIOP-BFM-ALL 2009.26 Nevertheless, HSCT outcomes are also affected by persistent MRD, but these studies measure MRD later, primarily in the peritransplant period. MRD that remains detectable at a level of 10−3 or 0.1% pre-HSCT increases the risk of relapse,23,27 prompting several groups to incorporate intensive HR chemotherapy blocks before HSCT. Although the clearance or reduction of MRD to a low level before HSCT may improve DFS, data on MRD reduction are limited, and these blocks are associated with a high rate of grade 3 to 4 toxicities, notably infections in two-thirds of patients.28 Finally, post-HSCT MRD was more predictive of relapse risk on a multicenter observational study.23 It is not clear whether the level of MRD itself is prognostic, or if MRD is a marker of the underlying leukemia biology. Although chemotherapy refractory leukemias may be responsive to the immune surveillance provided by allogeneic HSCT, aggressive leukemias may not tolerate the delay in immune surveillance before donor T-cell engraftment.
Toxicity
The risk-benefit analysis for HSCT necessarily considers the morbidity and mortality associated with HSCT, both of which have improved dramatically over the past 30 years.21,29 These improvements are due in part to improved matching of donors and recipients, leading to decreased graft-versus-host disease (GVHD), improved treatments for GVHD, and improved supportive care.21,27,29 Nevertheless, rates of grade 3 to 4 acute GVHD and non-relapse mortality remain substantial, between 10% and 15% and between 5% and 15%, respectively.27,29,30 In addition, long-term toxicities related to chronic GVHD or conditioning regimen include endocrinopathies, growth delay, organ dysfunction, and infertility. The role of HSCT in pediatric ALL continues to be a moving target, as survival benefit and significant but improving toxicities are balanced against novel and targeted therapies.
CAR T-cell therapy for VHR B-ALL in CR1
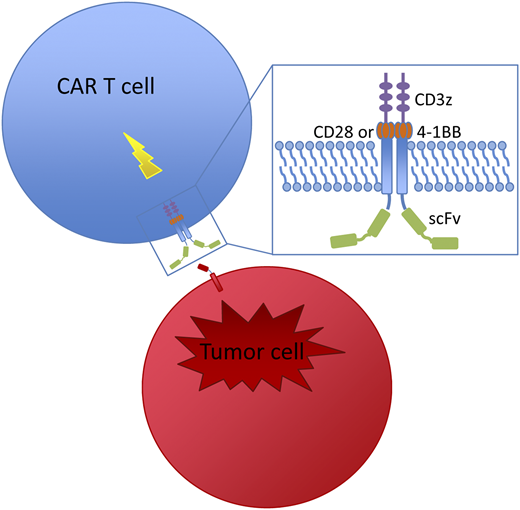
Chemotherapy refractory disease remained an insurmountable challenge for most novel therapies and HSCT until the advent of CAR T-cell therapy. T cells engineered to express a CAR, which links an antigen recognition domain with T-cell signaling domains, are activated by CAR engagement by their target, producing a cytotoxic T-cell response that kills the bound antigen-expressing cell (Figure 1). In clinical trials for multiply relapsed/refractory (R/R) B-ALL, CAR-modified T cells targeting CD19 produced CR rates exceeding 80% to 90% (Table 2).31-37 Tisagenlecleucel (CTL019) became the first CAR T-cell therapy approved by the US Food and Drug Administration in August 2017. This CAR T-cell product, developed by the University of Pennsylvania (Penn), Children’s Hospital of Philadelphia (CHOP), and Novartis, uses an anti-CD19 scFv domain for B-cell targeting and the 4-1BB domain for costimulation.38
Structure and mechanism of CAR-modified T cells. T cells are engineered to express a CAR, which links an extracellular antibody domain (scFv) to intracellular T-cell signaling domains, the CD3 zeta cytoplasmic domain and a costimulatory domain (CD28 or 4-1BB). Once engaged by their target, CARs activate a cytotoxic T-cell response that kills the bound antigen-expressing cell.
Structure and mechanism of CAR-modified T cells. T cells are engineered to express a CAR, which links an extracellular antibody domain (scFv) to intracellular T-cell signaling domains, the CD3 zeta cytoplasmic domain and a costimulatory domain (CD28 or 4-1BB). Once engaged by their target, CARs activate a cytotoxic T-cell response that kills the bound antigen-expressing cell.
Summary of CD19-directed CAR T-cell trials in pediatric R/R ALL
| Trial . | Costimulatory domain . | Population . | N* . | CR rate (%) . | Subsequent HSCT n (%)† . | Outcome . | CAR T-cell persistence . |
|---|---|---|---|---|---|---|---|
| Penn/CHOP phase 1/2a37,39 | 4-1BB | R/R ALL: refractory, relapse after HSCT, ineligible for HSCT | 6039 | 9339 | 7 (13)39 | 60% RFS at 12 months (95% CI, 48-75%)39 | 68% at 6 months (95% CI, 50-92%)‡37 |
| NCI phase 136,52 | CD28 | R/R B-ALL: second relapse or greater, refractory, ineligible for HSCT | 5152 | 60.852 | 21 (75)52 | 49.5% LFS at 18 months52 | Longest, 68 days§36 |
| Seattle phase 1/232 | 4-1BB | R/R ALL: second relapse or greater, refractory, MRD after HSCT, ineligible for HSCT | 43 | 93 | 11 (28) | 50.8% EFS at 12 months (95% CI, 36.9-69.9%) | Median, 3 months (range, 2.07-6.44 months)|| |
| MSKCC phase 153 | CD28 | R/R B-ALL: second relapse or greater, very early (CR1 <18 months) BM relapse, refractory, ineligible for HSCT | 25 | 75 | 15 (83) | 8 of 18 in remission, median follow-up, 28.6 months | Median, 7 days (range, 0-234 days) |
| ELIANA phase 233 | 4-1BB | R/R B-ALL: second relapse or greater, refractory, relapse after HSCT | 75 | 81 | 8 (13) | 80% RFS at 6 months, 59% RFS at 12 months | Median, 168 days (range, 20-617 days) |
| Trial . | Costimulatory domain . | Population . | N* . | CR rate (%) . | Subsequent HSCT n (%)† . | Outcome . | CAR T-cell persistence . |
|---|---|---|---|---|---|---|---|
| Penn/CHOP phase 1/2a37,39 | 4-1BB | R/R ALL: refractory, relapse after HSCT, ineligible for HSCT | 6039 | 9339 | 7 (13)39 | 60% RFS at 12 months (95% CI, 48-75%)39 | 68% at 6 months (95% CI, 50-92%)‡37 |
| NCI phase 136,52 | CD28 | R/R B-ALL: second relapse or greater, refractory, ineligible for HSCT | 5152 | 60.852 | 21 (75)52 | 49.5% LFS at 18 months52 | Longest, 68 days§36 |
| Seattle phase 1/232 | 4-1BB | R/R ALL: second relapse or greater, refractory, MRD after HSCT, ineligible for HSCT | 43 | 93 | 11 (28) | 50.8% EFS at 12 months (95% CI, 36.9-69.9%) | Median, 3 months (range, 2.07-6.44 months)|| |
| MSKCC phase 153 | CD28 | R/R B-ALL: second relapse or greater, very early (CR1 <18 months) BM relapse, refractory, ineligible for HSCT | 25 | 75 | 15 (83) | 8 of 18 in remission, median follow-up, 28.6 months | Median, 7 days (range, 0-234 days) |
| ELIANA phase 233 | 4-1BB | R/R B-ALL: second relapse or greater, refractory, relapse after HSCT | 75 | 81 | 8 (13) | 80% RFS at 6 months, 59% RFS at 12 months | Median, 168 days (range, 20-617 days) |
CHOP, Children’s Hospital of Philadelphia; EFS, event-free survival; LFS, leukemia-free survival; MSKCC, Memorial Sloan Kettering Cancer Center; NCI, National Cancer Institute; Penn, University of Pennsylvania; RFS, relapse-free survival; R/R, relapsed/refractory.
*Numbers of patients infused.
†Percentage of patients in remission.
‡Probability of persistence.
§Longest duration in any patient.
Measured by B-cell aplasia.
Efficacy in R/R ALL
In a phase 1/2a single-institution trial of tisagenlecleucel conducted at CHOP (Clinical Trials.gov identifier: NCT01626495), a CR rate of 93% was observed in 60 patients with R/R ALL; relapse-free survival was 60% (95% confidence interval [CI], 48%-75%) at 12 months and 53% (95% CI, 39%-70%) at 24 months, with a median follow-up of 15 months.37,39 A phase 2 single-arm, multicenter, global registration trial (ELIANA; Clinical Trials.gov identifier: NCT02435849) conducted across 25 centers demonstrated a CR rate of 81% in 75 patients with R/R B-ALL treated with tisagenlecleucel, with undetectable MRD in 100% of responses.33 At 12 months, relapse-free survival was 59% (95% CI, 41%-73%) and overall survival was 76% (95% CI, 63%-86%), with a median follow-up of 13 months. Remissions were achieved in patients with chemotherapy refractory disease who had high leukemic burden in the bone marrow and across a wide range of disease burden. In the phase 1/2a trial, 73% of patients had detectable disease: 20% with MRD, 53% with >5% leukemic blasts, and 38% with >50% leukemic blasts in the bone marrow at the time of infusion. Both of these trials demonstrated durable tisagenlecleucel persistence (as long as 39 months at data cutoff) and durable remissions without consolidative HSCT (11% underwent HSCT in remission after tisagenlecleucel).33,39 On the basis of data from the ELIANA trial, with supporting data from the Penn/CHOP phase 1/2a trial and a Novartis US multicenter phase 2 trial (Clinical Trials.gov identifier: NCT02228096), tisagenlecleucel was granted approval by the US Food and Drug Administration for children and young adults up to age 25 years with B-ALL that is refractory or in second or greater relapse.
AALL1721/Cassiopeia
For patients with poor early response to chemotherapy, a therapy with a distinct mechanism of action and demonstrated efficacy in chemotherapy refractory disease is desirable. To improve outcomes in the VHR population with persistent MRD, the COG chose to study CAR T-cell therapy using tisagenlecleucel on the basis of the excellent MRD-negative remission rates and durable remission rates reported in B-ALL refractory to standard chemotherapy. Although HSCT is often considered for patients with anticipated poor survival with chemotherapy alone, relevant to this population, detectable MRD at the time of HSCT has been consistently associated with an increased risk of relapse, as is MRD post-HSCT.23,25,27,40,41 Further intensification of chemotherapy to decrease or eliminate MRD and HSCT itself are not without significant risk of morbidity and mortality.3,22,28,42 Therefore, the possibility of durable remission without consolidative HSCT is one aim of an ongoing trial developed by the COG and Novartis.
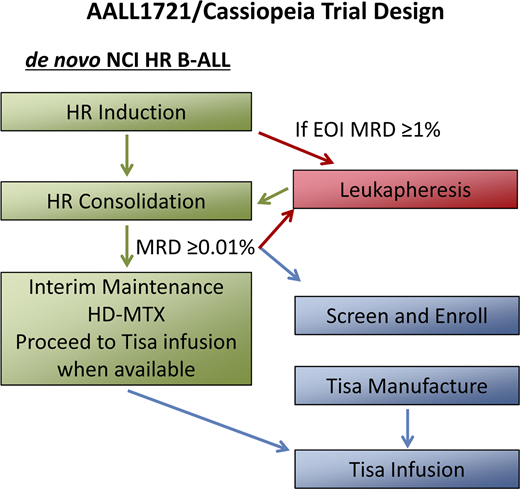
AALL1721/Cassiopeia (Clinical Trials.gov identifier: NCT03876769), a phase 2 single-arm trial of tisagenlecleucel in children and young adults with National Cancer Institute HR B-ALL and persistent MRD at EOC, is the first trial of CAR T-cell therapy in CR1. Participating sites include COG centers in the United States and Canada as well as pediatric centers in the United Kingdom and Europe. Patients age 1 to 25 years diagnosed with CD19-expressing National Cancer Institute HR B-ALL are eligible in CR1 after induction/protocol IA and consolidation/protocol IB chemotherapy if MRD is detected by central multiparameter flow cytometry at ≥0.01% (Figure 2). To closely match the population studied on AALL0232, patients with Ph+ ALL and hypodiploid ALL are excluded, as are those who have received tyrosine kinase inhibitor therapy. AALL1721/Cassiopeia, which opened in March 2019, aims to determine the efficacy and safety of tisagenlecleucel in CR1 in this patient population through a primary end point of 5-year DFS and secondary end points including overall survival, safety, the percentage of patients in remission without HSCT at 1 year, and time to B-cell recovery, a surrogate marker of CD19 CAR T-cell functional persistence. A potential advantage of CAR T-cell therapy is rapid, targeted immune surveillance; however, durability of this surveillance is determined by persistence, which can vary between products and individuals (Table 2). In addition, the potential for antigen escape leading to relapse is a concern with targeted immunotherapy that has been observed in ∼20% to 25% of patients.33,39 Poor T-cell expansion resulting in manufacture failure or toxicities precluding infusion are also potential limitations to CAR T-cell therapy; therefore, these rates will be monitored.
AALL1721/Cassiopeia trial design. AALL1721/Cassiopeia is a phase 2, single-arm, international multicenter trial of tisagenlecleucel in children and young adults with persistent MRD. Patients age 1 to 25 years diagnosed with CD19-expressing National Cancer Institute (NCI) HR (age 10 years or older or presenting with a white blood cell count ≥50 × 109/L) B-ALL are eligible in first remission after induction/protocol IA and consolidation/protocol IB chemotherapy if MRD is detected by central multiparameter flow cytometry at ≥0.01%. Leukapheresis can occur after induction, if EOI MRD ≥1%, or after consolidation, once a patient has a qualifying MRD result. Enrolled patients proceed to the next phase of standard-of-care therapy, interim maintenance (IM), during the period of tisagenlecleucel manufacture. After stopping IM chemotherapy, patients will receive a lymphodepleting chemotherapy regimen of fludarabine and cyclophosphamide followed by a single infusion of tisagenlecleucel. After infusion, no further cancer-directed chemotherapy (including intrathecal chemotherapy) will be administered per protocol. HD-MTX, high-dose methotrexate; tisa, tisagenlecleucel.
AALL1721/Cassiopeia trial design. AALL1721/Cassiopeia is a phase 2, single-arm, international multicenter trial of tisagenlecleucel in children and young adults with persistent MRD. Patients age 1 to 25 years diagnosed with CD19-expressing National Cancer Institute (NCI) HR (age 10 years or older or presenting with a white blood cell count ≥50 × 109/L) B-ALL are eligible in first remission after induction/protocol IA and consolidation/protocol IB chemotherapy if MRD is detected by central multiparameter flow cytometry at ≥0.01%. Leukapheresis can occur after induction, if EOI MRD ≥1%, or after consolidation, once a patient has a qualifying MRD result. Enrolled patients proceed to the next phase of standard-of-care therapy, interim maintenance (IM), during the period of tisagenlecleucel manufacture. After stopping IM chemotherapy, patients will receive a lymphodepleting chemotherapy regimen of fludarabine and cyclophosphamide followed by a single infusion of tisagenlecleucel. After infusion, no further cancer-directed chemotherapy (including intrathecal chemotherapy) will be administered per protocol. HD-MTX, high-dose methotrexate; tisa, tisagenlecleucel.
Toxicity
The principle toxicity of CAR T-cell therapy, cytokine release syndrome (CRS), is anticipated to be less severe in this population in CR, based on experience in early-phase clinical trials.37 CRS is a hyperinflammatory syndrome associated with rapid exponential proliferation of CAR T cells.43-45 On previous clinical trials of tisagenlecleucel, CRS was observed in close to 90% of ALL patients, with grade 4 CRS reported in 25% of patients on the ELIANA trial.33,39 CRS symptoms range from mild flu-like symptoms, including persistent high fevers, myalgias, headache, fatigue, nausea/vomiting, and anorexia, that are self-limited and typically fully resolve in the first month, to life-threatening complications and multiorgan system failure. In patients with ALL, high bone marrow disease burden is associated with an increased risk of severe CRS, an association reproduced with several CD19 CAR T-cell products.31,35-37,45 Conversely, the risk of severe CRS is low for patients in morphologic remission.
Neurotoxicity is a second common toxicity associated with T-cell–engaging immunotherapies, reported in 40% to 45% of patients on clinical trials of tisagenlecleucel.33,46 The spectrum of neurotoxicity symptoms is broad and includes confusion, delirium, hallucinations, global encephalopathy, aphasia, tremor and, less commonly, seizure.33,37,46-48 Although neurotoxicity has been observed at low disease burden and can occur in the absence of CRS symptoms,47,48 increased incidence and severity of neurotoxicity has been associated with higher-grade CRS.33,46,48
The association of CRS severity with disease burden and with incidence and severity of neurotoxicity suggests improved tolerance in a low disease burden state of MRD during frontline therapy. However, the long-term effects of CAR T-cell therapy, its acute toxicities, and chronic B-cell aplasia remain unknown; therefore, it will be important to monitor patients for late toxicities.
Clinical case update
After discussing the risk of relapse with EOC MRD, HSCT or enrollment on the AALL1721/Cassiopeia trial is offered, and the patient and family elect to enroll on the study. The patient continues standard-of-care chemotherapy while tisagenlecleucel is manufactured, receiving 2 courses of high-dose methotrexate. Before infusion, MRD remains stably positive. The patient receives tisagenlecleucel and achieves an MRD-negative remission 1 month after infusion.
Conclusions
The data for poor outcomes with standard chemotherapy in HR B-ALL with persistent MRD are strong; however, the data for alternative therapy approaches are limited or lacking. HSCT improves outcomes in specific ALL populations, and CD19 CAR T-cell therapy has demonstrated efficacy in patients who are not candidates for HSCT. It is reasonable to hypothesize that either approach will improve outcomes relative to chemotherapy alone, but it is difficult to directly compare these approaches with differing eligibility criteria and toxicity profiles. The AALL1721/Cassiopeia trial aims to address part of the question of whether outcomes for VHR B-ALL can be improved without the toxicity of HSCT. Results of this trial may inform broader study of CAR T-cell therapy in frontline therapy for poor-risk B-ALL.
Correspondence
Shannon L. Maude, Children’s Hospital of Philadelphia, 3012 Colket Translational Research Building, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: maude@chop.edu.
References
Competing Interests
Conflict-of-interest disclosure: S.L.M. received clinical trial support from Novartis and participated in consulting, advisory boards, or study steering committees for Novartis, Kite, and Wugen. C.D. declares no competing financial interests.
Author notes
Off-label drug use: Tisagenlecleucel is investigational in the frontline setting.
ORCID profile: C.D., 0000-0002-8005-3836.