Abstract
The pathophysiology, clinical presentation, and natural history of acute pain in sickle cell disease are unique and require a disease-centered approach that also applies general principles of acute and chronic pain management. The majority of acute pain episodes are managed at home without the need to access health care. The long-term consequences of poorly treated acute pain include chronic pain, adverse effects of chronic opioid usage, psychological maladjustment, poor quality of life, and excessive health care utilization. There is no standard protocol for management of an acute pain crisis in either the hospital or the community. The assumptions that severe acute pain must be managed in the hospital with parenteral opioids and that strong opioids are needed for home management of pain need to be questioned. Pain management in the emergency department often does not meet acceptable standards, while chronic use of strong opioids is likely to result in opioid-induced hyperalgesia, exacerbation of chronic pain symptoms, and opioid dependency. We suggest that an integrated approach is needed to control the underlying condition, modify psychological responses, optimize social support, and ensure that health care services provide safe, effective, and prompt treatment of acute pain and appropriate management of chronic pain. This integrated approach should begin at an early age and continue through the adolescent, transition, and adult phases of the care model.
Learning Objectives
To conceptualize acute sickle pain from a holistic perspective, taking into account non–disease factors that determine the response to pain and recognizing the emergence of symptoms of chronic pain in older patients
To review the problems with current models of treatment and formulate a lifetime management plan based on disease modification, psychosocial support, optimal use of analgesics, and better organization of care in both the hospital and the community
Introduction
The acute painful crisis is the most common acute complication of sickle cell disease (SCD). Although several influential management guidelines have recently been published,1,2 local protocols differ substantially and reflect a range of different approaches to pain management. Sickle pain experience is influenced by psychological response to pain, the socioeconomic context, and the organization of health care services delivering pain management programs and protocols. Furthermore, because most pain episodes are managed without accessing health care services, patients need support in their daily lives to cope with pain and avoid adverse long-term effects of repeated pain events, including chronic pain syndromes and complications of chronic opioid treatment.
This review will address how these broader aspects of acute pain in SCD can be incorporated into an overall management strategy. We have concentrated on experience in the United States and the United Kingdom to examine the outcomes in contrasting health care systems: US health care consists of a range of different providers covering primary, secondary, and tertiary care, whereas the UK National Health Service (NHS) aims to provide a more integrated service that is funded by the taxpayer, free of charge to the patient, and encompasses primary, secondary, and tertiary care.
Definition of an acute pain episode
The term “crisis” is confusing and has a variety of different meanings for specialists as well as for patients and carers. The Cooperative Study of Sickle Cell Disease defines an acute sickle cell pain crisis as an episode of pain in the extremities, back, abdomen, or head lasting >2 hours that leads to a clinic visit and cannot be explained by a cause other than SCD. This definition excluded episodes managed at home and other acute painful complications of SCD such as dactylitis, acute chest syndrome, priapism, right upper quadrant syndrome, and osteomyelitis.3 It also did not take into account readmissions with pain occurring within 14 days. In the BABY HUG trial, the definition of pain as an adverse event included any mention of pain, regardless of whether analgesics were used, and did not require treatment in a hospital or clinic. This was to support US Food and Drug Administration approval for a pediatric indication for hydroxyurea4 and preempted a consensus that the definition based on health care utilization alone is inadequate. Regulatory authorities such as US Food and Drug Administration and the UK Medicines and Healthcare Products Regulatory Agency are now encouraging the development of tools to enable patients to report their experience of pain on a day-by-day basis and to use changes in this patient-reported outcome as a measure of therapeutic effectiveness in clinical trials. This measurement of daily pain is most appropriate for patient assessment, but it requires further standardization for widespread use in the clinic.
Natural history and treatment effects
Pain episodes recorded using a daily diary are relatively infrequent in infants and young children but increase in the adolescent years.5,6 Pain episodes resulting in health care utilization increase in frequency with age, reaching a peak in adolescents7 and in adults between the age of 20 and 30 years.3 Although health care utilization for pain decreases in older adults, diary-reported pain increases.3,8 In the Pain in Sickle Cell Epidemiology Study (PiSCES), 232 patients aged ≥16 years were asked to record pain episodes in a daily diary over a 6-month period. Pain without health care utilization was reported on an average of 38.3% of days, “crisis” without health care utilization on 12.7% of days, and crisis with health care utilization on only 3.5% of days.8 A diary study comparing pain experiences in children in contrasting settings of inner-city London and a Caribbean island showed that children in the United Kingdom experience more pain than their Caribbean counterparts,9 and the authors suggest that cultural and/or environmental factors may have an important role in the causation and experience of pain.
Efficacy of hydroxyurea and regular transfusion in preventing pain episodes
Hydroxyurea is still the only licensed pharmacological agent used for modification of disease severity and has significantly altered the natural history of pain in SCD, reducing acute episodes and hospital attendances by ∼50%. For subjects enrolled in the Multicentre Study of Hydroxyurea in Sickle Cell Anemia, average daily pain intensity was lower for patients taking hydroxyurea (2.5 vs 2.8 on a 10-point scale recorded in a daily diary). Although small, the difference appeared early and was sustained. There were also small but significant reductions in analgesic use and health care utilization.10 The NHLBI recommendation to initiate hydroxyurea at an early age for the prevention of acute pain episodes is welcome for children who have had hospital admissions with pain or who are experiencing recurrent pain at home.11 Clinical trials of therapy to prevent ischemic brain damage in children have shown that regular transfusion is also effective in preventing acute pain episodes,12,13 and may be superior to hydroxyurea.14 There is ample clinical experience and published data to justify recommending regular transfusion for patients who, for whatever reason, continue to have significant pain episodes despite optimization of hydroxyurea therapy.
Chronic pain in SCD
In addition to chronic musculoskeletal and neuropathic pain related to bone, joint, or peripheral nerve damage, there is another form of chronic pain seen in SCD that is more typical of a central sensitization pain syndrome. Typical characteristics of such pain include allodynia and hyperalgesia. Chronic pain in SCD is relatively poorly understood and characterized.15 A recent consensus proposal for a definition requires pain to be ongoing, present on most days, lasting at least 6 months, and located at a single site or multiple sites.16 The evolution of chronic SCD pain has been shown by increased sensitivity to noxious stimuli elicited in subjects who have not yet developed characteristic symptoms of chronic pain.17 Evidence from a mouse model of severe SCD is compatible with a mechanism of sensitization through chronic afferent pain fiber stimulation due to repeated severe acute pain episodes.18
This type of pain is common. In the PiSCES study, almost one-third of adult patients experienced pain nearly every day,8 and up to 40% of children with SCD in one center were categorized as suffering from chronic pain.19 It is likely that many of the frequent attenders in emergency departments (EDs) are suffering from chronic pain as well as an acute painful vaso-occusive crisis.
Opioid analgesia for chronic pain in SCD
Chronic pain is often treated ineffectively and inappropriately, with dosage escalation and rotation of strong opioid analgesics, overreliance on a combination of pain-modifying drugs, and insufficient attention to nonpharmacological approaches. While judicious use of opioids under expert supervision and careful monitoring does have a role in chronic noncancer pain,20 there is a significant risk of adverse effects. Of particular concern is the possibility of exacerbating pain through opioid-induced hyperalgesia. This is well described in animal models, human volunteers given opioid infusions, and also different clinical scenarios of recurrent acute pain.21 Opioid use is associated with higher rates of pain intensity, hyperalgesia symptoms, stress, and mental health problems. High usage is associated with increased health care utilization,22 but it is generally unclear whether opioid use is a cause or consequence of these symptoms.
Some studies have documented very frequent usage of strong opioids outside the hospital. In the PiSCES study, patients used opioids on 78% of home pain days, and 44% of patients used opioids on >50% of diary days.8,23 In the PROACTIVE feasibility study, (a US trial of preemptive blood transfusion for the prevention of acute chest syndrome), 36.7% of patients reported using strong opioids prior to admission with acute pain, most commonly oxycodone, hydromorphone, morphine, and hydrocodone.24 This is a worrying trend, because unsupervised treatment with strong opioids at home may result in acute overdose and will inevitably cause tolerance, hyperalgesia, and dependency. In the absence of systematic studies on alternative approaches to chronic pain treatment or use of opioid sparing analgesic drugs, the patient and care team often have no option but to rely on a pharmacological approach based on long-term opioid prescriptions.
Psychological responses to sickle cell pain
The psychological response is fundamental to the pain experience. Stress, low mood, and negative coping strategies such as negative thinking, somatization, and catastrophizing all have an adverse effect on the pain experience. Coexisting mental health problems are common but may be overlooked. Depression has been reported in between 2% and 57% of patients in different studies using different methodologies, and a systematic review suggests at least a modest association between depression and higher health care utilization.25 Psychological responses are probably learned at an early age and become increasingly difficult to modify at a later age. It is likely that there is a window period in childhood and adolescence when intervention is most likely to be effective.
Psychological therapies include group and individual therapy; education covering SCD, pain, and its effects; and instruction in relaxation techniques, self-hypnosis, and cognitive and behavioral therapies to improve coping skills. It has been difficult to draw firm conclusions on the best form of therapy as there are relatively few studies in this area, and methodologically, these are quite varied in terms of therapeutic input and study design.26
Guidelines for managing acute sickle pain make very little reference to psychological inputs in the acute situation or as a long-term intervention, and it is not possible to make overall recommendations on the specific type of input at present. There are ongoing trials of psychological interventions, including randomized studies of stress management and self-relaxation techniques, which may inform practice over the next few years.
Modification of psychological responses should form an important part of a long-term pain management strategy for lessening suffering during acute events, reducing the risk of emerging chronic pain symptoms, minimizing exposure to analgesic drugs, and avoiding the utilization of emergency health care services. Patients should be screened for psychological comorbidities and, if present, should be referred to a psychologist or psychiatrist for further evaluation and treatment.
Socioeconomic factors impacting pain severity
Socioeconomic deprivation has a strong impact on health outcomes, but the evidence for an impact on pain-related outcomes in SCD is difficult to assess. One early study demonstrated several aspects of social disadvantage were more common in SCD patients than in non-SCD controls. These included a single-parent household, low family income, a fragmented or absent supportive network of family and friends, and inadequate health insurance.27 Socioeconomic factors have been shown to have a negative impact on health-related quality of life, including some measures of pain. It is unclear, however, how the frequency and severity of painful episodes managed at home are related to socioeconomic factors,8 and in the case of ED attendance and hospital admission, analysis is confounded, because patients or families without health insurance avoid hospital care for financial reasons.28 The UK NHS provides universal health care. Analysis of NHS hospitalization episodes for SCD showed that patients living in the most socioeconomically deprived areas were at highest risk of admission and readmission and there was increased inpatient mortality among readmissions in patients living in the most deprived areas.29 This suggests that improving accessibility to health care does not necessarily improve outcomes.
Interventions to improve socioeconomic status are generally outside of the scope of the SCD care team, but there are examples of beneficial interventions. Legal issues associated with low socioeconomic status contribute to poor health outcomes, and one scheme in a US pediatric service incorporating access to legal services as part of the care plan resulted in a positive impact on patients, parents, and guardians that was attributable to legal assistance as part of the care partnership team.30 In the United Kingdom, SCD specialists spend considerable time filling out forms and writing letters in support of applications for welfare payments. This is not only an altruistic gesture, as it may help form a positive relationship with the patient or carer and probably does improve the chance of a successful application, and access to illness-related welfare payments may well improve long-term outcome by relieving the patient or carer of some financial distress.
A risk assessment is important at the earliest stage to identify children from very low-income families, those who have insecure family support, and those who have inadequate or temporary accommodation in order to offer closer monitoring and encourage social support packages with the aim of avoiding long-term maladaptive responses to acute pain.
Addiction, dependency, and substance misuse
There is a widespread belief among health care workers that that opioid addiction in the sickle cell population is common.31-35 Patients often feel that they are labeled as opioid addicts and treated inconsiderately and inappropriately. The term “addiction” itself can be perceived as pejorative, and it might be constructive to use the term “dependency” in place of addiction.
Formal assessment of addiction/dependency in patients with pain is complex, and traditional diagnostic criteria are probably not appropriate. Physiological dependence, manifested by tolerance and withdrawal symptoms, is expected for any patient using opioids on a regular basis, and the psychiatric definition of substance dependence requires additional symptoms or behaviors.33 Traditional diagnostic criteria for addiction can apply to patients exhibiting an appropriate response to pain and attempts to control it. This can lead to misperceptions of substance dependence. Non–pain-related symptoms and behaviors (eg, opioid use in the absence of pain or in attempts to alter mood, obtain euphoria, or reduce nonpain distress) are a more reliable indication of opioid dependency in this context.
Symptoms of opioid withdrawal (eg, anxiety, agitation, sweating, nausea, vomiting, diarrhea, abdominal cramps, muscular spasms, and generalized discomfort) can potentially merge with symptoms of an acute complication of SCD. Well-documented examples of this occurrence were reported in a long-term single-center study in the United States, where 5 out of 55 patients readmitted within 1 week after an acute painful episode had symptoms of opioid withdrawal that then precipitated a further painful episode.36
The older literature contains several studies attempting to record and interpret the experiences of patients and the staff involved in their care. Common themes are feelings of anger and powerlessness on the part of the patient, who may believe they he or she is not being treated with sufficient analgesia to control the pain, and a breakdown of trust between the patient and health care worker. Doctors describe having no option but to continue inappropriate treatment with large doses of opiates despite being unconvinced that they are treating a severe vaso-occlusive crisis.31,33,37 A recent study suggests that there has been no major change in these perceptions over time.35
There are no specific trials or consensus protocols for management of opioid dependency in SCD, but some general themes emerge from the literature. An individualized approach based on a relationship of trust between the health care provider and the patient is recommended. The coordinator is usually the specialist (hematologist) who has long-term responsibility for care and understands the specific medical, social, and psychological contexts for his or her patient. It is important to ensure an awareness of opioid adverse events and expected symptoms of dependency. Once an agreement has been reached that opioid dependency is a problem, a specialist in substance abuse should be included in formulating a management plan. Specific advice on management of dependency and withdrawal symptoms should be combined with more general measures such as single-provider prescriptions of opioids and a focus on developing a healthy lifestyle. There are often social and psychological comorbidities that need to be addressed.
Problems with delivering acute care in the ED
Most EDs are noisy, stressful, and overcrowded. There is an urgent demand to prioritize life-threatening cases over treatment of pain, and competing priorities make it difficult for ED staff to adhere to the basic requirements of care. Rapid administration of analgesia, mandatory monitoring of vital signs for recognition of opioid toxicity, and prompt diagnosis of other acute complications of SCD may therefore be compromised.
Recent national guidelines produced in the United States and United Kingdom both recommend that analgesia is given rapidly (60 minutes after registration and 30 minutes after triage in the NHLBI guideline1 and within 30 minutes of arrival in hospital in the National Institute for Health Care Excellence guideline2 ). Studies and audits are consistent in showing delays in initiating and continuing analgesia therapy.38,39 A recent national peer review of specialist centers in the United Kingdom found that most services were not yet able to demonstrate that they were providing analgesia within 30 minutes. Patient feedback was better when adult patients were admitted straight under hematologists rather than via the general medical team.40
In a US study, delays have been related to inappropriate allocation to low-level triage despite recommendations for higher priority in cases of severe pain. Additional problems include overcrowding and understaffing of EDs and difficulty in obtaining IV access for opioid administration.41,42
When inadequate care is recognized, quality improvement projects have had only limited success in meeting analgesia standards and improving patient satisfaction. In one study, 3 US institutions participated in a learning collaborative model to identify the gaps in quality of care and develop and evaluate local solutions, including nurse-initiated analgesia protocols. This study did not show any improvement in time to first analgesia or in patient-reported satisfaction with pain management.39 The consequences of unsatisfactory care in ED include a reluctance to attend the hospital or maladaptive behavior leading to repeated attendances and conflict between the patient and staff.
In summary, although ED remains the default option because it provides open-access, 24-hour care and expert emergency health care staff, it is not the ideal environment for management of an acute pain event. Other entry points into acute health care should be explored to improve outcomes for patients and health care providers.
Prospects for bypassing the ED
Day care
Managing acute pain in a specialized day unit setting has many advantages over the ED. Pain can be managed in an environment that is less busy, noisy, and stressful. The specialist day care team is likely to be more knowledgeable and expert in SCD management and have a greater awareness of the individual patient’s care needs.
The care model for an acute painful episode would typically include administration of strong opioid analgesia and discharge to home with a prescription of oral analgesia even if still reporting a severe pain score. The patient can then benefit from rest at home and maintenance of a normal evening and nighttime routine with family support. Following this, they can reattend the following day to continue day care pain management.
There are several examples of day care pain management in the literature demonstrating better pain management, reduction in hospital admission, reduction in hospital bed occupancy, reduced rate of readmission, and a health economic benefit.43-46 There have been significant barriers to universal adoption of this model, including difficulty in sustained funding of expert nursing and medical staff over an extension of the working day. Furthermore, there is a risk of inappropriate use of day care, particularly by frequent attenders with chronic pain and high opioid use or opioid dependency. This risk needs to be carefully managed, and it may be necessary to place restrictions on access to certain patients.
Primary care
Management of acute sickle cell pain in a primary care setting could be an alternative means of avoiding ED and hospital admission. In the United Kingdom, all NHS patients are registered with a family doctor and a primary care practice. SCD is a condition that requires specialist medical input, and although general practitioners are expected to provide routine primary care to children and adults with SCD, they are not expected to provide care for severe painful events. There is nevertheless scope for avoidance of ED attendances and hospital admissions with effective collaboration between the SCD specialist and general practitioners, and good examples of collaboration include regular prescription of analgesics for home use, integrated management of patients on discharge after an acute event, and multidisciplinary community-based management of chronic pain from a community setting.40 These, however, are local initiatives rather than standard NHS care.
NHS primary care fulfills most of the aspirations of the patient-centered medical home, including care that is accessible, continuous, comprehensive, family centered, coordinated, compassionate, and culturally effective. In the United States, many children have access to services that fulfill some of the components of the patient-centered medical home, and this probably helps in reducing the need for ED and hospital treatment.47 There is less information on the access for adults with SCD, and it is not clear from the published literature how patient-centered medical homes can provide care for acute severe pain as an alternative to the ED.
Community outreach by specialist teams
Most of the large pediatric and adult specialist SCD services in England employ nurse specialists as part of the multidisciplinary team, and some of these nurses have a community outreach role. In one home-based program, which has run for many years in in a North London borough, SCD nurse specialists are able to visit adult patients at home and administer injected opioids up to 2 times per day. This has resulted in a substantial reduction of hospitalizations and length of stay (LOS).40 There is a potential for a collaborative approach between a community-outreach SCD team and other community nursing services. For instance, teams that provide pain management to terminally ill patients in their homes could potentially adapt to management of acute sickle pain episodes. Clinical studies of health service delivery are needed to evaluate the safety, efficacy, and acceptability of different approaches to management of an acute painful episode in the patient’s home and address this unmet need.
Hospital pain management
First analgesia
Time to first analgesia dose is an important standard of care. The problems with meeting this standard have been discussed in a previous section. The National Heart, Lung, and Blood Institute (NHLBI) guidelines recommend use of strong opioid given intravenously (or subcutaneously when IV access is not available). Administration of IV opioid is often delayed during the process of prescribing, checking, drawing up, and insertion of the IV line. Drugs administered across the nasal or buccal mucosa are alternative options for rapid-onset analgesia and could be offered by suitably trained nursing staff in the ED almost as soon as the patient presents. Table 1 shows the time to maximal plasma concentration for opioids given by different routes.
Time to peak plasma concentration for short-acting opioids
| Drug and formulation . | Population . | Tmax (h) . |
|---|---|---|
| IV morphine (5 mg) | Healthy adults | 0.03 |
| Oral morphine liquid (10 mg) | Healthy adults | 0.75 |
| Intranasal diamorphine (1 mg/kg) | Children with isolated limb fracture | 0.17 |
| Oral oxycodone liquid (10-20 mg) | Healthy adults | <1.0 |
| Sublingual fentanyl (Abstral) | Opioid-tolerant cancer patients | 0.3-2 |
| Buccal fentanyl lozenge (Actiq; 800 μg) | Healthy adults | 1.5 |
| Buccal fentanyl effervescent tablet (Effentora; 400 μg) | Healthy adults | 0.78 |
| Drug and formulation . | Population . | Tmax (h) . |
|---|---|---|
| IV morphine (5 mg) | Healthy adults | 0.03 |
| Oral morphine liquid (10 mg) | Healthy adults | 0.75 |
| Intranasal diamorphine (1 mg/kg) | Children with isolated limb fracture | 0.17 |
| Oral oxycodone liquid (10-20 mg) | Healthy adults | <1.0 |
| Sublingual fentanyl (Abstral) | Opioid-tolerant cancer patients | 0.3-2 |
| Buccal fentanyl lozenge (Actiq; 800 μg) | Healthy adults | 1.5 |
| Buccal fentanyl effervescent tablet (Effentora; 400 μg) | Healthy adults | 0.78 |
Adapted from Telfer et al48 with permission.
Tmax, time to peak plasma concentration.
Diamorphine is highly soluble, rapidly absorbed, and clinically efficacious when administered across the nasal mucosa. At our institution, a study in children aged 2 to 14 years showed that intranasal diamorphine was effective for rapid analgesia on first arrival in the ED and could be used as an alternative to IV morphine. In this study, 54% of children received the first dose of intranasal diamorphine within 5 minutes and 79% within 10 minutes of arrival in the ED.49,50 Transmucosal fentanyl preparations have a rapid onset of analgesia and are licensed for management of breakthrough pain in cancer. They have the potential to produce rapid analgesia for acute sickle pain without the need for injection and within a short time after arrival in the ED.48 The Sickle Cell Analgesia Protocol Evaluation (SCAPE) trial, which is currently being conducted at our institution, is a phase 1 trial of sublingual fentanyl for acute analgesia in adolescents and young adults.
“Effective” analgesia until resolution of a painful event
The majority of adults and older children will have severe pain (pain score >7 on a 10-point scale) in the early stages of a hospitalized episode of pain. The most rapid reduction in pain score is during the early stages, followed by a plateau, and many patients still have a pain score >7 at time of discharge.51 Attempts to control pain completely are unrealistic and result in excessive analgesic dosing. Pain management aimed at reducing pain to a moderate level (4-6) is more realistic, and patients should be counseled that they may not be completely pain-free when discharged from the hospital.
It is accepted that opioid analgesics are required as part of the management of severe pain, though there is no consensus on the type of opioid or route of delivery. There is a general assumption that injected opioids are needed to treat acute severe pain, and indeed, the NHLBI guidelines make no mention of oral opioids.1 However, there is evidence from a randomized controlled trial that an oral morphine protocol can be as effective as IV morphine. Children aged 5 to 17 years presenting to the ED with a severe acute pain episode were allocated to initial IV bolus morphine followed either by long-acting oral morphine for background or short-acting oral morphine boluses for breakthrough analgesia or to continued IV background and IV bolus morphine. Both regimes had a similar effect in reducing pain scores, and there were no significant differences in overall opioid usage or LOS.
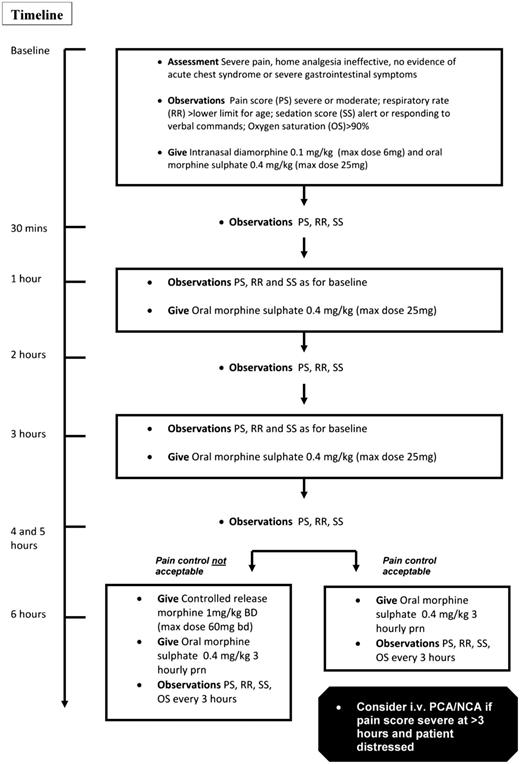
Managing patients with oral opioids using careful monitoring of the pain score, proactive prescheduled boluses, and a long-acting oral opioid for background analgesia may circumvent problems associated with delivery of parenteral opioids and enable earlier discharge into the community. In an audit of a protocol of intranasal diamorphine (single dose) combined with oral short and long-acting morphine given at our institution over a 1-year period, there were 96 admissions for 38 children; 92% of admissions were managed without the need for injected opioid, and there were no safety issues.50 Children were managed in the ED for the first 2 hours and then transferred to a pediatric ward specializing in hematological disorders. In some cases, treatment continued in the ED for more than 2 hours, depending on bed availability. Full details of this protocol are provided in Figure 1.
How I treat an uncomplicated acute painful event in a child aged 1 to 14 years. NCA, nurse-controlled analgesia; PCA, patient-controlled analgesia. Adapted from Telfer et al48 with permission.
How I treat an uncomplicated acute painful event in a child aged 1 to 14 years. NCA, nurse-controlled analgesia; PCA, patient-controlled analgesia. Adapted from Telfer et al48 with permission.
There is limited evidence for efficacy and safety of similar protocols for adolescents and young adults after transition to adult services. The SCAPE study is evaluating a protocol of sublingual fentanyl combined with oral short- and long-acting oxycodone in this age group. At present, SCAPE is in the dose-escalation phase where an optimal safe and effective dose of sublingual fentanyl is being evaluated, and full results of the study are expected in late 2018.
Avoidance of opioid-induced adverse effects
The most serious opioid adverse effects are respiratory suppression and oversedation. UK National Institute for Health Care Excellence guidelines recommend monitoring (including sedation score) every 1 hour for the first 6 hours and at least every 4 hours thereafter.2 The NHLBI guidelines are less specific and only recommend monitoring for excessive sedation with an objective measurement sedation scale and oxygenation levels.1 Certain categories of patients are particularly at risk. These include children, patients who rarely suffer acute pain crises and are opioid naive, and patients with renal impairment. The risk of opioid accumulation and toxicity is higher with continuous IV infusions or high doses of oral long-acting opioids. Evidence of inadequate monitoring is provided in a UK National Confidential Inquiry into Patient Deaths in the United Kingdom.52 Of 35 patients with SCD who died in the hospital, 19 had pain as the presenting complaint. In at least 50% of these cases, there was evidence of excessive opioid dosing and inadequate monitoring. In many of these cases, junior trainees were responsible for the prescribing of analgesia and no attempt was made to seek expert advice.
Protocols for opioid monitoring need to be very specific and should be regularly audited. In addition, a record should be kept of patient education concerning potential dangers of inappropriate and unsupervised opioid use.
Minimization of opioid exposure
Dosage of strong opioids should be kept to the minimum necessary to ensure a reduction or stabilization of pain score. There is some data to suggest that dosage and frequency of bolus analgesia is reduced when used in combination with continuous IV infusion or long-acting oral opioids for background analgesia.53,54 Nonsteroidal anti-inflammatory agents are recommended as adjuncts in most guidelines on the basis of an old systematic review, but a more recent randomized trial of IV ketoprofen did not show any effect in reducing morphine usage or duration of crisis.55 Patients on hydroxyurea have reduced opioid requirements,56 and phase 2 trial results of the experimental synthetic pan-selectin inhibitor GMI-1070 (rivipansel) showed an 83% reduction in cumulative parenteral opioid consumption. This effect was apparent within 24 hours of initiation of treatment.57 Ongoing trials of opioid-sparing treatment in acute painful episodes include the phase 3 trial of rivipansel and a trial of gabapentin.58
Adjunctive nonpharmacologic approaches include heat pads, acupuncture, massage, and psychological techniques such as relaxation techniques, self-hypnosis, and distraction. In general, these have not been evaluated in a robust clinical trial setting, but many patients find them useful, and their use should be encouraged.1
Minimization of LOS
Between 1989 and 1993, the estimated average LOS for pain-related admissions in the United States was ∼5 days for children, 7 days for adults, and 6 days overall.59 LOS increases with age during childhood.7 In an NHS-based study of hospital episode statistics of admissions with SCD between 2001 and 2010, 57.9% of all patients were discharged within 24 hours of being admitted to hospital, while only 15.4% of admissions stayed for longer than 1 week.60
There is likely to be variability in LOS related to the health care system and access to care, but this has not been systematically assessed in the literature. It is also not clear whether there have been any trends in LOS over the past 20 years. LOS has been assessed as a clinical end point in several clinical trials. In the Multicentre Study of Hydroxyurea in Sickle Cell Anemia study, there was a significant reduction in LOS in patients randomized to hydroxyurea compared with placebo, and there was a nonsignificant reduction in the phase 2 trial of rivipansel vs placebo.57 Low-demand high-background opioid patient-controlled analgesia dosing also appeared to reduce LOS compared with high-demand low-background dosing.53
Discharge from the hospital is contingent on at least a partial resolution of the painful event with the pain score consistently reduced from severe to moderate. Furthermore, a clinical judgment should be made that the risk of developing additional acute complications (such as acute chest syndrome) is low. There may be opportunities for earlier discharge if parenteral opioid use can be shortened and replaced with a suitable regime of short- and long-acting oral opioids. Brandow et al found that earlier initiation of oral opioids was correlated with shorter LOS for children admitted for acute pain.61 Successful early discharge requires preassessment and planning for continued pain management,62 and this should usually include a specialist outpatient assessment within 1 week of discharge into the community.
Avoidance of readmission
The definition of a readmission is not consistent. In the NHS, a readmission within 30 days of discharge has been considered an indicator of poor discharge planning and poorer quality of care and may incur a financial penalty.63 Analysis of NHS repeat hospitalization episodes over the period 2005-2011 showed that readmissions were more likely in those living in more socioeconomically deprived regions.29 Readmission rates within 30 days have been reported in the United States in 17% to 33% of episodes and are more common in those aged 18 to 30 years.51 In a study of adult patients attending a specialist center, 15% of episodes were readmissions within 1 week of discharge. These patients were often discharged while still in severe pain (the average pain score at discharge was 8.1). The authors suggested that readmission may represent a recurrence of vaso-occlusive pathology during the natural course of an acute painful crisis.36,51
These observations suggest that readmissions may be avoided if pain management and analgesic dosing were better planned prior to and after discharge. Specialist hematology review within 30 days of discharge is important both for coordination of care between the hospital and the community and for monitoring analgesic requirements after discharge.51,64
Conclusions
Acute pain in SCD is a unique clinical problem. The long-term consequences of repeated acute pain episodes include a chronic pain syndrome, adverse effects of chronic opioid usage, psychological maladjustment, poor quality of life, and excessive health care utilization.
There is no standard protocol for management of an acute pain episode, and it is not clear how long-term complications of acute pain can best be avoided. We suggest that an integrated approach is needed to control the underlying condition, modify psychological responses, optimize social support, and ensure that health care services provide safe, effective, and prompt treatment of acute pain and appropriate management of chronic pain. This integrated approach should begin at an early age and continue through adolescent, transition, and adult care.
Introduction of hydroxyurea or regular transfusions should be considered during early childhood to prevent acute pain episodes and reduce the risk of later development of a chronic pain syndrome. This should be combined with a program of regular and long-term psychological interventions to encourage effective coping mechanisms and prevent negative and harmful illness behaviors.
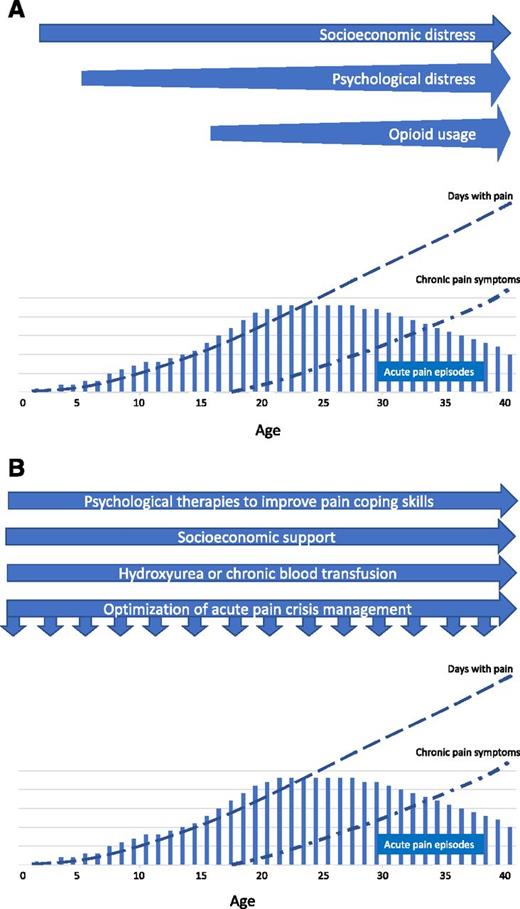
Figure 2 is a schematic diagram depicting the evolution of acute and chronic complications of pain in a severely affected patient and a broad range of interventions that should be implemented to impact on the different domains of pain experience. Services would need to adapt these principles to their own circumstances and assess long-term outcomes through analysis of trends in pain-related outcomes in age cohorts of their service users.
Evolution of acute pain and chronic complications of pain in a severely affected patient. (A) Timeline of non–disease factors impacting the pain experience. (B) Timeline of proposed interventions for modifying the pain experience. There is no numerical scale on the y-axis, and quantitative comparisons of pain episodes, days with pain, and chronic pain symptoms are not intended.
Evolution of acute pain and chronic complications of pain in a severely affected patient. (A) Timeline of non–disease factors impacting the pain experience. (B) Timeline of proposed interventions for modifying the pain experience. There is no numerical scale on the y-axis, and quantitative comparisons of pain episodes, days with pain, and chronic pain symptoms are not intended.
Correspondence
Paul Telfer, Department of Hematology, Royal London Hospital, 80 Newark St, London E1 2ES, United Kingdom; e-mail: paul.telfer@bartshealth.nhs.uk.
References
Competing Interests
Conflict-of-interest disclosure: P.T. is on the board of directors or an advisory committee for Pfizer; has received research funding from Napp and Kyowakirin; has consulted for Novartis, Global Blood Therapeutics, and Bluebird Bio; and has received honoraria from Novartis, Global Blood Therapeutics, Apopharma, and Bluebird Bio. B.K. is on the board of directors or an advisory committee for Astra Zeneca and has received honoraria from Novartis.
Author notes
Off-label drug use: None of the analgesic drugs discussed in the text are specifically licensed for use in managing acute painful episodes in sickle cell disease. Sublingual fentanyl and hydromorphone injection are only licensed for treatment of severe pain in cancer.