Abstract
Pain is a complex multidimensional experience and the most common morbidity in patients with sickle cell disease (SCD). Tools to assess pain can be of use not only to guide pain treatment but also to provide insight into underlying pain neurobiology. Mechanisms of pain in SCD are multifactorial and are not completely elucidated. Although vaso-occlusion of microcirculation by sickled red cells is believed to be the underlying mechanism of acute vaso-occlusive pain, mechanisms for chronic pain and the transition from acute to chronic pain are under investigation. A number of modalities can be used in clinical practice and/or research to capture various dimensions of pain. Selection of a pain-assessment tool should be directed by the purpose of the assessment. Pain-assessment tools, many of which are currently in the early stages of validation, are discussed here. Development and validation of these multimodal tools is crucial for developing improved understanding of SCD pain and its management.
Learning Objectives
To increase knowledge of pain-measurements tools, which can be of use in both assessing pain and understanding the complex neurobiology of sickle cell pain
To understand that pain is a complex multidimensional experience and comprehensive assessment should include all dimensions of pain
To review existing and investigational pain-measurement tools available to assess the multidimensional nature of pain in patients with SCD
Background
Tools to measure pain are critical for pain management and research in sickle cell disease (SCD). Pain is the most common morbidity associated with SCD. Recurrent pain is the leading cause of SCD-related hospitalizations, contributing to direct annual health care costs of $1.1 billion in the United States.1 Pain is associated with poor health-related quality of life,2,3 and increased frequency of hospitalizations for pain is a key predictor of early mortality.4 Although the hallmark feature of SCD is recurrent episodes of acute severe pain typically referred to as vaso-occlusive crises, chronic pain is common and has been recently classified using a common set of criteria.5 It is important to recognize that, even within the same genotype, significant diversity of pain phenotype exists, resulting in large variability in pain burden whether defined by number of hospitalizations for pain or degree and days in pain at home.4,6 Features of chronic pain start to emerge in adolescents and young adults. Similar to other non-SCD conditions associated with pain, patients with SCD and high burden of pain are more likely to experience functional disability and higher somatic burden, depression, and anxiety.7 SCD pain has been proposed to be mechanistically classified as: inflammatory, peripheral/nociceptive, peripheral neuropathic/sensitization, central neuropathic/sensitization, or centralized pain; any of these mechanisms may be operative simultaneously in the same patient.8 Despite pain being the most common manifestation of SCD, the neurobiological mechanism of pain and especially factors responsible for the transition from acute to chronic pain are not completely clear. Thus, age and developmentally appropriate tools and methodologies are needed to assess pain, determine the impact of pain, and investigate underlying pain mechanisms that could lead to improved understanding and ultimately improved management of pain in SCD.
Pain experience is always subjective. The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”9 Individuals learn to define pain through experiences generally associated with injury in early life; thus, pain is often reported in the context of actual or potential tissue damage. It is now well recognized that people may report pain in the absence of tissue damage or pathophysiological cause. Nonetheless, if these individuals regard their experience as pain, and report it in the same ways as pain caused by tissue damage, it should be accepted as pain based on the current definition, which avoids associating pain to the stimulus.9
Because pain is a subjective, complex, and multidimensional experience, its assessment should include all of its dimensions. This review will discuss unidimensional and multidimensional tools that assist in the measurement of pain. In addition, investigational pain-assessment tools used to reveal underlying mechanisms of pain that could be used to develop and guide therapies will also be discussed.
Unidimensional tools for assessment of pain intensity
The most traditional tools used to assess pain in SCD are focused on the assessment of pain intensity; they include the Numeric Rating Scale (pain ratings from 0 to 10; for patients 8 years of age or older)10 and the Bieri Faces Pain Scale (sequential facial expressions that represent pain anchored by smiling and crying; for patients 3 years of age or older).11 The Visual Analog Scale (VAS) (for patients 8 years of age or older) is also used to assess pain intensity.12 The VAS requires patients to draw a line along a 10-cm horizontal or vertical axis that best describes their pain with the extremes of the lines representing no pain and worst pain possible. The VAS is then scored by measuring the distance between the patient’s line and the no-pain anchor.12 The Numeric Rating Scale, Faces Pain Scale, and VAS ask patients to rate momentary pain intensity on a spectrum, forcing the patient to choose a single number/face or line that represents his/her pain at the time the assessment is completed. The Numeric Rating Scale and Faces Pain Scale are the most traditionally used metrics in most hospitals and outpatient clinics. The VAS is used less traditionally for clinical pain assessment due to the need for paper/pencil or an electronic device; it also requires an additional step in interpretation. In general, pain-intensity scales are limited in their ability to evaluate the spectrum and multidimensional nature of the pain experience.13 A significant amount of interindividual variability also exists in pain-intensity scores because pain tolerance between patients can be highly variable.13 For example, one patient may rate her worst pain bearable a 9 whereas another patient may rate his worst pain bearable a 5; this does not necessarily mean that the patient with the score of 5 is in less pain than the one with a score of 9.13 Additionally, a patient admitted to the hospital may be up and walking around the unit and asking to go home with a pain score of 6 whereas another is writhing in bed in pain with a pain score of 6. This interindividual variability in pain-intensity scores makes them a poor outcome measure for pain research when used in isolation, however, the Δ or change in pain-intensity scores could be a useful research outcome measure for acute interventions.
Tools that primarily assess pain intensity are most informative when used to assess the acute clinical change in pain within a patient in response to an intervention. For example, pain-intensity scores are useful at detecting the response of a patient’s acute pain to IV morphine in the emergency department or inpatient unit. It is important to note that the Δ or change in pain score (ie, pain changed from 7 to 4, Δ of 3) is much more informative than the actual number/faces rating before and after the intervention. Aside from this context, pain-intensity scores should be used in combination with more expansive and multidimensional tools that assess the functional impact of pain on the daily lives of patients as outlined in the next section. Furthermore, pain-intensity scores are less useful for assessing the phenotypic characteristics of pain, which can direct investigations into the biology of pain and inform treatment decisions.
Multidimensional patient-reported outcome tools that measure pain impact
A more robust way to measure pain is to determine the impact that pain has on patients’ function through the use of patient-reported outcome tools. Patient-reported outcome tools are multidimensional and allow patients to quantitate the impact of pain on daily functioning and behavior. As described in the previous section, traditional unidimensional pain-intensity assessment tools have limitations. Determining the extent to which pain impacts the patients’ ability to live their daily lives is more informative in directing treatment than pain-intensity ratings alone. Thus, patient-reported outcome tools that assess health-related quality-of-life domains focused on pain impact can be robust pain-measurement tools that incorporate the patients’ voice in a systematic way. Fortunately, 3 currently available health-related quality-of-life tools exist that have pain-focused domains for use in SCD. Two of the tools are disease-specific: (1) Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-ME; for patients 18 years of age or older)14 and (2) Pediatric Quality of Life Inventory Sickle Cell Disease (PedsQL SCD) Module (for patients 2-18 years of age).15,16 One tool is general: National Institutes of Health (NIH) HealthMeasures/Patient-Reported Outcomes Measurement Information Systems (PROMIS; for patients of all ages).17 Each of these 3 tools includes domains that are specific to the assessment of the impact of pain. Specifically, ASCQ-ME has 1 such domain entitled “Pain Impact”14,17 (http://www.healthmeasures.net/administrator/components/com_instruments/uploads/ASCQ-Me%20Bank%20v2.0%20-%20Pain%20Impact_5-9-2017.pdf); the PedsQL SCD Module has 2 such domains entitled “About my pain impact” and “About my pain management and control”15,16 (https://eprovide.mapi-trust.org/instruments/pediatric-quality-of-life-inventory-sickle-cell-disease-module). NIH HealthMeasures/PROMIS has 1 established domain for pain-impact assessment entitled “Pain Interference”17-19 (http://www.healthmeasures.net/search-view-measures?task=Search.search) and 2 newer domains entitled “Pain Behavior”17 (http://www.healthmeasures.net/search-view-measures?task=Search.search) and “Pain Quality-Affective” that are currently being studied in children with SCD.20 Examples of questions from these health-related quality-of-life measures for assessing pain impact are included in Tables 1 and 2.
Patient-reported outcome measures for multidimensional assessment of pain impact in patients with SCD: health-related quality-of-life measures
| Measure . | Pain domain . | Example questions . |
|---|---|---|
| ASCQ-ME14 (ages ≥18 y) | “Pain impact” | In past 7 d: “How often did you have pain so bad that you could not do anything?”; “How often did you have pain so bad that you could not get out of bed?”; “How often did you have severe pain?”; “How often did you have pain so bad that you had to stop what you were doing?”; “How often did you have pain so bad that it was hard to finish what you were doing?” |
| PedsQL SCD Module15,16 (ages 2-18 y) | “About my pain impact” | In past 1 mo: “It is hard for me to do things because I might get pain”; “I miss school when I have pain”; “It is hard for me to run when I have pain”; “It is hard for me to have fun when I have pain”; “I have trouble moving when I have pain”; “It is hard for me to do what others can do because I might get pain” |
| “About my pain management and control” | In past 1 mo: “It is hard for me to manage my pain”; “It is hard for me to control my pain” | |
| PROMIS/ HealthMeasures17 (all ages) | “Pain interference” | In past 7 d “I had trouble sleeping when I had pain”; “It was hard for me to pay attention when I had pain”; “It was hard for me to run when I had pain”; “It was hard for me to walk one block when I had pain” |
| “Pain behavior” | Asks whether the following occurred when in pain: “It showed on my face”; “I talked about my pain”; “I asked for medicine”; “I moved slower”; “I had to stop what I was doing”; “I lay down” | |
| “Pain quality-affective” | Asks whether pain made patient feel: “miserable, awful, horrible, unbearable, worrying, unending, annoying, unpleasant” |
| Measure . | Pain domain . | Example questions . |
|---|---|---|
| ASCQ-ME14 (ages ≥18 y) | “Pain impact” | In past 7 d: “How often did you have pain so bad that you could not do anything?”; “How often did you have pain so bad that you could not get out of bed?”; “How often did you have severe pain?”; “How often did you have pain so bad that you had to stop what you were doing?”; “How often did you have pain so bad that it was hard to finish what you were doing?” |
| PedsQL SCD Module15,16 (ages 2-18 y) | “About my pain impact” | In past 1 mo: “It is hard for me to do things because I might get pain”; “I miss school when I have pain”; “It is hard for me to run when I have pain”; “It is hard for me to have fun when I have pain”; “I have trouble moving when I have pain”; “It is hard for me to do what others can do because I might get pain” |
| “About my pain management and control” | In past 1 mo: “It is hard for me to manage my pain”; “It is hard for me to control my pain” | |
| PROMIS/ HealthMeasures17 (all ages) | “Pain interference” | In past 7 d “I had trouble sleeping when I had pain”; “It was hard for me to pay attention when I had pain”; “It was hard for me to run when I had pain”; “It was hard for me to walk one block when I had pain” |
| “Pain behavior” | Asks whether the following occurred when in pain: “It showed on my face”; “I talked about my pain”; “I asked for medicine”; “I moved slower”; “I had to stop what I was doing”; “I lay down” | |
| “Pain quality-affective” | Asks whether pain made patient feel: “miserable, awful, horrible, unbearable, worrying, unending, annoying, unpleasant” |
Patient-reported outcome measures for multidimensional assessment of pain impact in patients with SCD: other multidimensional pain tools
| Tool . | Age range, y . | Description . |
|---|---|---|
| McGill Pain Questionnaire (MPQ)29 | Adults, ≥18 | Patients choose from 3 categories of words that are divided into sensory, affective, and evaluative aspects of pain; measured/scored based on number of words chosen from each category |
| Brief Pain Inventory (BPI)31 | Adults, ≥18 | Assesses pain location, intensity, treatments, generalized functional impact on 0-10 Likert scales |
| Adolescent Pediatric Pain Tool (APTT)30 | Ages ≥8 | Assesses pain intensity, pain pattern, and location; pain quality assessed by asking child to choose from list of words grouped into the following domains: sensory, affective, evaluative, and temporal |
| PAINReportIt60,66 | Adults, ≥18 | Assesses pain location, intensity, quality, and patterns. Also evaluates analgesic use, patient-related barriers to effective pain management, and items to asses the following 9 concepts: amount of time that pain is greater than the tolerable level; patient satisfaction with pain level; expectations about the pain; effectiveness of previous pain treatments; pain medication treatment pattern; nondrug treatments used for pain; tendency to tell or not tell others about the pain; onset of pain; and belief about the cause of the pain |
| Tool . | Age range, y . | Description . |
|---|---|---|
| McGill Pain Questionnaire (MPQ)29 | Adults, ≥18 | Patients choose from 3 categories of words that are divided into sensory, affective, and evaluative aspects of pain; measured/scored based on number of words chosen from each category |
| Brief Pain Inventory (BPI)31 | Adults, ≥18 | Assesses pain location, intensity, treatments, generalized functional impact on 0-10 Likert scales |
| Adolescent Pediatric Pain Tool (APTT)30 | Ages ≥8 | Assesses pain intensity, pain pattern, and location; pain quality assessed by asking child to choose from list of words grouped into the following domains: sensory, affective, evaluative, and temporal |
| PAINReportIt60,66 | Adults, ≥18 | Assesses pain location, intensity, quality, and patterns. Also evaluates analgesic use, patient-related barriers to effective pain management, and items to asses the following 9 concepts: amount of time that pain is greater than the tolerable level; patient satisfaction with pain level; expectations about the pain; effectiveness of previous pain treatments; pain medication treatment pattern; nondrug treatments used for pain; tendency to tell or not tell others about the pain; onset of pain; and belief about the cause of the pain |
These health-related quality-of-life measures are psychometrically robust. The SCD-specific measures have been validated in patients with SCD.14,15 The NIH PROMIS measures have been validated in healthy people; ongoing studies are validating them in patients with SCD.17-21 These measures allow for comparisons between both patients (ie, how is my patient doing compared with other patients) and within a patient (ie, how is my patient doing today compared with 6 months ago). Furthermore, these tools, in general, have a recall period of 7 days to 1 month14,15,17 and are designed to measure changes over time22 whereas traditional pain-intensity tools measure momentary change and can vary from hour to hour (ie, after a dose of morphine, a patient’s pain score changes from 8 to 4).13 Health-related quality-of-life tools can also be used to help patients living with chronic pain establish functional goals. For example, a patient may never live pain-free, however, he or she could set functional goals that can then be measured by health-related quality-of-life tools. These tools can be used in any health care setting, including the inpatient unit,19,23,24 outpatient clinic, and at home, as patients are now routinely completing these measures remotely.19 Furthermore, these measures can be used as research outcomes.19,25,26 Ongoing research is being done to determine how to use these tools in real time to guide clinical care.27,28 Other multidimensional patient-reported pain-measurement tools that have been used in SCD include the McGill Pain Questionnaire,29 the Adolescent Pediatric Pain Tool,30 and the Brief Pain Inventory31 (see Tables 1 and 2 for descriptions of these tools).
Diagnosis of medical and psychological comorbidities
Patients with SCD can experience pain caused by complications of the disease such as avascular necrosis of joints, gallstones, and leg ulcers. These comorbidities should be evaluated by appropriate clinical examination and imaging, which are not the focus of this paper. Readers should refer to guidelines to screen for complications of SCD. In addition to assessing pain intensity and the functional impact of pain, a thorough psychological assessment should be considered an adjunct pain-assessment tool. Depression, anxiety, and sleep disturbances are some of the most common psychological comorbidities that coexist with acute and chronic pain.7,32,33 These psychological comorbidities can exacerbate pain experience as shown in a prospective study of adults with SCD in which participants with depression and anxiety had higher somatic symptom burden that was associated with a higher percentage of daily pain days.7 Validated age-appropriate surveys exist to evaluate these comorbidities and should be included in the comprehensive pain assessment. A detailed discussion of the assessment and treatment of these psychological comorbidities is not the focus of this paper; however, it is important to note that the input of a trained psychologist or psychiatrist is an important and necessary adjuvant tool to the assessment of pain patients with SCD. Additional factors such as racial bias, injustice, and health-related stigma, which may impact pain burden and quality of life, should also be considered.34
Assessment of pain at home
The biologic differences between acute and chronic pain will not be discussed in this paper. However, it is important to note that the tools used for pain assessment at home for recurrent acute and chronic pain compared with the assessment of acute severe pain in the inpatient setting may need to be different. Whereas pain-intensity measures could be useful for the measurement of momentary changes in acute pain and response to analgesics, they are likely less useful in the assessment of chronic pain. For example, patients suffering from chronic pain may report persistent daily pain scores ranging from 4 to 6 but are functioning well (ie, going to school, working, etc.) Thus, using a patient-reported outcome measure could better assist the provider in assessing his/her patient’s pain. Electronic pain diaries and smartphone applications that incorporate patient-reported outcome measures. and could facilitate real-time assessment of pain and subsequent pain management at home, are increasingly being used to follow patients’ pain at home.35 These applications are currently being evaluated in patients with SCD.35
Investigational pain-measurement tools that assess pain biology
There are a variety of tools currently under investigation that address pain measurement in SCD. Many of these investigational tools are targeted at better understanding the mechanisms of pain in patients with SCD and are specifically focused on studying alterations in the central and peripheral nervous system. The goal of these investigational tools is to delineate the neurobiological mechanism of pain and better phenotype pain. As previously discussed, pain in SCD is complex and has components of various pain types (neuropathic, inflammatory, etc); being able to differentiate these components in the clinic is therapeutically important. These tools can also help with understanding the interindividual variability in pain that exists between patients with SCD. Ultimately, these investigational tools could lead to testing of targeted pain therapies and allow for patient selection of these therapies based on pain phenotype as shown in other non-SCD pain conditions such as fibromyalgia.36,37 In a study of acupuncture as a treatment of fibromyalgia, Zuker et al tested analgesic response in a cohort of 114 patients who were randomized to receive either verum or sham acupuncture. In this study, responsiveness to acupuncture correlated with pain-pressure threshold, suggesting that quantitative sensory testing (QST) findings could be used to personalize pain treatment.37 Furthermore, these tools can assist in testing of targeted therapies as shown by Harris et al who used brain-neuroimaging techniques to demonstrate that pregabalin, not placebo, was able to rectify altered brain chemistry, connectivity, and response to experimental pain in patients with fibromyalgia.36
Quantitative sensory testing
A pain-assessment tool currently used in investigational studies is QST. The conduct of QST involves a psychophysical evaluation of the somatosensory system through application of various physical sensory stimuli (cold, heat, mechanical pressure) to patients in order to interrogate the peripheral and/or central nervous system.38,39 These various physical sensory stimuli activate specific receptors that subsequently generate signals in the sensory nervous system that correspond to pain-sensing nerve fibers. QST can measure sensory loss (hyposensitivity) or gain (hypersensitivity) in these various physical sensory stimuli. QST can assess for impaired pain sensitivity or pain hypersensitivity when the application of the same physical sensory stimuli (cold, heat, mechanical pressure) produces more pain in a patient with SCD compared with healthy controls.38,39 Ultimately, this pain hypersensitivity suggests the existence of pain-processing abnormalities at the level of the peripheral and/or central nervous system. To date, 10 studies utilizing QST have been completed in patients with SCD with variable findings.40-49 Results of 5 of these studies are summarized in detail in a recent review.50 In general, patients with SCD were found to have increased sensitivity to cold, heat, and/or mechanical pain compared with healthy controls to varying degrees at different body sites.40-50 Recent studies in adults with SCD using QST have also provided evidence for central mechanisms of pain (central sensitization).48 Furthermore, patients with heightened central sensitization profile were shown to experience more clinical pain, vaso-occlusive crises, catastrophizing, poorer sleep, and negative mood. Collectively, these QST data support the existence of abnormalities in pain processing at the level of the central and/or peripheral nervous system. The reason for this pain hypersensitivity is an active area of investigation. Currently, QST has only been used in the context of research studies in patients with SCD. Additional work is required before QST is used as a tool to assess pain in the clinic and direct treatment. Despite the current limitations in real-time clinical utility, QST can be a powerful research tool to dissect the underlying neurobiology of sickle cell pain, especially because the laboratory methodologies of QST for human subjects research closely parallel those used in the sickle cell mouse model to study pain.51
Neuroimaging
Brain-imaging methodologies such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and scalp electroencephalography (EEG) are noninvasive and useful in understanding the neural basis of pain in a living human being. The findings presented in this section are derived largely from non-SCD populations as these methodologies have only recently begun to be used in SCD. Neuroimaging studies have identified a network of diverse regions of brain that are activated by painful stimuli and play a role in pain perception and modulation. This “pain network” includes primary and secondary somatosensory, insular, anterior cingulate, and prefrontal cortices and thalamus. These areas receive input from multiple nociceptive pathways and contribute to the multidimensional experience of pain.52 Studies have also shown that brain network activation patterns associated with acute pain in healthy subjects are different from those in individuals with chronic pain.53 Furthermore, there is involvement of brain regions associated with cognition and emotion in chronic pain suggesting that these comorbidities could be a distinctive feature of chronic pain.54
fMRI, also known as blood-oxygen-level–dependent (BOLD) MRI, is one of the most often used functional imaging modalities due to its noninvasiveness and widespread availability. Its principle is based on the hemodynamic response. Brain activity is measured by detecting changes associated with blood flow, which parallels increased neuronal activity associated with a task. The BOLD signal is generated because of differences in the magnetic properties of deoxygenated and oxygenated hemoglobin, which is then mapped to show which neurons are active. In recent years, resting-state functional connectivity MRI (rs-fcMRI) studies of brain activity are being increasingly used to provide insight into functionally interconnected networks.55 Some of the other magnetic resonance–based measures such as spectroscopy, diffuse tensor imaging, and volumetric imaging can also be used to assess changes in metabolic activity, white matter tracts, or brain volume in different areas of the brain that may be associated with pain.
fMRI studies in SCD have demonstrated differences in brain-network connectivity in patients with SCD compared with healthy controls.56 Furthermore, the resting-state brain-connectivity patterns have been shown to be different in patients with high pain burden (more connectivity to pronociceptive structures) compared with patients with low pain burden (more connectivity to antinociceptive structures). The association between pain burden and altered brain connectivity patterns suggests the possible role of central mechanisms in SCD pain.57 A fMRI-EEG study designed to assess temporal patterns of activation areas showed that compared with controls, SCD patients had increased activity in pain-processing regions during rest.58 It is important to note that at present, fMRI cannot be used as a tool to confirm whether a person is experiencing pain for medical or legal purposes and currently is only used in the context of investigational pain studies.
Patient-reported measurement tools that assess pain biology
Questionnaires focused on the phenotypic characterization of pain are currently under investigation in patients with SCD. These questionnaires systematically collect data that carefully describe the phenotypic characteristics of pain such as qualitative pain descriptors, aggravating and alleviating factors, sensory symptoms, pain pattern, and radiation of pain. The importance of tools that measure phenotypic pain characteristics cannot be underestimated both in their ability to assist in understanding pain biology and to differentiate between various types of pain (ie, neuropathic, inflammatory, nociceptive) to direct treatment. Currently, these tools are not at the stage where they are being used to direct treatment, however, they have been primarily used in research.45,48,59 Some of these tools that have been studied in patients with SCD that specifically screen for neuropathic pain include the PAINReportIt,60 painDETECT,61 Leeds Assessment of Neuropathic Pain Symptoms and Signs (LANSS),40,62 and the Neuropathic Pain Symptom Inventory.40 Table 3 includes a brief description of these measures including how they are scored. Data reveal 25% to 40% of patients with SCD score positive for neuropathic pain based on these measures.40,50,61,62 The application of how screening for neuropathic pain can be used in a clinical trial is demonstrated in a phase 1 trial of an antipsychotic, trifluoperazine, which was used to treat neuropathic pain. One of the inclusion criteria for this trial was patients reporting chronic pain with ≥4 neuropathic pain descriptors. The drug revealed preliminary efficacy with 44% of subjects reporting at least a 50% reduction in chronic pain without supplemental analgesics supporting a larger efficacy trial.59 Many of these tools are limited by age and are only available for use in older adolescent and adult populations due to the nature of the questions asked and vocabulary used (Table 3). There are 2 new NIH HealthMeasures/PROMIS domains focused on the phenotypic characterization of pain entitled “Neuropathic Pain Quality” (adults) and “Nociceptive Pain Quality” (adults).17 Currently, these phenotypic pain-assessment tools are not yet used clinically to direct treatment but could be used as a research outcome measure in clinical trials of novel pain treatments; someday, they could be used as screening tools to direct clinical care.
Patient-reported screening tools to phenotype pain in patients with SCD
| Tool . | Age range, y . | Description . | Scoring of tool . |
|---|---|---|---|
| PAINReportIt60 | Adults, ≥18 | Electronic version of McGill | Tool asks patients to choose from 78 pain descriptors that are grouped into neuropathic, nociceptive, or other categories and the mean number of descriptors from each category is calculated |
| painDETECT61 | Adolescents/ adults, ≥14 | Cross-sectional questionnaire that elicits pain phenotype through questions targeted at pattern of pain, sensory symptoms of pain, aggravating and alleviating factors | Tool yields total score: 0-38 ≥19: definite neuropathic pain 13-18: probable neuropathic pain ≤12: no neuropathic pain |
| Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS)40,62 | Adolescents/ adults, ≥14 | Cross-sectional questionnaire of 7 items that screen for neuropathic pain | Tool yields total score: 0-24 ≥12 indicative of neuropathic pain |
| Neuropathic Pain Symptom Inventory (NPSI)40 | Adults, ≥18 | Cross-sectional questionnaire of 12 items that investigate neuropathic pain symptoms | Tool yields total score: 0-100 Higher scores indicate increased likelihood of neuropathic pain. No cutoff score established to differentiate neuropathic from nonneuropathic pain. |
| PROMIS: “Neuropathic Pain Quality”17 | Adults, ≥18 | In past 7 d: “Pain feels like pins and needles”; “Pain feels tingly”; “Pain feels stinging”; “Pain feels electrical”; “Pain feels numb” | Use HealthMeasures Scoring Service or HealthMeasures data collection tool to calculate scores. Generally, higher T-scores indicate more neuropathic pain. |
| PROMIS: “Nociceptive Pain Quality”17 | Adults ≥18 | In past 7 d: “Pain feels sore”; “Pain feels tender”; “Pain feels achy”; “Pain feels deep” | Use HealthMeasures Scoring Service or HealthMeasures data collection tool to calculate scores. Generally, higher T-scores indicate more nociceptive pain. |
| Tool . | Age range, y . | Description . | Scoring of tool . |
|---|---|---|---|
| PAINReportIt60 | Adults, ≥18 | Electronic version of McGill | Tool asks patients to choose from 78 pain descriptors that are grouped into neuropathic, nociceptive, or other categories and the mean number of descriptors from each category is calculated |
| painDETECT61 | Adolescents/ adults, ≥14 | Cross-sectional questionnaire that elicits pain phenotype through questions targeted at pattern of pain, sensory symptoms of pain, aggravating and alleviating factors | Tool yields total score: 0-38 ≥19: definite neuropathic pain 13-18: probable neuropathic pain ≤12: no neuropathic pain |
| Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS)40,62 | Adolescents/ adults, ≥14 | Cross-sectional questionnaire of 7 items that screen for neuropathic pain | Tool yields total score: 0-24 ≥12 indicative of neuropathic pain |
| Neuropathic Pain Symptom Inventory (NPSI)40 | Adults, ≥18 | Cross-sectional questionnaire of 12 items that investigate neuropathic pain symptoms | Tool yields total score: 0-100 Higher scores indicate increased likelihood of neuropathic pain. No cutoff score established to differentiate neuropathic from nonneuropathic pain. |
| PROMIS: “Neuropathic Pain Quality”17 | Adults, ≥18 | In past 7 d: “Pain feels like pins and needles”; “Pain feels tingly”; “Pain feels stinging”; “Pain feels electrical”; “Pain feels numb” | Use HealthMeasures Scoring Service or HealthMeasures data collection tool to calculate scores. Generally, higher T-scores indicate more neuropathic pain. |
| PROMIS: “Nociceptive Pain Quality”17 | Adults ≥18 | In past 7 d: “Pain feels sore”; “Pain feels tender”; “Pain feels achy”; “Pain feels deep” | Use HealthMeasures Scoring Service or HealthMeasures data collection tool to calculate scores. Generally, higher T-scores indicate more nociceptive pain. |
Plasma biomarkers for pain measurement
Currently, there are no plasma biomarkers that can be used in isolation to measure pain in patients with SCD. Multiple inflammatory cytokines and neuropeptides have been shown to be increased in patients with SCD both at baseline and during acute pain and are associated with increased numbers of painful events.63-65 To date, plasma biomarkers should be used to investigate SCD pain biology and should not be used in isolation as a pain-measurement tool. The use of plasma biomarkers could be used in conjunction with patient-reported outcome tools to anchor these tools to baseline health and disease exacerbations.
Conclusions
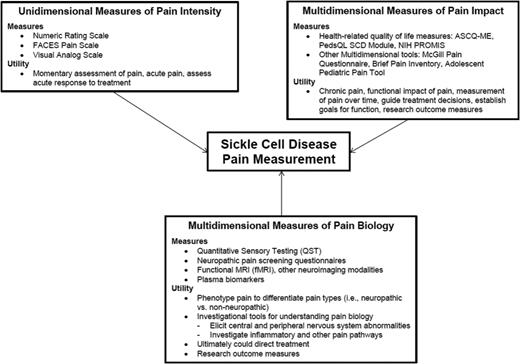
The assessment and measurement of pain is a complex phenomenon that relies on patient report. Nonetheless, a variety of experimental pain models and tools exist that allow for the study of pain in humans under controlled conditions. These pain-assessment tools have provided new insight into pain processing and perception in SCD. Due to the subjective and multidimensional nature of pain, which is unique to the individual, multimodal assessment tools are needed to fully elucidate the underlying mechanism, degree, and impact of pain. Pain is a subjective symptom experienced by patients that does not always have an objective measurement. This overriding principle needs to be kept at the forefront when approaching the measurement of pain in patients with SCD. Pain is multidimensional and is not experienced the same way in each patient and may not always be experienced the same way within a patient at different points in time. Therefore, a multifaceted approach should be used to measure pain in patients with SCD. This approach is summarized in Figure 1. There are inherent dangers in allowing pain assessment and measurement to become too objectified, however, having objective assessments is necessary to systematically study pain robustly in order to improve clinical care. It is imperative that we always allow the patients’ voice to be heard to ensure empathetic and comprehensive assessment of pain in patients who are suffering. Ultimately, the goals of pain assessment and measurement are to guide the understanding of pain biology, which can lead to the development of innovative therapies, and to then be able to measure the impact of these innovative therapies from the perspective of the patient to decrease suffering.
Conceptual framework for pain measurement in patients with SCD. the measurement of pain in SCD patients of has 3 main components: unidimensional measurement of pain intensity, multidimensional measurement of pain impact, and multidimensional measures of pain biology. The specific measures in each category and their utility are depicted.
Conceptual framework for pain measurement in patients with SCD. the measurement of pain in SCD patients of has 3 main components: unidimensional measurement of pain intensity, multidimensional measurement of pain impact, and multidimensional measures of pain biology. The specific measures in each category and their utility are depicted.
Acknowledgments
This work was supported by the American Society of Hematology (A.M.B.) and National Institutes of Health National Heart, Lung, and Blood Institute grant 1K23 HL114636-01A1 (A.M.B.).
Correspondence
Amanda Brandow, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: abrandow@mcw.edu.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.