Abstract
While iron deficiency remains the most common cause of anemia worldwide, low iron stores are associated with symptoms regardless of the presence of typical microcytic, hypochromic anemia and may be hard to recognize in patients with concurrent inflammation. Diagnosing and treating iron deficiency become more of a challenge because markers of iron status are influenced by low-grade inflammation present in common conditions, such as chronic kidney disease, cirrhosis, or heart failure. Here I present a pragmatic way of interpreting diagnostic lab tests to help clinicians recognize patients who are most likely to benefit from iron supplementation, choose between oral and parenteral administration, and make personalized decisions when patients do not fit usual guidelines.
Learning Objectives
Recognize chronic inflammatory conditions that affect the interpretation of laboratory markers of iron status
Identify patients most likely to benefit from iron supplementation using ferritin and transferrin saturation
Understand risks and benefits of oral and IV iron preparations
Clinical case
A 56-year-old woman was referred for evaluation of anemia. She had a medical history of rheumatoid arthritis treated with methotrexate, hypertension treated with lisinopril, type 2 diabetes mellitus treated with pioglitazone, nonalcoholic fatty liver disease, and stage 3 chronic kidney disease with an estimated creatinine clearance of 32 mL/min per 1.73 m2. She reported progressive fatigue, dyspnea on exertion, and mental fogginess in the past 6 months. She had hemoglobin, 7.9 g/dL; hematocrit, 24%; mean corpuscular volume, 83 fL; and mean corpuscular hemoglobin, 29 pg, with reticulocytes at 2%. Additional laboratory results showed ferritin of 89 μg/L (reference range, 20-200 μg/L) and C-reactive protein (CRP) of 1.8 mg/L (reference value, <5 mg/L). Her rheumatologist was concerned that the patient’s anemia was too severe to be explained by her autoimmune disease, which was under control, or by her comorbidities and requested a hematologist’s opinion.
Distinguishing IDA from iron deficiency
Iron deficiency anemia (IDA) is the most common acquired anemia and should be the first consideration in a patient with unexplained anemia. The World Health Organization (WHO) defines anemia as hemoglobin <13 g/dL and <12 g/dL in adult men and nonpregnant women, respectively,1 a well-known trigger for an investigation of ID. Low red cell mass occurs secondary to chronic reduction in iron availability, impairing the incorporation of the metal into the porphyrin ring to form heme, making hemoglobinization of erythroid precursors in the bone marrow (BM) incomplete.2 In IDA, mature erythrocytes are typically hypochromic (with low mean corpuscular hemoglobin [MCH; <28 pg]) and microcytic (with low mean corpuscular volume [MCV; <80 fL]). A percentage of hypochromic red cells >6% and a reticulocyte hemoglobin equivalent (CHr or Ret-He) <29 pg, as provided by some modern cell counters, also supports iron-restricted erythropoiesis.
ID is defined as “a health-related condition in which iron availability is insufficient to meet the body’s needs and which can be present with or without anemia,”3(p1069) and it is fundamentally recognized that IDA is simply the most advanced stage of ID. In ID, iron stores are progressively exhausted before red cell morphology of hemoglobin levels are affected, and patients may experience early symptoms such as fatigue, reduced cognitive performance, and exercise intolerance. Overlap of ID and other disorders, such as chronic liver or kidney disease, may prevent the MCH and MCV from decreasing, and such indices also become unreliable for use in screening for ID in the presence of thalassemia trait, a frequent hereditary anemia.
We therefore recommend investigating ID in all patients with unexplained signs and symptoms of ID, regardless of the presence of anemia, low MCH, or low MCV, and in those patients with conditions that pose a higher risk for ID, either by increased iron loss (caused by chronic or recurrent bleeding and use of anticoagulants) or by reduced iron absorption (related to, eg, gastrointestinal [GI] disorders, surgical resections, or chronic use of proton pump inhibitors) (Table 1).
Mechanisms contributing to ID in CICs
| Condition . | Reduced iron absorption . | Increased iron loss . |
|---|---|---|
| CKD | Anorexia/GI tract edema; frequent use of proton pump inhibitors; use of phosphate chelators; high hepcidin with blockade of duodenal absorption | Uremic platelet dysfunction; antiplatelet therapy and anticoagulation; blood loss from hemodialysis |
| HF | Anorexia/GI tract edema; high hepcidin with blockade of duodenal absorption | Antiplatelet therapy and anticoagulation |
| IBDs | High hepcidin with blockade of duodenal absorption; small bowel resection | Chronic diarrhea with high epithelial turnover; GI tract bleeding; use of corticosteroids |
| Obesity | High hepcidin due to adipose tissue inflammation; bariatric surgery | Increased uterine bleeding (when associated with polycystic ovarian syndrome) |
| Liver disease | Anorexia/GI tract edema; diarrhea caused by laxatives | Variceal bleeding; thrombocytopenia; coagulopathy |
| Rheumatologic disorders | High hepcidin with blockade of duodenal absorption | Use of corticosteroids and NSAIDs |
| Condition . | Reduced iron absorption . | Increased iron loss . |
|---|---|---|
| CKD | Anorexia/GI tract edema; frequent use of proton pump inhibitors; use of phosphate chelators; high hepcidin with blockade of duodenal absorption | Uremic platelet dysfunction; antiplatelet therapy and anticoagulation; blood loss from hemodialysis |
| HF | Anorexia/GI tract edema; high hepcidin with blockade of duodenal absorption | Antiplatelet therapy and anticoagulation |
| IBDs | High hepcidin with blockade of duodenal absorption; small bowel resection | Chronic diarrhea with high epithelial turnover; GI tract bleeding; use of corticosteroids |
| Obesity | High hepcidin due to adipose tissue inflammation; bariatric surgery | Increased uterine bleeding (when associated with polycystic ovarian syndrome) |
| Liver disease | Anorexia/GI tract edema; diarrhea caused by laxatives | Variceal bleeding; thrombocytopenia; coagulopathy |
| Rheumatologic disorders | High hepcidin with blockade of duodenal absorption | Use of corticosteroids and NSAIDs |
NSAIDs, nonsteroidal anti-inflammatory drugs.
Ferritin in absolute and functional ID
Measuring ferritin levels is the recommended approach to initiating an investigation of ID, because serum ferritin correlates well with body iron determined by serial phlebotomies.4 Ferritin is primarily an intracellular iron-binding protein with main functions of sequestering iron and ensuring correct incorporation into enzymes and hemoglobin, while preventing the generation of reactive species through Fenton chemistry.5 Nevertheless, only a minute fraction circulates as serum ferritin, which is rather iron poor and is secreted by macrophages through a nonclassic lysosomal pathway.6,7 Thus, the serum ferritin level should not be regarded as a direct measurement of iron stores.
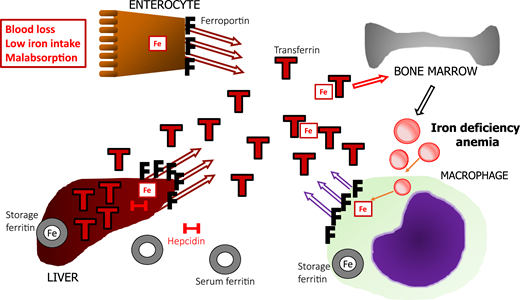
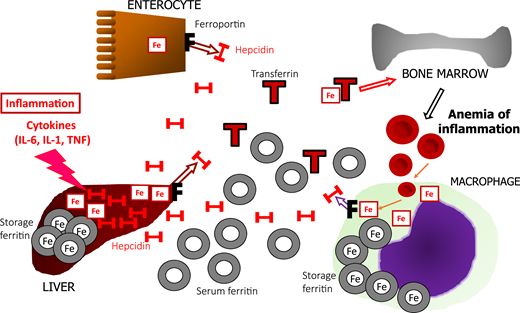
WHO guidelines recommend a ferritin level <15 μg/L as a sign of absolute ID in adults,8 although a cutoff of 30 μg/L is more often used because of its higher sensitivity (∼92%) and high specificity (98%).9 Unfortunately, its high accuracy is lost in the presence of inflammation. An acute-phase reaction is triggered by proinflammatory cytokines, particularly interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF), in response to infection or tissue injury, making hepatocytes increase the synthesis of acute-phase proteins,5 including ferritin and hepcidin. One of the functions of an acute-phase reaction is to prevent iron from being scavenged by pathogens. Hepcidin is a predominantly liver-derived regulator of iron trafficking. It binds to ferroportin, the only iron exporter found on the membrane of mammalian cells and reduces iron export, lowering iron in circulation. Hepcidin-mediated ferroportin blockade traps iron inside cells, such as hepatocytes and macrophages, which in turn produce ferritin to store iron safely. That mechanism underlies functional iron deficiency (FID; pathogenesis and management are reviewed elsewhere10 ). In absolute ID, mechanisms are activated to replenish iron: low hepcidin production keeps ferroportin on the membranes to facilitate iron absorption, and transferrin is upregulated to increase total iron binding capacity (TIBC) and transport of iron to the tissues. A comparison between absolute ID (Figure 1) and FID (Figure 2) shows that both have low serum iron and elevated ferritin, and low TIBC characterizes FID. Their opposing reactions to low and high intracellular iron render ferritin levels of limited help in distinguishing between isolated FID and the association between absolute ID and FID.4 Other biomarkers, such as soluble transferrin receptor, the soluble transferrin receptor/log ferritin index, and hepcidin levels, have been regarded as improving the ability to detect absolute ID in combination with FID, but there is a lack of standardization and limited availability for broader use.11
Schematic representation of the regulation of iron metabolism in absolute ID. Iron depletion occurs commonly and is related to associations among blood loss, low dietary iron intake, and malabsorption. Low iron decreases hepcidin (H) production, allowing for ferroportin (F) activity in duodenal enterocytes, to transfer iron (Fe) absorbed from the diet to transferrin (T), and mobilize iron stored in hepatocytes and macrophages. With progressive iron depletion, the intracellular store of ferritin (iron-rich) is depleted, and serum ferritin (iron-poor) release by macrophages decreases proportionately, along with a progressive decrease in circulating transferrin-bound iron. Low iron also upregulates hepatic production of transferrin, resulting in high TIBC, contributing to low TSAT. Lack of iron available to the BM eventually manifests as hypochromic, microcytic anemia.
Schematic representation of the regulation of iron metabolism in absolute ID. Iron depletion occurs commonly and is related to associations among blood loss, low dietary iron intake, and malabsorption. Low iron decreases hepcidin (H) production, allowing for ferroportin (F) activity in duodenal enterocytes, to transfer iron (Fe) absorbed from the diet to transferrin (T), and mobilize iron stored in hepatocytes and macrophages. With progressive iron depletion, the intracellular store of ferritin (iron-rich) is depleted, and serum ferritin (iron-poor) release by macrophages decreases proportionately, along with a progressive decrease in circulating transferrin-bound iron. Low iron also upregulates hepatic production of transferrin, resulting in high TIBC, contributing to low TSAT. Lack of iron available to the BM eventually manifests as hypochromic, microcytic anemia.
Schematic representation of the regulation of iron metabolism in FID in CICs. Inflammation with increased cytokine production causes upregulation of liver hepcidin (H), which binds to ferroportin (F). Enterocytes are prevented from exporting absorbed iron (Fe) to transferrin (T) in the bloodstream. In hepatocytes and macrophages, iron is also trapped intracellularly and is stored as iron-rich ferritin, whereas macrophages increase iron-poor serum ferritin in circulation. High intracellular iron also downregulates transferrin production, lowering TIBC. Iron release is so restricted that the decrease in serum iron still lowers TSAT despite low TIBC. Iron restriction eventually leads to the anemia of inflammation. Both ID and FID have hypoferremia but low TIBC, and high ferritin characterizes FID.
Schematic representation of the regulation of iron metabolism in FID in CICs. Inflammation with increased cytokine production causes upregulation of liver hepcidin (H), which binds to ferroportin (F). Enterocytes are prevented from exporting absorbed iron (Fe) to transferrin (T) in the bloodstream. In hepatocytes and macrophages, iron is also trapped intracellularly and is stored as iron-rich ferritin, whereas macrophages increase iron-poor serum ferritin in circulation. High intracellular iron also downregulates transferrin production, lowering TIBC. Iron release is so restricted that the decrease in serum iron still lowers TSAT despite low TIBC. Iron restriction eventually leads to the anemia of inflammation. Both ID and FID have hypoferremia but low TIBC, and high ferritin characterizes FID.
Serum inflammatory markers in CICs
Despite their limitations, markers of inflammatory activity, such as erythrocyte sedimentation rate (ESR) and CRP levels have survived the test of time and are often used in clinical practice to help interpret ferritin levels, because ferritin is an acute-phase reactant. Overt inflammation with high ESR and CRP levels has usually been found in active autoimmune disorders (eg, Still’s disease, rheumatoid arthritis, and inflammatory bowel disorders [IBDs]) and in chronic infections (eg, tuberculosis and chronic osteomyelitis). Nevertheless, ESR varies with hematocrit and is driven mostly by the production of fibrinogen and immunoglobulins, which last for several days in the circulation, whereas CRP is mainly produced by the liver in response to cytokines, particularly IL-6, and has a much shorter half-life; discrepancies between ESR and CRP are unsurprisingly common.12 CRP >50 mg/L is frequent in bacterial infections, making it an excellent marker of acute inflammation, whereas the less-noted α-1-acid glycoprotein (AGP) increases later in the inflammatory process and is more suitable for confirming chronic inflammation.13 Because ferritin increases >5 times in patients with CRP >80 mg/L than in those with CRP <10 mg/L,14 studies have examined the possibility of correcting ferritin for inflammatory activity. The Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia (BRINDA) research group found that a regression correction of ferritin using CRP >5 mg/L and AGP >1 g/L increased the prevalence of ID by 3% to 7%, even in countries with a low burden of infection, such as the United States,15,16 and a different regression correction using CRP and albumin increased the prevalence of ID from 7% to 24% in another study.17 Therefore, in areas of widespread inflammation or infection, the 2020 WHO guidelines18 strongly endorse the measurement of CRP and AGP, but make a conditional recommendation to use a ferritin threshold of 70 μg/L to define iron deficiency in patients with CRP >5 mg/L or AGP >1 g/L or to implement arithmetic or regression correction of ferritin levels based on those markers. The guideline may not apply to all patients with chronic inflammatory conditions (CICs), such as obesity, chronic kidney disease (CKD), liver disease, and heart failure (HF), in whom an increase in CRP is frequently absent, or where AGP measurements are not routinely available. Low-grade inflammation in a CIC is enough to disrupt iron metabolism by increasing hepcidin, but does not necessarily correlate with inflammatory markers. Moreover, other mechanisms put patients with CICs at higher risk of ID and underscore the need to make a correct diagnosis despite interference in iron parameters (Table 1).
Ferritin in CIC: making the best of an imperfect tool
Various ferritin cutoff values have been recommended to help detect ID in different patient populations, such as in those with CKD, HF, and IBD.3 There is a general consensus that the usual ferritin cutoff of 30 μg/L is inappropriate in the presence of a CIC but the recommended ferritin values range between 50 and 500 μg/L across guidelines.
A pragmatic way of understanding the implications of a certain ferritin threshold is to examine studies comparing ferritin levels with BM iron, the gold-standard test for determination of iron stores. Bone marrow iron deficiency (BMID) is ID confirmed by the absence of granules of hemosiderin in macrophages and erythroblasts and requires an invasive procedure to obtain an adequate BM sample stained with Prussian blue (or Perls’ stain). Its indication in clinical practice by itself has become rare with the ease of the use of ferritin, but it may occasionally prove useful in patients who undergo BM sampling for other reasons.
A systematic review19 examined 38 studies of BM iron in nonhealthy adults with rheumatoid arthritis, liver disease, hematologic disorders, and other CICs. They found 1023 people with confirmed BMID with mean ferritin between 33.6 and 158.3 μg/L, whereas individuals with detectable BM iron had a mean ferritin >171.6 μg/dL. In the CKD population, ferritin values vary more broadly. Ten deceased patients with dialytic CKD and BMID had ferritin values between 537 and 3994 μg/L; the researchers acknowledged that 4 of the patients had rare minute deposits of iron, but even assuming they would have the highest ferritin values, the maximum value of ferritin in a patient with BMID with dialytic CKD would be in the 1000 to 2000 μg/L range.20 Another study found that 3 of 96 patients were receiving hemodialysis with BMID, with ferritins in the 100 to 1100 μg/L range.21 More recent studies reported ferritin of 36 to 100 μg/L in HIV+ patients with BMID, of whom half had a diagnosis of tuberculosis or Epstein-Barr viremia, and >25% had CMV viremia.22 In HF, patients with true BMID were found to have ferritin levels ranging from 44 to 162 μg/L (interquartile range).23 Except in patients with CKD and some with HF, patients with BMID in CICs appear to have a ferritin level rarely >200 μg/L.
Transferrin saturation in CIC: a helping hand
Transferrin saturation (TSAT) <6% in combination with low ferritin is diagnostic of ID, but in the presence of inflammation, a seemingly arbitrary TSAT <20% is often used to diagnose ID. Because there is a significant overlap in ferritin levels between samples with BMID and normal BM iron (range, 50-500 μg/L), TSAT helps identify patients who are more likely to benefit from iron supplementation. Normal TSAT of 20% to 45% is associated with adequate iron stores in most CICs, because hepcidin is expected to lower TSAT by blocking iron export. In patients with HF who undergo coronary artery bypass graft, TSAT <19.8% and serum iron <13 μmol/L were independently associated with mortality and were most accurate for BMID. In patients with HF, TSAT >20% essentially excluded the possibility of BMID, regardless of ferritin levels.23 In patients with nondialysis CKD (ndCKD) who underwent BM evaluation, TSAT below 20% had only 50% sensitivity but 83% specificity to detect BMID, and the specificity for BMID improved to 98% if associated with a ferritin level <100 μg/L, with a reduction in sensitivity to 33%. TSAT <25% yielded maximum sensitivity of 71%.24 In another study, BMID was identified in only 50% of patients with both TSAT <20% and ferritin <100 μg/L, but TSAT <20% alone had a sensitivity of 85% and specificity of 48%.25 Those data suggest that underlying ID can still be considered in patients with CKD with TSAT of 20% to 25%, whereas for other CICs, TSAT <20% along with judicious evaluation of ferritin to diagnose ID seems appropriate.
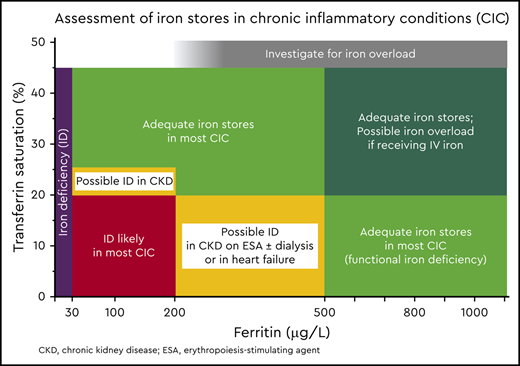
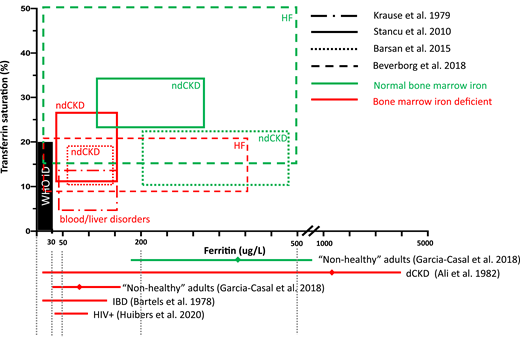
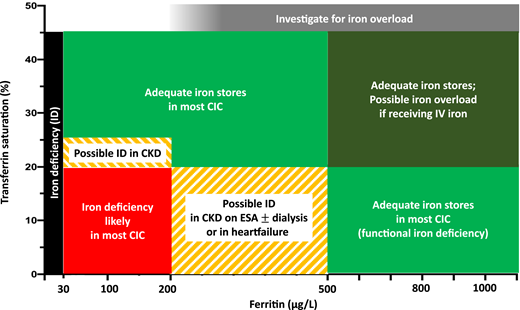
Figure 3 shows TSAT and ferritin levels found in patients with different CICs, with and without BMID. BMID is found in patients with a ferritin range between 30 and 200 μg/dL and TSAT between 10% and 20%. Based on the available evidence from BMID studies, the map in Figure 4 has been designed to help estimate the adequacy of iron stores and to aid in interpreting ferritin and TSAT in patients with CIC. Patients with iron stores estimated to be low (in black and red) should be considered for iron supplementation. Yellow striped areas represent areas in which iron supplementation may be considered, depending on the CIC; only patients with CKD are likely to benefit from iron supplementation with ferritin <200 μg/L and TSAT of 20% to 25%, whereas patients with HF or CKD treated with erythropoiesis-stimulating agents (ESAs) and/or hemodialysis may be considered for iron supplementation if TSAT is <20% and ferritin is up to 500 μg/L. Patients in the green areas most likely have adequate stores and should not receive supplemental iron.
Ferritin and TSAT ranges reported by studies that evaluated BM iron in patients with CICs. Data include patients with HF, dialytic CKD or ndCKD, HIV infection, IBDs,36 and data from a systematic review of 38 studies in nonhealthy patients, including blood disorders, liver conditions, rheumatoid arthritis, among others.19 The area in red represents the thresholds for absolute ID recommended by WHO (ferritin >30 μg/L and TSAT >16%). Patients with BMID have ferritin <160 μg/L and TSAT <20%. TSAT 20% to 25% is still associated with BMID in CKD, and TSAT <20% may still predict BMID in patients with ferritin up to 500 μg/L with HF or CKD treated with ESAs, with or without hemodialysis. Studies that reported only ferritin levels are represented by red lines beneath the x-axis that encompass the range, and means are represented by diamonds situated on the lines.
Ferritin and TSAT ranges reported by studies that evaluated BM iron in patients with CICs. Data include patients with HF, dialytic CKD or ndCKD, HIV infection, IBDs,36 and data from a systematic review of 38 studies in nonhealthy patients, including blood disorders, liver conditions, rheumatoid arthritis, among others.19 The area in red represents the thresholds for absolute ID recommended by WHO (ferritin >30 μg/L and TSAT >16%). Patients with BMID have ferritin <160 μg/L and TSAT <20%. TSAT 20% to 25% is still associated with BMID in CKD, and TSAT <20% may still predict BMID in patients with ferritin up to 500 μg/L with HF or CKD treated with ESAs, with or without hemodialysis. Studies that reported only ferritin levels are represented by red lines beneath the x-axis that encompass the range, and means are represented by diamonds situated on the lines.
Assessment of iron stores using ferritin and TSAT in CICs. Ferritin <30 μg/L in the presence of TSAT <45% is indicative of absolute low iron stores (black). Most patients with CICs in association with true ID are found to have TSAT<20% and ferritin <200 μg/L (red). Patients in the yellow region may be considered for iron supplementation if TSAT is 20% to 25% in CKD, or if TSAT is <20% and ferritin is up to 500 μg/L in HF, if they are receiving dialysis, and/or if they are using ESAs. Adequate iron stores are expected in the green areas, but caution is recommended for patients in the dark green area (TSAT >20% and ferritin >500 μg/L) if they are receiving parenteral iron, because they may be at risk of iatrogenic iron overload.
Assessment of iron stores using ferritin and TSAT in CICs. Ferritin <30 μg/L in the presence of TSAT <45% is indicative of absolute low iron stores (black). Most patients with CICs in association with true ID are found to have TSAT<20% and ferritin <200 μg/L (red). Patients in the yellow region may be considered for iron supplementation if TSAT is 20% to 25% in CKD, or if TSAT is <20% and ferritin is up to 500 μg/L in HF, if they are receiving dialysis, and/or if they are using ESAs. Adequate iron stores are expected in the green areas, but caution is recommended for patients in the dark green area (TSAT >20% and ferritin >500 μg/L) if they are receiving parenteral iron, because they may be at risk of iatrogenic iron overload.
Back to the case
A complete iron panel showed low serum iron (54 mg/dL), normal TIBC (300 mg/dL), and low TSAT (18%). Despite a ferritin level of 89 μg/L (considered normal for healthy individuals), the presence of ferritin <200 μg/L, a TSAT <20% in the presence of several CICs (liver disease, controlled rheumatoid arthritis, and stage 3 ndCKD), and hypoproliferative normochromic, normocytic anemia supported a diagnosis of IDA. The patient underwent an upper endoscopy and colonoscopy, and a bleeding gastric ulcer was detected, for which omeprazole was prescribed. She asked whether she could take iron tablets or should receive “iron injections,” which she had heard carry a risk for allergic reactions.
Management of ID in CICs
The treatment of absolute IDA has been extensively reviewed elsewhere,26,27 but the mainstay of the recommendations for ID in patients with CIC must include investigating underlying causes and implementing appropriate iron supplementation.
Investigation of underlying causes of ID
Patients should always be investigated for blood loss, such as uterine and GI bleeding. Hematuria and epistaxis should be included in the inquiry because patients frequently fail to mention them. Vegetarianism or veganism should not be considered to cause ID, because compensatory upregulation of the absorption of nonheme iron occurs. In CICs, polypharmacy is the rule, and chronic use of some medications can predispose patients to GI bleeding (eg, corticosteroids, nonsteroidal anti-inflammatory drugs, aspirin, and anticoagulants), and use of other medications can impair iron absorption (eg, proton pump inhibitors and laxatives). In patients with chronic diarrhea, high epithelial turnover impairs absorption and mucosal inflammation can cause bleeding. Blood loss may also increase with frequent blood draws during an admission or in equipment circuits, in patients on hemodialysis, for example.
Iron supplementation: oral or IV?
The choice of route of administration of iron should take comorbidities and the patient’s preference into consideration. Oral treatment is cost effective, easily available, and should always be considered. There is no specific iron-containing preparation recommended to treat ID (Table 2), and evidence in pure ID/IDA supports that a single minimum dose of 60 mg of elemental iron administered on alternate days can be adequate and maximize tolerability,26,28,29 but studies in patients with CIC who are following such a regimen are lacking.
Characteristics and side effects of most commonly available oral iron supplements
| Iron formulation (example US brand names) (dose per tablet/Fe dose) . | Usual dose . | Most common side effects (>1%) . | Observations . |
|---|---|---|---|
| Ferric citrate (Auryxia) (1000 mg/210 mg Fe) | 2 tablets once daily (LR) | >20%: fecal discoloration, diarrhea; 10-20%: constipation, nausea; 1-10%: hyperkalemia, cough. | Phosphate binder, approved for use in ID in ndCKD. |
| Ferrous fumarate (Ferretts, Ferrimin, Hemocyte) (324 or 325 mg/106 mg Fe) | 1 tablet every other day (>100 mg Fe per dose) | >10%: constipation, fecal discoloration, nausea, stomach cramps, vomiting; 1-10%: dental discoloration, diarrhea, urine discoloration | Cereals, dietary fiber, tea, coffee, eggs, and milk may decrease absorption. |
| Ferrous gluconate (Ferate) (240 mg/27 mg Fe; 324 mg/38 mg Fe) | 3-4 tablets every other day (>100 mg Fe per dose) | >10%: constipation, fecal discoloration, nausea, stomach cramps, vomiting; 1-10%: dental discoloration, diarrhea, urine discoloration. | |
| Polysaccharide iron complex (EZFE, Ferrex, NovaFerrum) (50 mg Fe) | 2 tablets every other day (>100 mg Fe per dose) | >10%: constipation, fecal discoloration, nausea, stomach cramps, vomiting; 1-10%: dental discoloration, diarrhea, urine discoloration. | |
| Ferrous sulfate (several) (324 or 325 mg/65 mg Fe) | 2 tablets every other day (>100 mg Fe) | >50%: fecal discoloration, abdominal pain, nausea; 20-50%: constipation, vomiting, diarrhea. | |
| Ferric polymaltose (Maltofer; not available in the US) (357 or 370 mg/100 mg Fe) | 1-3 tablets once daily (LR) | >10%: fecal discoloration; >1%: diarrhea, nausea, abdominal pain, constipation. | — |
| Heme iron polypeptide (Proferrin) (10.5-12 mg Fe) | 1 tablet once daily (LR) | Incidence unknown: constipation, abdominal pain, diarrhea, muscle cramps. | — |
| Ferric maltol (Accrufer) (30 mg Fe) | 1 tablet twice daily (LR) | 1-10%: fecal discoloration, constipation, diarrhea, abdominal pain, nausea, vomiting. | Absorption may be decreased with food. |
| Iron formulation (example US brand names) (dose per tablet/Fe dose) . | Usual dose . | Most common side effects (>1%) . | Observations . |
|---|---|---|---|
| Ferric citrate (Auryxia) (1000 mg/210 mg Fe) | 2 tablets once daily (LR) | >20%: fecal discoloration, diarrhea; 10-20%: constipation, nausea; 1-10%: hyperkalemia, cough. | Phosphate binder, approved for use in ID in ndCKD. |
| Ferrous fumarate (Ferretts, Ferrimin, Hemocyte) (324 or 325 mg/106 mg Fe) | 1 tablet every other day (>100 mg Fe per dose) | >10%: constipation, fecal discoloration, nausea, stomach cramps, vomiting; 1-10%: dental discoloration, diarrhea, urine discoloration | Cereals, dietary fiber, tea, coffee, eggs, and milk may decrease absorption. |
| Ferrous gluconate (Ferate) (240 mg/27 mg Fe; 324 mg/38 mg Fe) | 3-4 tablets every other day (>100 mg Fe per dose) | >10%: constipation, fecal discoloration, nausea, stomach cramps, vomiting; 1-10%: dental discoloration, diarrhea, urine discoloration. | |
| Polysaccharide iron complex (EZFE, Ferrex, NovaFerrum) (50 mg Fe) | 2 tablets every other day (>100 mg Fe per dose) | >10%: constipation, fecal discoloration, nausea, stomach cramps, vomiting; 1-10%: dental discoloration, diarrhea, urine discoloration. | |
| Ferrous sulfate (several) (324 or 325 mg/65 mg Fe) | 2 tablets every other day (>100 mg Fe) | >50%: fecal discoloration, abdominal pain, nausea; 20-50%: constipation, vomiting, diarrhea. | |
| Ferric polymaltose (Maltofer; not available in the US) (357 or 370 mg/100 mg Fe) | 1-3 tablets once daily (LR) | >10%: fecal discoloration; >1%: diarrhea, nausea, abdominal pain, constipation. | — |
| Heme iron polypeptide (Proferrin) (10.5-12 mg Fe) | 1 tablet once daily (LR) | Incidence unknown: constipation, abdominal pain, diarrhea, muscle cramps. | — |
| Ferric maltol (Accrufer) (30 mg Fe) | 1 tablet twice daily (LR) | 1-10%: fecal discoloration, constipation, diarrhea, abdominal pain, nausea, vomiting. | Absorption may be decreased with food. |
Fe, elemental iron; LR, label recommendation.
Parenteral iron is often used because numerous systematic reviews have identified the superiority of parenteral iron over oral iron for patients with IBD, HF, CKD, or perioperative anemia. Other indications for parenteral iron include GI tract resection (including bariatric surgery), prolonged use of inhibitors of iron absorption (eg, proton pump inhibitors), and GI intolerance to oral iron (reported in 30% to 70% of patients).
CIC cause hepcidin elevation and may preclude GI absorption. In the future, hepcidin measurement may help identify patients with significant blockade of duodenal iron absorption indicating upfront parenteral iron. Patients with several comorbidities may also prefer parenteral iron to avoid adding another pill to their routine.
A growing portfolio is currently available in the United States: low-molecular-weight iron dextran, iron sucrose, ferric gluconate, ferumoxytol, ferric carboxymaltose (FCM), and ferric derisomaltose (previously known as iron isomaltoside; Table 3). Low-molecular-weight iron deficiency, iron sucrose, and ferric gluconate may require several shorter infusions, whereas the remainder have become increasingly popular because of the lower number of visits required to administer high-dose infusions, despite the higher cost of the medication.
IV iron preparations: test dose, dosage, side effects, and average wholesale pricing
| Iron preparation . | Test dose . | Usual dosage for adults . | Most common side effects . | Estimated AWP (US dollars) . | Observations32 . |
|---|---|---|---|---|---|
| Iron sucrose/iron saccharate | Not recommended; If history of drug allergies, consider test dose with 25-mg IV, slow push. | 1000 mg per treatment; 10 doses of 100 mg IV 2-5 min in consecutive hemodialysis sessions; 5 doses of 200 mg within 14 d or weekly, by IV 2-5 min or in 100 mL NS infusion for >15 min. | >20%: hypotension and muscle cramps in hemodialysis patients; 10-20%: nausea, headache, nasopharyngitis; 1-10%: hypotension, hypertension, edema, chest pain, dizziness, pruritus, GI symptoms, injection site reaction, myalgia, conjunctivitis, dyspnea, cough, fever. | $432.00 (1000 mg Venofer) | 1-wk interval recommended before MRI. |
| LMW iron dextran | Recommended before the first dose; 25 mg (0.5 mL) IV push for 30 s; observe for 1 h. | 1000 mg per treatment; 10 doses of 100 mg >2-min undiluted IV infusion daily; Single dose of 1000 mg 1-h IV infusion in 250 mL NS (off label).35 | Incidence unknown: hypotension, flushing, headache, urticaria, GI symptoms, anaphylaxis, injection site reaction, myalgia, dyspnea, wheezing, fever. | $350.60 (1000 mg INFeD) | Not to be confused with high-molecular-weight dextran (discontinued); 4-wk interval recommended before MRI. |
| Ferric gluconate | Previously recommended, but currently not on label; If history of drug allergies, consider test dose with 25 mg (2 mL) in 50 mL NS for 60-min infusion. | 1000 mg per treatment; 8 doses of 125 mg IV bolus undiluted for 10 min for patients in hemodialysis or 1-h infusion in 100 mL NS. | >20%: hypotension, vomiting, nausea, headache, diarrhea, injection site reaction, muscle cramps; 10-20%: dizziness, tachycardia, hypertension, dyspnea; 1-10%: chest pain, thrombosis, edema, pruritus, hyperkalemia, abdominal pain, pharyngitis, cough, fever; <1%: flushing, hypersensitivity reaction. | $610.40 (1000 mg Ferrlecit) | 1-wk interval recommended before MRI. |
| Ferumoxytol | Not recommended | 1020 mg per treatment; 2 doses of 510 mg, >15 min infusion in 50-250 mL NS or 5% dextrose, 3-8 d apart or single 1020-mg dose, 30-min infusion; Observation for 30 min after infusion recommended. | 1-10%: hypotension, edema, chest pain, hypertension, dizziness, headache, pruritus, rash, diarrhea, nausea, constipation, vomiting, abdominal pain, hypersensitivity reaction, cough, dyspnea, fever. | $2571.42 (1020 mg of Feraheme) | 12- (US) to 24- (Europe) wk interval recommended before MRI. |
| FCM | Not recommended | 1500 mg per treatment; 2 doses of 750 mg undiluted IV at 100 mg/min, or in 250 mL NS 15-min infused 7 d apart; Observation recommended for 30 min after infusion. | >10%: hypophosphatemia (<2 mg/dL); 1% to 10%: hypertension, hypotension, flushing, skin discoloration at injection site, nausea, vomiting, abdominal pain, increased ALT, dizziness, headache; <1%: abdominal pain, anaphylaxis, diarrhea, increased GGT, injection site reaction, paresthesia, skin rash, sneezing. | $2735.10 (1500 mg of Injectafer) | Verification of phosphate levels is recommended for repeated infusions; 1-wk interval recommended before MRI. |
| Ferric derisomaltose | Not recommended | 1000-mg single dose in 100-500 mL NS with a final concentration >1 mg iron/mL, infused over >20 min. | 1-10%: hypophosphatemia, skin rash, nausea; <1%: asthma, severe hypersensitivity reaction. | $2957.10 USD (1000 mg of Monoferric) | Previously iron isomaltoside; verification of phosphate levels is recommended for repeated infusions; 4-wk interval recommended before MRI. |
| Iron preparation . | Test dose . | Usual dosage for adults . | Most common side effects . | Estimated AWP (US dollars) . | Observations32 . |
|---|---|---|---|---|---|
| Iron sucrose/iron saccharate | Not recommended; If history of drug allergies, consider test dose with 25-mg IV, slow push. | 1000 mg per treatment; 10 doses of 100 mg IV 2-5 min in consecutive hemodialysis sessions; 5 doses of 200 mg within 14 d or weekly, by IV 2-5 min or in 100 mL NS infusion for >15 min. | >20%: hypotension and muscle cramps in hemodialysis patients; 10-20%: nausea, headache, nasopharyngitis; 1-10%: hypotension, hypertension, edema, chest pain, dizziness, pruritus, GI symptoms, injection site reaction, myalgia, conjunctivitis, dyspnea, cough, fever. | $432.00 (1000 mg Venofer) | 1-wk interval recommended before MRI. |
| LMW iron dextran | Recommended before the first dose; 25 mg (0.5 mL) IV push for 30 s; observe for 1 h. | 1000 mg per treatment; 10 doses of 100 mg >2-min undiluted IV infusion daily; Single dose of 1000 mg 1-h IV infusion in 250 mL NS (off label).35 | Incidence unknown: hypotension, flushing, headache, urticaria, GI symptoms, anaphylaxis, injection site reaction, myalgia, dyspnea, wheezing, fever. | $350.60 (1000 mg INFeD) | Not to be confused with high-molecular-weight dextran (discontinued); 4-wk interval recommended before MRI. |
| Ferric gluconate | Previously recommended, but currently not on label; If history of drug allergies, consider test dose with 25 mg (2 mL) in 50 mL NS for 60-min infusion. | 1000 mg per treatment; 8 doses of 125 mg IV bolus undiluted for 10 min for patients in hemodialysis or 1-h infusion in 100 mL NS. | >20%: hypotension, vomiting, nausea, headache, diarrhea, injection site reaction, muscle cramps; 10-20%: dizziness, tachycardia, hypertension, dyspnea; 1-10%: chest pain, thrombosis, edema, pruritus, hyperkalemia, abdominal pain, pharyngitis, cough, fever; <1%: flushing, hypersensitivity reaction. | $610.40 (1000 mg Ferrlecit) | 1-wk interval recommended before MRI. |
| Ferumoxytol | Not recommended | 1020 mg per treatment; 2 doses of 510 mg, >15 min infusion in 50-250 mL NS or 5% dextrose, 3-8 d apart or single 1020-mg dose, 30-min infusion; Observation for 30 min after infusion recommended. | 1-10%: hypotension, edema, chest pain, hypertension, dizziness, headache, pruritus, rash, diarrhea, nausea, constipation, vomiting, abdominal pain, hypersensitivity reaction, cough, dyspnea, fever. | $2571.42 (1020 mg of Feraheme) | 12- (US) to 24- (Europe) wk interval recommended before MRI. |
| FCM | Not recommended | 1500 mg per treatment; 2 doses of 750 mg undiluted IV at 100 mg/min, or in 250 mL NS 15-min infused 7 d apart; Observation recommended for 30 min after infusion. | >10%: hypophosphatemia (<2 mg/dL); 1% to 10%: hypertension, hypotension, flushing, skin discoloration at injection site, nausea, vomiting, abdominal pain, increased ALT, dizziness, headache; <1%: abdominal pain, anaphylaxis, diarrhea, increased GGT, injection site reaction, paresthesia, skin rash, sneezing. | $2735.10 (1500 mg of Injectafer) | Verification of phosphate levels is recommended for repeated infusions; 1-wk interval recommended before MRI. |
| Ferric derisomaltose | Not recommended | 1000-mg single dose in 100-500 mL NS with a final concentration >1 mg iron/mL, infused over >20 min. | 1-10%: hypophosphatemia, skin rash, nausea; <1%: asthma, severe hypersensitivity reaction. | $2957.10 USD (1000 mg of Monoferric) | Previously iron isomaltoside; verification of phosphate levels is recommended for repeated infusions; 4-wk interval recommended before MRI. |
ALT, alanine transferase; AWP, average wholesale price (reported on UpToDate.com; last accessed 25 September 2020); GGT, γ-glutamyl transferase; LMW, low-molecular-weight; MRI, magnetic resonance imaging; NS, normal saline (0.9% sodium chloride); USD, US dollars.
The route of administration in CICs may shift back to oral with the ongoing success of trials of novel iron formulations that have better absorption and tolerance, such as ferric citrate (a phosphate binder approved for use in ndCKD) and ferric maltol, or those that do not depend on ferroportin (eg, Sucrosomial iron) and are currently in clinical trials.30
Back to the case
IV iron was indicated because the use of a proton pump inhibitor precludes adequate oral iron absorption, and the patient’s concerns about side effects of parenteral iron were addressed. She received an infusion of FCM uneventfully. A week later, she called the office to report that she was still feeling weak and wondered whether her anemia was getting worse. Her laboratory results showed that her hemoglobin had had a minor increase from 7.9 to 8.2 g/dL, but her phosphate levels were moderately decreased at 1.6 mg/dL. She eventually completed her treatment with hemoglobin of 10.4 g/dL, ferritin of 359 μg/L, and TSAT of 35%.
Adverse events with IV iron supplementation and management
Before starting parenteral iron, patients should be informed about potential adverse events. Parenteral iron still enjoys the bad reputation of causing severe allergic reactions, mostly because of frequent reactions to high-molecular-weight iron dextran, which has been discontinued, but some manufacturers still recommend a test dose for some formulations (Table 3). The most common side effects of current IV iron formulations are hypotension, headache, injection site reactions, and GI symptoms. Skin discoloration from extravasation is also a possible complication and patients should be informed of that particular risk. FCM and ferric derisomaltose have been associated with the development of hypophosphatemia in 27% to 90% and 4% of treatments, respectively, attributable to an increase in fibroblast growth factor 23 with renal phosphate wasting. Hypophosphatemia is usually asymptomatic, but exacerbation of symptoms of anemia may be caused by lower levels of 2,3-diphosphoglycerate in erythrocytes, an increase in hemoglobin’s affinity for oxygen, and limited oxygen delivery to the tissues.31 Verifying phosphate levels is recommended in symptomatic patients, in those who require repeated infusions with those compounds, or in those at higher risk for low phosphate levels (eg, patients treated with renal replacement therapy, those with chronic diarrhea, and those who have undergone a parathyroidectomy secondary to end-stage renal disease), or in those on medications associated with low absorption or increased excretion of phosphate (antacids, phosphate binders, niacin, acetazolamide, imatinib, and sorafenib). Mild (1.8-2.5 mg/dL or 0.6-0.8 mmol/L) to moderate (1.0-1.8 mg/dL or 0.3-0.6 mmol/L) decreases in phosphate levels can be managed with dietary changes to increase ingestion of phosphate-rich food (eg, dairy, poultry) and/or oral potassium phosphate. Severe hypophosphatemia (below 1.0 mg/dL or 0.3 mmol/L) is exceedingly rare but requires parenteral phosphate infusion to prevent seizures and arrhythmias.
Iatrogenic iron overload is another concern in the absence of reliable ferritin levels. Infused iron is captured by Kupffer cells, which become overloaded and gradually shuttle the iron to hepatocytes. Liver iron overload has been diagnosed by MRI in up to 84% of patients with dialytic CKD and is associated with the infusion of more than 250 mg of iron per month.32 Kidney Disease Improving Global Outcomes 2012 guidelines33 warn against iron supplementation in patients with CKD with ferritin >500 μg/L, but MRIs have shown that patients with ferritin in that range may have significant iron overload. Recently, a large randomized clinical trial favored the use of a high-dose regimen of 400 mg/mo of iron to lower risk of death and nonfatal cardiovascular events in patients in hemodialysis within a 2-year time frame, but did not report incidence of liver iron overload, so concerns for late effects of excess iron remain.34 If iatrogenic iron overload is suspected, MRI can be used, but different intervals for each iron formulation are recommended before MRI scans, to prevent interference with imaging (Table 3).32 In patients on hemodialysis with confirmed iron overload, the discontinuation of iron infusions has been shown to correct it slowly over several months without the need for iron chelators.
Conclusion
CICs caused by CKD, HF, and other disorders make the diagnosis of ID more difficult, but knowledge of how ferritin and TSAT measurements behave in concurrent CICs and ID helps identify patients who are more likely to benefit from iron supplementation. Patients and physicians should discuss risks and benefits of oral and parenteral iron preparations to make personalized treatment decisions, especially when patients have multiple comorbidities and do not fit the available guidelines.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.
Correspondence Kleber Yotsumoto Fertrin, University of Washington, 825 Eastlake Ave, E MS CE3-300, Seattle, WA 98109; e-mail: kleber@uw.edu.