Abstract
Immune thrombocytopenia (ITP) has historically been thought to occur in 2 distinct forms: childhood ITP and adult ITP. This division is based largely on the presumption that childhood ITP is often benign and self-limited, whereas ITP in adults tends to be more chronic and difficult to treat. Although data exist to justify a different approach to the diagnosis and treatment in young children and the elderly, ITP in older children, adolescents, and younger adults is likely to share more similar pathology. This article will highlight the most recent data describing the natural history, diagnostic approach, management strategies, and disease-related outcomes in children and adults with ITP. These data reveal many unexpected similarities between the 2 groups, while confirming some of the more well-described differences. Discussion of these findings aims to highlight similarities and differences between ITP in children and adults, which will underscore important areas of future research and/or changes in management guidelines.
Learning Objectives
Describe the natural history of immune thrombocytopenia (ITP) in children and adults
Recognize the similarities and differences between ITP in children and adults
Determine the implications that similarities and differences of disease may have in the approach to diagnosis and management of ITP in children vs adults
Introduction
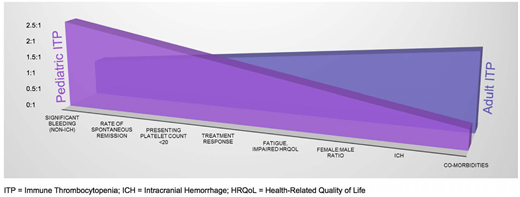
Immune thrombocytopenia (ITP) is one of the most common acquired bleeding disorders, occurring in ∼5 to 10 per 100 000 children per year1,2 and 3.3 per 100 000 adults per year.1 Although the pathophysiology of ITP is incompletely understood, it is believed to result from the production of autoreactive antibodies targeting endogenous platelets for destruction and a number of alterations in cellular immunity, as well as impaired megakaryopoiesis and platelet production, resulting in often profound thrombocytopenia and variable bleeding symptoms. Goals of treatment generally focus on cessation or prevention of bleeding. There are clear differences in standard clinical practices and guideline recommendations regarding the management of pediatric vs adult ITP patients. Many of these differences are based on traditional knowledge of the natural history of disease in children vs adults with ITP, including a lower likelihood of spontaneous remission and a greater bleeding tendency and need for treatment in adults.3,4 However, inconsistent definitions and resultant variability in reporting have made data interpretation difficult, and newer data are now challenging some of our traditional presumptions.5,6 Although key differences between children and adults with ITP remain, important similarities in clinical features in the 2 groups have been uncovered as well (Figure 1) and may lead to significant paradigm changes in the diagnosis and management of all patients with ITP.
Characteristic differences and similarities between pediatric and adult ITP.
Natural history of ITP in children and adults
Epidemiology
One of the most well-documented epidemiological distinctions between childhood and adult ITP is the predominance of females among adults affected with ITP. This female predominance (∼2:1 ratio) is consistently documented throughout the literature.6-8 The increased percentage of female patients in the adult ITP population is generally thought to be related to the increased incidence of systemic autoimmune disease in adult females.
Another major historical distinction between pediatric and adult ITP is the incidence of comorbid medical conditions. Although more data regarding the relationship and/or role of comorbidities in ITP outcomes are needed, several studies have confirmed the increased incidence of comorbidities in the adult ITP population.8 Data from the Pediatric and Adult Registry on Chronic ITP (PARC-ITP) showed the presence of ≥1 comorbidity in just 3.9% of children and >30% of adults at presentation5 and in 10.7% of children and 30.7% of adults at 2 years of follow-up,6 with an uptrending incidence as age increased among the adult population. Diabetes, gastrointestinal disease, hypertension, and thyroid disease appear to be the most common comorbid conditions among adults with ITP, whereas cancer and cardiovascular disease occurred with similar (and lower) frequency in children and adults with ITP.5,6
Remission
One of the major paradigms in ITP decision making is that children with ITP are much more likely to achieve spontaneous remission than adults with ITP, whose disease is generally thought to be more insidious in onset and chronic in nature. Although the natural history of ITP in adults is less well-studied than that in children, several studies have shown this to be true. In a 1995 study, long-term observation of 208 adults with chronic ITP revealed complete remission (off all therapy) in only 20.7% of patients after 48 to 151 months of follow-up.9 Notably, however, spontaneous recovery without intervention was seen in 9% of patients. In the first evidence-based practice guidelines for ITP developed for the American Society of Hematology (ASH),3 data pooled from 12 case series spanning from 1928 to 1989 showed the overall remission rate in adults with ITP to be 64% at the time of last follow-up. The majority of these patients had received therapy, including corticosteroids and/or splenectomy.3 A later study (2006) of 114 adults with ITP showed complete remission in 30% of patients by 6 months, in 53% of patients between 6 months and 3 years, and in 61% of patients by 5 years who received corticosteroids, splenectomy, or no treatment.10 Meanwhile, ITP in children tends to be acute, short-lived, and much more likely to resolve spontaneously.2,11,12 The Intercontinental Childhood ITP Study Group registry (n = 1496) reported complete remission in 69% of children with ITP by 6 months.13 These differences in remission rates for childhood and adult ITP were confirmed in a recent analysis of 2-year follow-up data from prospectively enrolled children (n = 3360) and adults (n = 420).6 (Table 1) Surprisingly, however, the rates of late remission among children and adults with chronic ITP were similar in the 2 groups. Among children with chronic ITP (defined as ongoing/active disease at 12-month follow-up), 28% achieved remission by 24 months; among adults with chronic ITP, 30% achieved remission by 24 months.6
Rates of spontaneous remission in children and adults with ITP
| Time point (mo) . | Children (%) . | Adults (%) . |
|---|---|---|
| 6 | 1559/2233 (70) | 145/324 (45) |
| 12 | 1160/1639 (71) | 133/271 (49) |
| 24 | 744/1045 (71) | 111/197 (56) |
| Time point (mo) . | Children (%) . | Adults (%) . |
|---|---|---|
| 6 | 1559/2233 (70) | 145/324 (45) |
| 12 | 1160/1639 (71) | 133/271 (49) |
| 24 | 744/1045 (71) | 111/197 (56) |
Data are from the PARC-ITP study.6 Numbers at each time point included only those prospectively enrolled patients with data available at the specified time point and did not include patients lost to follow-up.
Bleeding manifestations in children and adults with ITP
As noted before, the impression of ITP among hematologists tends to be one in which pediatric ITP and adult ITP encompass 2 separate and very distinct clinical entities. However, recent data show more similarities in the clinical presentation and bleeding tendencies in the 2 groups than was previously thought.5,6 These similarities include presenting platelet counts (mean, 18.1 × 109/L in children and 25.4 × 109/L in adults), incidence and type of bleeding when platelet counts are <20 × 109/L, family history of thrombocytopenia (2% in children and 3% in adults), rates of treatment (80% of children and 71% of adults at presentation and 58% of children and adults at 6 months, with similarly decreasing rates within both groups at 12 and 24 months),5,6 and, surprisingly, as noted above, similar late remission rates among children and adults with persistent and chronic ITP at 12 and 24 months.6
However, differences have been noted in bleeding manifestations. Recent data consistently describe higher rates of bleeding reported in children compared with adults with ITP, although this contradicts the previously held notion that adults are more likely than children to have significant bleeding. In the recently described prospectively enrolled cohort of children and adults with ITP (PARC-ITP), bleeding symptoms were reported in 91% of children at diagnosis compared with 69% of adults.5 Although bleeding severity within the 2 groups is more difficult to describe as a result of inconsistent or incomparable reporting methodologies, a large systematic review of prospective studies among children and adults with primary ITP recently reported a higher incidence of severe bleeding in children (20.2%) than in adults (9.6%).14 Only those studies with high-quality reporting of bleeding, using bleeding-assessment tools developed specifically for ITP patients and demonstrated to have good reliability and validity, and with clearly defined severity grades were included in this study.14 Another large study among pediatric patients hospitalized with ITP reports bleeding complications at a rate of 15.2% in this population.15 In this study, the Observational Medical Outcomes Partnership definition for bleeding was used.16 Conversely, however, intracranial hemorrhage (ICH) is consistently demonstrated to occur at a higher frequency in adults with ITP than children with ITP: ∼1.5% vs 0.5%17,18 (Table 2). Furthermore, the risk for ICH appears to be most increased at ≥60 years of age.19,20 In early 2018, data from the PARC-ITP cohort confirmed an increase in ICH among adults with ITP: 1.7% of adults developed ICH compared with only 0.6% of children.6 Among adult ITP patients experiencing ICH, 71% reported comorbidities, and 29% reported taking relevant medications (anti-inflammatory agents, antiplatelet agents, vitamin K antagonists, or other anticoagulants) at the time ICH occurred (among a group of patients in which 10% of the total adult population reported taking relevant medications at the time of diagnosis). Among pediatric ITP patients experiencing ICH, 7% reported comorbidities, and 14% reported taking relevant medications at the time ICH occurred (among a group of patients in which 1.1% of the total pediatric population reported taking relevant medications at the time of diagnosis). Mean age at the time of ICH among adult patients was 60 years. All ICH events in adult ITP patients occurred in the newly diagnosed phase, and 70% of pediatric ICH events occurred in newly diagnosed ITP patients, whereas 15% occurred within the first year of diagnosis, and another 15% occurred between 12 and 24 months. There has been consistent evidence over the years to support the hypothesis that risk for ICH is highest during the newly diagnosed phase of ITP,21-25 but evidence remains to support the risk for ICH during persistent and chronic phases of disease as well.14,19,26-28 The majority of ICH appears to occur at a platelet count < 20 × 109/L in children and adults (Table 2). Definitive associations or predictors of this dreaded ITP complication are difficult to ascertain, given the small number of patients affected. Ongoing investigation is crucial, because these data naturally portend important implications for the management of ITP in children and adults.
Studies reporting ICH in children and adults with ITP
| First author, year . | Methodology . | Subjects . | ICH incidence (%) . | ITP phase at ICH . | Platelet count at ICH (×109/L) . |
|---|---|---|---|---|---|
| Lilleyman, 199421 | Retrospective | Children | 14/∼11 000 (∼0.1) | 72% ND, 14% P, 14% C | <15 |
| Iyori, 200022 | Retrospective | Children | 4/772 (0.5) | 75% ND, 25% C | <10 |
| Kühne, 200113 | Prospective | Children | 2/1 496 (0.1) | ND* | |
| Neunert, 200823 201324 | Prospective | Children | 1/863 (0.1) [0-28 d]; 0/854 (0) [6-24 mo] | 100% ND, 0% P/C | <20 |
| Choudhary, 200925 | Retrospective | Children | 17/750 (2.3) | 59% ND, 41% C | Median, 12 (range, 20-50) |
| Psaila, 200926 | Retrospective | Children | 40 (0.19-0.78) | 45% ND, 25% P, 30% C | Median, 5 (<20 in 90%) |
| Elalfy, 201027 | Retrospective | Children | 10/1 840 (0.5) | 40% ND, 20% P, 40% C | <10 in 70% |
| Nørgaard, 201119 | Retrospective | Adults | 5/407 (1.2) | C* | <30 |
| Saeidi, 20148 | Retrospective | Children & adults | Children 0/223 (0); adults 0/100 (0) | N/A | N/A |
| Neunert, 201514 | Retrospective | Children & adults | Children (0.4); adults (1.4) | C (1.6%) > ND (0.4%) | |
| Zhou, 201528 | Retrospective | Children | 9/520 (1.7) | 45% ND, 22% P, 33% C | Median, 6 (<20 in 89%); range, 0-32 |
| Palandri, 201651 | Retrospective | Adults | 3/557 (0.5) | ||
| Altomare, 201618 | Retrospective | Adults | 74/6 651 (1.1) | ||
| Tsuda, 201720 | Retrospective | Adults | 5/169 (3) | <30 | |
| Schifferli, 20186 | Prospective | Children & adults | Children 20/3 360 (0.6); adults 7/420 (1.7) | 78% ND, 11% P, 11% C | <20 in 93% |
| First author, year . | Methodology . | Subjects . | ICH incidence (%) . | ITP phase at ICH . | Platelet count at ICH (×109/L) . |
|---|---|---|---|---|---|
| Lilleyman, 199421 | Retrospective | Children | 14/∼11 000 (∼0.1) | 72% ND, 14% P, 14% C | <15 |
| Iyori, 200022 | Retrospective | Children | 4/772 (0.5) | 75% ND, 25% C | <10 |
| Kühne, 200113 | Prospective | Children | 2/1 496 (0.1) | ND* | |
| Neunert, 200823 201324 | Prospective | Children | 1/863 (0.1) [0-28 d]; 0/854 (0) [6-24 mo] | 100% ND, 0% P/C | <20 |
| Choudhary, 200925 | Retrospective | Children | 17/750 (2.3) | 59% ND, 41% C | Median, 12 (range, 20-50) |
| Psaila, 200926 | Retrospective | Children | 40 (0.19-0.78) | 45% ND, 25% P, 30% C | Median, 5 (<20 in 90%) |
| Elalfy, 201027 | Retrospective | Children | 10/1 840 (0.5) | 40% ND, 20% P, 40% C | <10 in 70% |
| Nørgaard, 201119 | Retrospective | Adults | 5/407 (1.2) | C* | <30 |
| Saeidi, 20148 | Retrospective | Children & adults | Children 0/223 (0); adults 0/100 (0) | N/A | N/A |
| Neunert, 201514 | Retrospective | Children & adults | Children (0.4); adults (1.4) | C (1.6%) > ND (0.4%) | |
| Zhou, 201528 | Retrospective | Children | 9/520 (1.7) | 45% ND, 22% P, 33% C | Median, 6 (<20 in 89%); range, 0-32 |
| Palandri, 201651 | Retrospective | Adults | 3/557 (0.5) | ||
| Altomare, 201618 | Retrospective | Adults | 74/6 651 (1.1) | ||
| Tsuda, 201720 | Retrospective | Adults | 5/169 (3) | <30 | |
| Schifferli, 20186 | Prospective | Children & adults | Children 20/3 360 (0.6); adults 7/420 (1.7) | 78% ND, 11% P, 11% C | <20 in 93% |
C, chronic; N/A, not available; ND, newly diagnosed; P, persistent.
Study population included only patients in this phase of disease.
Diagnosis of ITP in children and adults
Childhood and adult ITP are similar in that major modifications in the extent of diagnostic investigation at the time of diagnosis have been made recently, given the increasingly convincing evidence against the need for exhaustive ancillary testing in the setting of typical ITP. In fact, the most recent ASH evidence-based practice guidelines for ITP recommend against routine bone marrow examination in children (even prior to corticosteroid therapy or splenectomy) and adults (irrespective of age) with typical features of ITP,12 and the international consensus guidelines published in 2010 recommend consideration of bone marrow examination only for patients aged >60 years old, prior to splenectomy, or in other atypical circumstances (ie, relapse following remission or first-line treatment failure).11 Both sets of guidelines recommend against the routine testing of anti-platelet, anti-phospholipid, and anti-nuclear antibodies in both populations.11,12 However, they do recommend routine HIV and hepatitis C virus (HCV) testing in adults, in addition to Helicobacter pylori testing if indicated, whereas routine HIV, HCV, and H. pylori testing are not recommended in children with ITP unless clinically indicated.11,12 Notably, however, recent data from the PARC-ITP cohort demonstrated significantly increased use of bone marrow examination, antinuclear antibody testing, and HIV and HCV testing in adults with ITP compared with children.5 The incidence of HIV among adults and children with ITP was similar (1%), whereas HCV (3% in adults vs 0% in children) and H. pylori (31% in adults vs 17% in children) were more common in adults with ITP. Antinuclear antibodies were more commonly positive in children (18%) than in adults (10%), whereas anti-phospholipid antibodies were not significantly different in children (10%) vs adults (6%).5 However, it must be noted that standardized testing for all patients was not performed, rather, it was completed among participating patients only as clinically indicated; therefore, incidences may be overestimated. Furthermore, the prevalence of chronic infections is clearly higher in the general adult population than in the general pediatric population, making it less likely that these incidences are definitively related to ITP etiology.
However, 1 laboratory feature of similar importance in children and adults with ITP at presentation is the platelet count. Studies have found that a presenting platelet count <20 × 109/L is predictive of remission and better overall outcomes in children29 and adults30 with ITP. Interestingly, presenting platelet count <20 × 109/L was reported at a higher frequency in children (79%) than in adults (58%) in the PARC-ITP cohort,5 which appears consistent with the higher likelihood of remission in children.
Management of ITP in children and adults
Observation vs treatment
Historically, platelet count thresholds were used in the decision models for treatment in pediatric and adult ITP. One of the most influential studies to mark platelet thresholds for bleeding was reported by Lacey and Penner in 1977,31 which showed that bleeding was rare with a platelet count > 30 × 109/L. Traditional management models subsequently moved forward with the clinical practice of treating all ITP patients with a platelet count < 30 × 109/L.3,7 This recommendation has been consistently carried forward among the adult population, whereas management recommendations for children with ITP continue to evolve toward a more conservative approach. This is likely due to a combination of factors, but most fundamentally the fact that the natural history of ITP in adults has been less well-studied than that in pediatrics. Empiric treatment at initial presentation has traditionally been the standard of care in adult ITP,3,11,12 further clouding any data regarding the natural history of untreated disease in adults. Providers’ propensity to treat is no doubt influenced by a perception of increased bleeding risk in adults. For one, adults are more likely to have comorbid conditions and ongoing antihemostatic therapies that are believed to place them at higher risk for bleeding,5,6 and increasing age has certainly been associated with an increased risk for bleeding.4 Furthermore, recent data consistently demonstrate a higher incidence of ICH in adults compared with children with ITP (Table 2), which generally increases with age.19,31 Simply stated, there is just not enough evidence to argue against this practice of platelet-based treatment; therefore, the most recent ASH evidence-based practice guidelines on ITP continue to suggest that “treatment be administered for newly diagnosed adults with a platelet count < 30 × 109/L.” Meanwhile, these same guidelines recommend that “children with no bleeding or mild bleeding be managed with observation alone regardless of platelet count,”12 and, in recent years, these recommendations have been increasingly incorporated into standard clinical practice among hematologists treating children.32-34 These recommendations are based on evidence that children with ITP presenting with only mild or no bleeding symptoms have an extremely low likelihood of developing significant bleeding, even in the setting of the lowest platelet counts.24 For example, among 863 children prospectively enrolled and followed from ITP diagnosis throughout the first 28 days, only 0.6% with a platelet count < 20 × 109/L and only mild or no bleeding symptoms went on to develop significant bleeding.23
Therefore, one of the biggest modern-day distinctions between pediatric and adult ITP lies in the recommendations for initial management: favoring observation in children vs treatment in adults. This clinical practice of observational management of children with ITP at presentation is happening despite consistent evidence for increased bleeding manifestations in children compared with adults with ITP (as discussed above in Bleeding manifestations in children and adults with ITP).5,6,14 This is likely due to an increased comfort level among providers, given the substantial evidence that has now accrued regarding the natural history of ITP in children and their extremely low likelihood of developing significant bleeding, regardless of therapy. However, data are emerging that there may be subpopulations of low-risk newly diagnosed adult patients who could be candidates for observation.35,36
First-line therapies
First-line treatment options include corticosteroids, IV immunoglobulin, and anti-D immunoglobulin, and they are used with similar efficacy and side effect profiles in children and adults with ITP.12 Recently, the most notable difference reported between the 2 groups is the choice of first-line treatment option, with IV immunoglobulin used more frequently in children and corticosteroids used more frequently in adults.5,6
Second-line therapies
Similar to first-line therapies, children and adults with ITP respond comparably to the most commonly used second-line agents. These therapies include rituximab, an anti-CD20 monoclonal antibody that targets B lymphocytes and, thereby, further antibody production; thrombopoietin receptor agonists (TPO-RAs), which stimulate increased platelet production; and splenectomy.
Rituximab generally yields initial response rates ∼ 60% in refractory ITP patients37 but with approximately twice as many complete responders within the adult ITP group compared with the pediatric ITP group.38 However, responses are often not sustained long-term, with a recent follow-up study showing that 33% of children and 38% of adults demonstrated a sustained response at 1 year, and 26% of children and 21% of adults demonstrated a sustained response at 5 years.38 Response rates are more favorable among certain subgroups of ITP patients, such as those with ITP secondary to systemic lupus erythematosus.39 Meanwhile, there are certain subgroups of ITP patients in whom more caution with the use of rituximab is warranted. For example, rituximab therapy has been associated with prolonged hypogammaglobulinemia, prolonged impairment and/or absence of antibody responses, and prolonged neutropenia in ITP patients with autoimmune lymphoproliferative syndrome (ALPS), common variable immunodeficiency, Evans syndrome, or other underlying immunodeficiency syndromes.40
TPO-RAs is a newer group of second-line therapies demonstrating excellent efficacy and safety in children and adults with ITP. Stimulation of platelet production became a therapeutic target in ITP following the substantiation of evidence for impaired megakaryopoiesis in ITP; including morphological evidence of abnormal thrombopoiesis, platelet auto-antibody effect on megakaryopoiesis, depressed or normal platelet production rates among ITP patients, and paradoxically normal levels of endogenous thrombopoietin (TPO) in the setting of very low platelet counts (especially compared with other conditions, such as aplastic anemia, in which TPO levels are very high in the setting of similarly low platelet counts).41 The 2 agents currently used in the treatment of ITP, eltrombopag olamine and romiplostim, are second-generation TPO-RAs that bind to the TPO receptor at different sites, but both are presumed to stimulate increased platelet production via mechanisms similar to endogenous TPO.42 Eltrombopag and romiplostim were approved by the US Food and Drug Administration for the treatment of chronic ITP in adults in 2008,43,44 and eltrombopag gained approval for the treatment of chronic ITP in children in 2015.43 Both agents are being widely used in the pediatric and adult ITP populations, with continued accumulation of excellent efficacy and safety data.45-47 Children and adults with ITP in the persistent or chronic phase of disease show excellent response rates (∼80%) with eltrombopag and romiplostim therapy. Furthermore, TPO-RA therapy results in decreased bleeding events and improved health-related quality of life (HRQoL) scores among ITP patients and does not appear to be associated with long-term toxicity or significant adverse effects, for children or adults with ITP.45-47
Prior to the advent of TPO-RA therapy, surgical splenectomy was the most reliable long-term treatment option for patients with refractory and/or chronic ITP, resulting in a sustained response rate of ∼70% in children48 and adults.49 In the recent 2-year prospective cohort of ITP patients comparing children and adults with ITP (PARC-ITP), splenectomy resulted in remission of ITP in 54% of children and 70% of adults.6 However, rates of splenectomy appear to be declining in adults and children as more medical therapies become available.50,51 Also, as with rituximab therapy, there are certain subpopulations of ITP patients in whom splenectomy should be considered with extreme caution. For example, patients with ITP secondary to ALPS are much less likely to respond to splenectomy, and they also have a significantly increased risk for infection-related morbidity and mortality following splenectomy.52 Therefore, in patients with ITP as a part of Evans syndrome, up to 50% of whom will have underlying ALPS53,54 or other disorders of immune regulation, splenectomy should be considered only as a last resort.
Other immunosuppressive agents, including cyclosporine, azathioprine, mycophenolate, and sirolimus, are used with varying degrees of efficacy in chronic refractory ITP. Although there are recent studies demonstrating benefit for specific immunomodulators in various subpopulations of ITP (such as sirolimus for children with refractory ITP due to ALPS),55 small patient numbers and lack of uniformity among studies make it difficult to draw definitive conclusions regarding most of these immunosuppressive therapies in children and adults with primary ITP.
HRQoL in children and adults with ITP
Although the relationship between ITP outcomes (such as bleeding) and HRQoL have not been optimally defined, there is significant evidence for a marked impact of ITP on patients’ HRQoL. One study among adults with chronic ITP showed significantly lower aggregate HRQoL scores compared with that of the general US population in nearly all domains (using the Medical Outcomes Study Short Form 36 Health Survey); surprisingly, it showed even lower scores than those reported by adults with hypertension, arthritis, or cancer.56
Fatigue is a significant complaint among children and adults with ITP57-59 ; in 1 study, 12.6% of adults and 8.1% of children often missed work or school because of fatigue.58 This is despite a similarly low bleeding frequency (∼10%) among children and adults with ITP.58 In another study among children with ITP starting second-line therapies, high fatigue was self-reported in 36% of children and 37% of adolescents with ITP, which was not significantly different from that reported by children and adolescents with cancer (34% and 21%, respectively), using the same fatigue scale.59 For children and adults with ITP, HRQoL scores appear to be the lowest at diagnosis and improve with time.57,60 Additionally, lower HRQoL scores were noted among children receiving treatment at diagnosis; however, only the persistence of ITP and lower platelet counts at 6 and 12 months were noted to consistently impact HRQoL beyond diagnosis for children with ITP and parents of pediatric ITP patients.60 Subtle differences in various HRQoL issues between children and adults with ITP were noted; children were more impacted by activity restrictions (23.3%, vs 9.5% for adults),58 and adults were more affected by glucocorticoid treatment side effects.57 However, there have been many similarities in the HRQoL reported by children and adults with ITP, most notably, a high prevalence of fatigue in both groups.
Conclusions
Clearly, there is evidence to define some of the more distinctive clinical qualities that characterize pediatric ITP (increased rates of acute bleeding and spontaneous remission) and adult ITP (increased rates of comorbidities, risk for ICH, and likelihood of chronicity) (Figure 1). However, closer inspection reveals what is becoming increasingly apparent about ITP in children and adults: heterogeneous treatment responses, remission rates, and clinical characteristics that result from a heterogeneous constellation of infectious, immunological, environmental, and genetic factors. Although classic spontaneously resolving childhood ITP continues to represent a significant proportion of disease, this homogeneous variety of ITP appears to be decreasing amid the spectrum of childhood and adult ITP that is evolving from an increasingly identifiable variety of immune aberrations and shifting toward increasing similarity in ITP among older children, adolescents, and young adults. Ongoing investigations into the biological determinants of disease will be key to understanding the full spectrum of ITP in children and adults and to further optimizing and individualizing management strategies.
Correspondence
Jenny M. Despotovic, Texas Children’s Cancer and Hematology Centers, 6701 Fannin St, Suite 1580, Houston, TX 77030 e-mail: jmdespot@txch.org.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.