Abstract
Conditional on surviving the first 2 to 5 years after allogeneic blood or marrow transplantation (BMT), the 10-year overall survival approaches 80%. Nonetheless, the risk of late mortality remains higher than the age- and sex-matched general population for several years after BMT. The higher mortality rates in transplant recipients translate into shorter projected life expectancies compared with the general population. Risk of relapse-related mortality reaches a plateau within 10 years after BMT. With increasing time from BMT, nonrelapse-related mortality becomes the leading cause of death, and continues to increase with time after BMT. The major causes of nonrelapse mortality include infection (with or without chronic graft-versus-host disease), subsequent neoplasms, and cardiopulmonary compromise. In this review, findings from large cohorts are summarized, identifying opportunities for risk-based anticipatory intervention strategies to reduce mortality.
Learning Objectives
Understand the magnitude of risk of late all-cause mortality
Understand the risk and causes of nonrelapse-related mortality
Clinical case
The patient is a 64-year-old woman with a history of acute myeloid leukemia (AML), with mixed-lineage leukemia rearrangement, diagnosed at 52 years of age. She was treated with 3 + 7 induction chemotherapy (daunorubicin, 270 mg/m2; and cytarabine, 1.4 g/m2) followed by consolidation with idarubicin (36 mg/m2), fludarabine (72 mg/m2), and cytarabine (5.3 g/m2). She subsequently underwent an allogeneic blood or marrow transplantation (BMT) from an HLA-identical unrelated donor, conditioned with cyclophosphamide (3.2 g/m2) and total-body irradiation (TBI; 1320 cGy in 11 fractions). She tolerated the treatment well and was discharged home after a relatively uneventful course in the hospital. Her medical care was transitioned to her primary care physician 5 years after BMT. At age 58 years, she developed type 2 diabetes, managed with metformin and diet. At age 60 years, she developed prehypertension (systolic blood pressure, 130 mm Hg; diastolic blood pressure, 80 mm Hg), but was not treated. One year later, she presented to her primary care physician with a 6-month history of worsening fatigue, shortness of breath, and lower extremity swelling. Her clinical examination was notable for rales over the lung bases, and pitting pedal edema extending up to her ankles. A chest radiograph was concerning for cardiomegaly and pulmonary interstitial edema. An echocardiogram revealed an ejection fraction of 32%, and the patient was referred to the cardiologist for management of systolic heart failure. Over the course of the following 4 years, her cardiac function worsened, and she died at the age of 68 years in overt decompensated heart failure, in completed continuous remission from her AML.
Allogeneic BMT is offered as curative therapy for leukemia, immune disorders, inborn errors of metabolism, and severe aplastic anemia. Long-term survival is now an expected outcome for such patients. However, there is ample evidence that allogeneic BMT recipients are at risk for premature death. Several studies have described late cause-specific mortality among allogeneic BMT recipients.1-7 These studies indicate that conditional on surviving the first 2 to 5 years, the probability of being alive 10 years after BMT is 70% to 85%.1,4-7 The risk of late mortality remains higher than the age- and sex-matched general population for several years after BMT. The higher mortality rates in transplant recipients translate into shorter projected life expectancies compared with the general population.4 Relapse is the major cause of death in the first few years after transplantation for hematologic malignancies, and then achieves a plateau. With increasing time from BMT, nonrelapse-related mortality becomes the leading cause, and continues to increase with time after BMT. The major causes of nonrelapse mortality include infection (with or without chronic graft-versus-host disease [GVHD]), subsequent neoplasms (SNs), and cardiovascular disease. In this review, findings from large cohorts are summarized; slight differences in findings are likely due to individual cohort characteristics (time from BMT, year of BMT, disease status at entry into cohort), and methods used to determine vital status and cause(s) of death.
Risk of all-cause late mortality in allogeneic BMT recipients
This section provides a summary of 4 large studies that have described late mortality in the past decade. These studies have included differing inclusion criteria and used different methods of ascertaining late deaths: the Center for International Bone Marrow Transplant Registry (CIBMTR),7 the Blood or Marrow Transplant Survivor Study (BMTSS),2 a nationwide study from Japan,8 and a single-center study (Fred Hutchison Cancer Research Center [FHCRC]).4 Although the CIBMTR, BMTSS, and the Japanese study included patients who had survived 2 or more years after BMT, the FHCRC study included patients who had survived 5 or more years after BMT. Unlike the other 3 studies, the BMTSS included patients irrespective of their disease status, as long as they had survived the first 2 years. Ascertainment of vital status and cause of death also differed across the studies. Thus, the CIBMTR study depended upon the 280 participating BMT sites to provide data regarding both survival and disease control. BMTSS, on the other hand, used National Death Index (NDI) Plus,9 medical records, and Accurint databases10 for this information. For the FHCRC study, annual attempts were made to contact all surviving patients, and, where such contact was not successful, linkage with NDI was made to determine vital status. The all-cause and cause-specific mortality are summarized in Table 1. After a median follow-up of 9 years, and conditional on surviving the first 2 years after BMT, the 10-year overall survival was 80% for myelodysplastic syndrome (MDS), 84% for AML, ,acute lymphoblastic leukemia (ALL) and lymphoma, and 92% for severe aplastic anemia (SAA) in the CIBMTR cohort of 10 632 cohort members who had undergone BMT between 1980 and 2003. In the BMTSS cohort of 2999 2-year survivors of allogeneic BMT who received transplants between 1974 and 2010 and were followed for a median of 12.4 years, the 20-year overall survival was 68.1%, conditional on surviving the first 2 years after BMT. Furthermore, the cohort was at a sixfold higher risk of all-cause mortality compared with the general population.2 In the FHCRC study of 2754 patients followed for 13 years after BMT, the overall survival was 80.4% at 20 years.4 Finally, in the Japanese study of 8571 patients followed for a median of 8.4 years, the overall survival was 83% at 15 years.8
Overall and cause-specific late mortality after allogeneic BMT
| Study . | Cohort characteristics . | Overall survival . | Relapse-related mortality . | Nonrelapse-related mortality . |
|---|---|---|---|---|
| Atsuta et al8 | 11 047 2-y survivors of allogeneic BMT | 83% at 15 y | 3.7% at 10 y | — |
| Holmqvist et al11 | 1388 2-y survivors of allogeneic BMT in childhood | 79.3% at 20 y | 4.5% | 13.2% at 10 y |
| Martin et al4 | 2574 5-y survivors (autologous or allogeneic BMT [n = 2160]) | 80.4% at 20 y | — | — |
| Wingard et al7 | 10 632 2-y allogeneic BMT survivors | 85% at 10 y | AML, 9%; MDS, 12%; ALL,9%; lymphoma, 11% | |
| Francisco et al2 | 2,999 2-y allogeneic BMT survivors | 68.1% at 20 y | 2.9% at 20 y | 13.9% at 20 y |
| Study . | Cohort characteristics . | Overall survival . | Relapse-related mortality . | Nonrelapse-related mortality . |
|---|---|---|---|---|
| Atsuta et al8 | 11 047 2-y survivors of allogeneic BMT | 83% at 15 y | 3.7% at 10 y | — |
| Holmqvist et al11 | 1388 2-y survivors of allogeneic BMT in childhood | 79.3% at 20 y | 4.5% | 13.2% at 10 y |
| Martin et al4 | 2574 5-y survivors (autologous or allogeneic BMT [n = 2160]) | 80.4% at 20 y | — | — |
| Wingard et al7 | 10 632 2-y allogeneic BMT survivors | 85% at 10 y | AML, 9%; MDS, 12%; ALL,9%; lymphoma, 11% | |
| Francisco et al2 | 2,999 2-y allogeneic BMT survivors | 68.1% at 20 y | 2.9% at 20 y | 13.9% at 20 y |
—, not available; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome.
Cause-specific late mortality
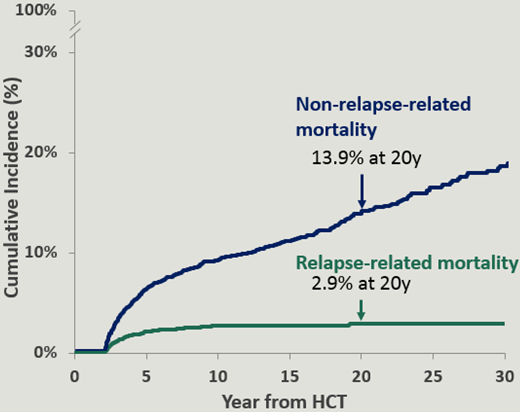
The causes of death included infection, chronic GVHD, primary disease, and SNs.2,7,8 The causes of late mortality are summarized in Table 2. The cumulative incidence of relapse-related mortality plateaued at 10 years, and was 2.9% at 20 years. On the other hand, the cumulative incidence of nonrelapse-related mortality continued to climb and was 13.9% at 20 years (Figure 1).2 Older age at BMT,2,7 use of TBI for conditioning,2 unrelated donor BMT,2 and chronic GVHD were associated with a higher risk of nonrelapse-related mortality.7 Oropharyngeal cancers, gastrointestinal cancers, and brain tumors accounted for the vast majority of the fatal nonhematologic SNs.4 Pulmonary fibrosis was the most common cause of fatal respiratory disease.4 All deaths related to hepatitis C infection occurred among patients who had received transplants prior to 1990, before hepatitis C screening became available.4 Deaths due to other infections were more prominent among patients with prior chronic GVHD.4
Causes of late mortality
| Study . | Causes of late mortality . | ||||
|---|---|---|---|---|---|
| Primary disease . | Infection . | Subsequent neoplasms . | Cardiovascular . | Pulmonary . | |
| Atsuta et al8 | 10-y cumulative incidence, 3.7% | 10-y cumulative incidence, 2.4% | 10-y cumulative incidence, 1.3% | — | 10-y cumulative incidence, 2.5% |
| Holmqvist et al11 | Prevalence, 24.6% | Prevalence, 49.6% | Prevalence, 18.4% | Prevalence, 9.8% | — |
| Martin et al4 | Prevalence, 29.7% | Hepatitis C, SMR = 35.6; other infections, SMR = 5.0 | SMR, 4.4 | SMR = 1.8 | SMR = 5.9 |
| Francisco et al2 | Prevalence, 9.6% | Prevalence: 18.5% | Prevalence, 7.2% | — | — |
| Study . | Causes of late mortality . | ||||
|---|---|---|---|---|---|
| Primary disease . | Infection . | Subsequent neoplasms . | Cardiovascular . | Pulmonary . | |
| Atsuta et al8 | 10-y cumulative incidence, 3.7% | 10-y cumulative incidence, 2.4% | 10-y cumulative incidence, 1.3% | — | 10-y cumulative incidence, 2.5% |
| Holmqvist et al11 | Prevalence, 24.6% | Prevalence, 49.6% | Prevalence, 18.4% | Prevalence, 9.8% | — |
| Martin et al4 | Prevalence, 29.7% | Hepatitis C, SMR = 35.6; other infections, SMR = 5.0 | SMR, 4.4 | SMR = 1.8 | SMR = 5.9 |
| Francisco et al2 | Prevalence, 9.6% | Prevalence: 18.5% | Prevalence, 7.2% | — | — |
—, not available; SMR, standardized mortality ratio.
Cumulative incidence of relapse-related mortality and nonrelapse-related mortality in patients with hematologic malignancies treated with allogeneic BMT as part of the BMTSS cohort.
Cumulative incidence of relapse-related mortality and nonrelapse-related mortality in patients with hematologic malignancies treated with allogeneic BMT as part of the BMTSS cohort.
Mortality in 2-year survivors of allogeneic BMTs performed in childhood
We present the late mortality experienced by patients who received transplants in childhood as a separate section because of the differences between this population as compared with the adults, with respect to the primary indications for transplantation, and the unique vulnerability of the organs to high-intensity therapeutic exposures. Among 1388 individuals who lived 2 years or more after allogeneic BMT performed in childhood, the median age at BMT was 14.6 years (range, 0-21 years) in the BMTSS.3 The primary diagnoses included ALL (25.1%), AML/MDS (23.5%), inborn errors of metabolism (13.8%), and SAA (10.6%). After a median follow-up of 14.9 years (range, 2.0-41.2 years), the overall survival was 79.3% at 20 years. Overall, the cohort had a 14.4-fold higher risk of death compared with the general population. Relative mortality remained elevated at 25 years or more after BMT (2.9-fold higher than the general population).
The cumulative incidence of nonrelapse-related mortality was 13.2% at 20 years after BMT, whereas that of relapse-related mortality was only 4.5% (Table 1). The cumulative incidence of nonrelapse-related mortality exceeded that of relapse-related mortality throughout follow-up. The leading causes of death were infection with or without chronic GVHD (50% of the deaths), primary disease (25%), and SNs (18%), cardiac disease (10%), and pulmonary disease (8%) (Table 2). Compared with patients with ALL, the hazard of relapse-related mortality was lower among patients who underwent BMT for AML/MDS (hazard ratio [HR] = 0.39; P = .01). On the other hand, the hazard of nonrelapse-related mortality was lower among those who underwent transplantation for SAA (HR = 0.36; P = .004) and immune disorders (HR = 0.14; P = .009). The hazard of nonrelapse-related mortality increased with age at BMT (HR = 1.03; P = .03) and was higher among those who received peripheral blood stem cells compared with those who received bone marrow (HR = 2.39; P = .01) and was high among those who were at a higher risk of relapse at BMT (HR = 2.05; P < .001).
Life expectancy after BMT
The study cohort included 2574 five-year survivors of allogeneic and autologous transplants performed before 2002 who were followed for a median of 13 years at FHCRC.4 Annual attempts were made to contact all surviving patients, and where such contact was not successful, linkage with NDI was made to determine vital status. The number of deaths per 1000 person-years inflected upward at age ∼50 years, which was 10 to 15 years earlier than expected in the general population. These mortality rates translated to shorter projected life expectancies compared with the general population. The absolute decrease in estimated residual life expectancy ranged from 17 years for survivors at 20 years of age to 6.4 years for survivors at age 60 years. The proportionate reduction in life expectancy was ∼30% across all attained ages. The average estimated reduction in residual life expectancy was higher for those with vs without prior chronic GVHD (35 years vs 21 years).
Late mortality trends
For patients whose BMT was performed in childhood, the 10-year cumulative incidence of late mortality declined by BMT era (BMTs performed before 1990, 18.9%; 1990-1999, 12.8%; 2000-2010, 10.9%; P = .002); this decline remained statistically significant after adjusting for demographic and clinical factors (referent group, <1990; 1990-1999 [HR = 0.64; P = .007]; 2000-2010 [HR = 0.49; P = .002]; P < .001 for trend).11 Furthermore, the hazard of late nonrelapse-related mortality declined by BMT era after adjusting for demographic and clinical factors (referent group, <1990; 1990-1999 [HR = 0.67; P = .06]; 2000-2010 [HR = 0.46; P = .01]).11 Patients who underwent BMT as adults experienced a significant decline in late mortality by transplant era (referent group, <1990; 1990-1999 [HR = 0.8; P = .01]; 2000-2004 [HR = 0.5; P < .001]; 2005-2010 [HR = 0.6; P = .005]).2 Furthermore, the hazard of late nonrelapse-related mortality also declined by BMT era (referent group, <1990; 1990-1999 [HR = 0.5; P < .001]; 2000-2004 [HR = 0.4; P < .001]).2 This decline is likely due to a change in transplant practice (and improvement in supportive care). Some of these changes have included an increase in the use of reduced-intensity conditioning, and decline in the use of TBI as a conditioning agent, both of which could possibly result in a decline in late mortality.
In summary, conditional on surviving 2 years in remission, the overall survival approaches 80% at 10 years,7 and 70% at 20 years2 after BMT. The major risk factors for all-cause mortality include older age at BMT, unrelated donor BMT, and high risk of relapse at BMT. Patients conditioned with TBI are at increased risk, whereas those conditioned with busulfan/cyclophosphamide are at reduced risk of all-cause mortality. BMT recipients are at sixfold to 14-fold higher risk of all-cause mortality when compared with the general population. Mortality rates remain elevated several years after BMT.3,4 Overall, there is a proportionate reduction in life expectancy of 30% across all attained ages, with the greatest reduction among those with prior chronic GVHD,4 and could possibly be due to accelerated aging previously reported in BMT recipients.12
Overall, disease recurrence is responsible for ∼25% of all late deaths. Although the cumulative incidence of relapse-related mortality plateaus at 10 to 15 years post-BMT at a modest 3% at 20 years, that for nonrelapse-related mortality continues to climb, with no evidence of a plateau, approaching 15% at 20 years after BMT. A major risk factor associated with nonrelapse-related late death is chronic GVHD.7 The prominence of chronic GVHD as a cause of late death provides evidence for prioritizing strategies to reduce the risk of GVHD. Infection in the absence of GVHD is another major cause of late deaths and suggests persistence of immunodeficiency for prolonged periods of time after BMT. SNs account for 2% to 10% of late deaths, usually due to epithelial cancers such as oropharyngeal cancers, gastrointestinal malignancies, and brain tumors that start developing 8 to 10 years after BMT, and account for a large proportion of the fatal subsequent malignancies. These findings emphasize the need for lifelong risk-based anticipatory screening for SNs (Table 3), appropriate immunizations, and aggressive management of infections several years after BMT.13 All survivors should get reimmunized after BMT to protect them from infection. Inactivated vaccines (such as the flu vaccine) can be initiated as early as 3 to 6 months after BMT, whereas live vaccines (such as measles, mumps, and rubella vaccine) are recommended at ∼2 years after BMT. However, some BMT survivors, such as those with active chronic GVHD, may need to wait longer to receive certain vaccines. The timeline in Table 4 outlines a typical schedule for immunization following BMT.
Risk-based anticipator screening
| Cause of nonrelapse late mortality . | Populations at highest risk . | Recommended interventions to reduce mortality . |
|---|---|---|
| Infection | ||
| Hepatitis C | • Active chronic GVHD | • Aggressive surveillance for, and appropriate management: fevers, respiratory/sinus infections, etc |
| Bacterial sepsis | • On immunosuppressive therapy | • Viral/bacterial prophylaxis per institutional policy |
| Fungal infections | • BMT <1990 | |
| Viral infections | ||
| Subsequent neoplasms | ||
| Oropharyngeal cancers | • Chronic GVHD | • Careful oral examination |
| • Human papillomavirus infection | • High index of suspicion, with referral to otolaryngologist | |
| • Tobacco, alcohol | ||
| Esophageal cancer | • Chronic GVHD | • High index of suspicion for patients with dysphagia, unintended weight loss–to initiate further workup |
| • TBI | ||
| Colorectal cancer | • TBI | • Colonoscopy as per US Preventive Services Task Force or American Cancer Society guidelines |
| Brain tumors | • TBI | • High index of suspicion for further workup |
| Hepatocellular carcinoma | • Chronic hepatitis B or hepatitis C infection | • High index of suspicion for further workup |
| • Chronic GVHD | ||
| Cardiovascular disease | ||
| Cardiomyopathy | • Prior exposure to anthracycline chemotherapy, chest radiation, neck radiation | • Aggressive management of diabetes, hypertension, and dyslipidemia |
| Coronary artery disease | • Presence of diabetes, hypertension, dyslipidemia | • Periodic screening for cardiomyopathy, coronary artery disease carotid artery stenosis (among those at high risk) |
| Stroke |
| Cause of nonrelapse late mortality . | Populations at highest risk . | Recommended interventions to reduce mortality . |
|---|---|---|
| Infection | ||
| Hepatitis C | • Active chronic GVHD | • Aggressive surveillance for, and appropriate management: fevers, respiratory/sinus infections, etc |
| Bacterial sepsis | • On immunosuppressive therapy | • Viral/bacterial prophylaxis per institutional policy |
| Fungal infections | • BMT <1990 | |
| Viral infections | ||
| Subsequent neoplasms | ||
| Oropharyngeal cancers | • Chronic GVHD | • Careful oral examination |
| • Human papillomavirus infection | • High index of suspicion, with referral to otolaryngologist | |
| • Tobacco, alcohol | ||
| Esophageal cancer | • Chronic GVHD | • High index of suspicion for patients with dysphagia, unintended weight loss–to initiate further workup |
| • TBI | ||
| Colorectal cancer | • TBI | • Colonoscopy as per US Preventive Services Task Force or American Cancer Society guidelines |
| Brain tumors | • TBI | • High index of suspicion for further workup |
| Hepatocellular carcinoma | • Chronic hepatitis B or hepatitis C infection | • High index of suspicion for further workup |
| • Chronic GVHD | ||
| Cardiovascular disease | ||
| Cardiomyopathy | • Prior exposure to anthracycline chemotherapy, chest radiation, neck radiation | • Aggressive management of diabetes, hypertension, and dyslipidemia |
| Coronary artery disease | • Presence of diabetes, hypertension, dyslipidemia | • Periodic screening for cardiomyopathy, coronary artery disease carotid artery stenosis (among those at high risk) |
| Stroke |
Vaccinations recommended in allogeneic BMT survivors
| Vaccine . | No. of doses . | Months or years after BMT . |
|---|---|---|
| Pneumococcal conjugate (PCV13) | 3-4 | 3+ mo |
| Pneumococcal polysaccharide (PPSV23) | 1 | 6+ mo |
| Haemophilus influenzae conjugate (Hib) | 3 | 6+ mo |
| Meningococcal conjugate (MCV4) | 2 | 6+ mo |
| Meningococcal type B (Men-B) | 2-3 | 6+ mo |
| Inactivated polio | 3 | 6+ mo |
| Hepatitis A | 2 | 6+ mo |
| Hepatitis B | 3 | 6+ mo |
| Human papillomavirus (ages 9-45 y) | 3 | 6+ mo |
| Inactivated influenza | Annually | 6+ mo |
| Measles-mumps-rubella | 1 (adults); 2 (children) | 2+ y |
| Varicella | 2 | 2+ y |
| Shingles | 1-2 | 2+ y |
| Vaccine . | No. of doses . | Months or years after BMT . |
|---|---|---|
| Pneumococcal conjugate (PCV13) | 3-4 | 3+ mo |
| Pneumococcal polysaccharide (PPSV23) | 1 | 6+ mo |
| Haemophilus influenzae conjugate (Hib) | 3 | 6+ mo |
| Meningococcal conjugate (MCV4) | 2 | 6+ mo |
| Meningococcal type B (Men-B) | 2-3 | 6+ mo |
| Inactivated polio | 3 | 6+ mo |
| Hepatitis A | 2 | 6+ mo |
| Hepatitis B | 3 | 6+ mo |
| Human papillomavirus (ages 9-45 y) | 3 | 6+ mo |
| Inactivated influenza | Annually | 6+ mo |
| Measles-mumps-rubella | 1 (adults); 2 (children) | 2+ y |
| Varicella | 2 | 2+ y |
| Shingles | 1-2 | 2+ y |
These interventional strategies to reduce late mortality stem from evidence garnered from observation studies; the magnitude of impact of these risk-reduction strategies would require randomized trials, and would be logistically impossible. Nonetheless, a summary of potential interventions to decrease late mortality is provided in Table 3; these could serve as a starting point, with evidence garnered from their adoption used to refine them further. Most importantly, the ongoing elevated risk of late mortality, especially due to nonrelapse causes emphasizes the need to maintain lifelong follow-up of allogeneic BMT survivors. This is illustrated by the case presented in this article, in which the combination of exposure to anthracycline chemotherapy with diabetes and hypertension resulted in congestive heart failure. This could perhaps have been prevented by aggressive management and anticipatory screening for cardiomyopathy.
Correspondence
Smita Bhatia, Institute for Cancer Outcomes and Survivorship, Department of Pediatrics, University of Alabama at Birmingham, 1600 7th Ave S, Lowder 500, Birmingham, AL 35233; e-mail: sbhatia@peds.uab.edu.
References
Competing Interests
Conflict-of-interest disclosure: S.B. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.