Abstract
Hematopoietic cell transplantation (HCT) remains a cornerstone of treatment of many hematologic malignancies but transplant-associated morbidity and mortality limit application to older patients. Biologic or chronologic age barriers to HCT have fallen, because patients in their 8th decade of life comprise the group with the greatest rise in transplant use over the past decade. Evaluating physiologic age or general health in older transplant candidates requires a systematic approach inclusive of functional and comorbidity assessment, which typically is accomplished through geriatric assessment (GA). GA incorporates measures of comorbidity, function, nutrition, social support, and other health-related domains to better describe physiologic age. Older allogeneic transplant patients have a surprisingly high prevalence of vulnerabilities by GA prior to transplant, and significant comorbidity or functional limitations heighten the risks of transplant-related mortality. Ultimately, incorporation of physiologic age can improve estimates of nondisease life expectancy, prognostic survival after HCT, and inform HCT candidacy. Future research on the optimal tools to characterize physiologic age and appropriate interventions in the context of transplant are needed.
Learning Objectives
Recognize methods to measure physiologic age in older candidates for HCT
Apply health assessment tools to risk-stratify patients for HCT
Biologic age
Aging is a complex process characterized by loss of function at the molecular, cellular, and tissue level, producing a progressive decline in organ function and ability to maintain homeostasis.1 Under the stress of advancing age, cellular senescence or loss of reserve results in age-related morbidity and mortality. We live in an era of unprecedented gains in longevity driven by improvements in public health, greater prosperity, and to some degree medical care, swelling the ranks of older people.
Biologic or chronologic age is an invaluable surrogate to gauge general health and life expectancy on a population basis. Yet, the heterogeneity of health in advancing age limits biologic age as the sole means to describe fitness for an individual patient.
Biologic age and transplant: conceptions and misconceptions
Hematologic malignancies in older age
The peak in incidence of common hematologic malignancies in older ages combined with improvements in life expectancy creates a growing number of older adults for whom transplant is an option. Older age consistently predicts for inferior overall survival (OS) from disease relapse due to a combination of more adverse disease, less aggressive treatment, and/or impaired health. Some diseases such as acute myeloid leukemia (AML) show consistently higher-risk genetic features reducing response in older age, whereas in multiple myeloma (MM) adverse karyotypes are not more frequent due to age.2,3
Hematopoietic cell transplantation (HCT) persists as one of the most effective therapies for hematologic malignancies. The common indications in older patients for high-dose chemotherapy followed by autologous rescue are MM and non-Hodgkin lymphoma, whereas allogeneic transplant is most often applied for AML and myelodysplastic syndromes (MDS). Transplant-related morbidity and mortality after autologous and particularly allogeneic transplant warrant careful weighing of the risks of transplant vs the expected benefit in disease-free survival among older patients and/or those with significant health impairments.
Older age and transplant utilization
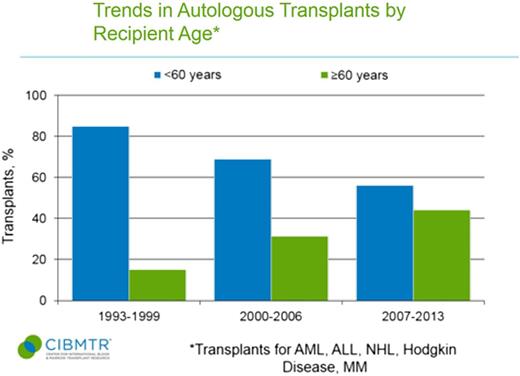
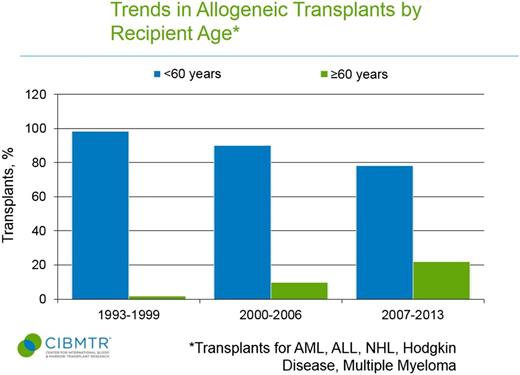
Use of autologous and allogeneic transplants for treatment of malignant diseases has grown markedly in older patients.4 Data from the Center for International Blood and Marrow Transplant Registry (CIBMTR) shows autologous and allogeneic transplant were rarely applied to patients ≥60 years in the 1990s (Figures 1 and 2).5 In the more recent 2007 to 2013 era, 44% of autologous transplant recipients and 22% of allogeneic transplant recipients were at least 60 years of age, and the entire rise in HCT uptake can be accounted for by patients ≥50 years.5 Patients in their 8th decade of life now represent the area of fastest growth of allogeneic and autologous grafts, and can have reasonable outcomes.6,7 The European registry showed that from 2006 to 2010, autografts for myeloma in those ≥70 years represented 3% of transplants compared with only 1.1% a decade before.7 Similarly, Muffly et al reported in abstract form that 3.3% of allogeneic HCTs in 2012 and 2013 derived from this age group vs 0.4% the decade before.6
Trends in autologous transplant by recipient age <60 years and ≥60 years. ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma. CIBMTR data is in the public domain in the United States.5
Trends in autologous transplant by recipient age <60 years and ≥60 years. ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma. CIBMTR data is in the public domain in the United States.5
Trends in allogeneic transplant by recipient age <60 years and ≥60 years. CIBMTR data is in the public domain in the United States.5
Trends in allogeneic transplant by recipient age <60 years and ≥60 years. CIBMTR data is in the public domain in the United States.5
Common transplant indications of MM, non-Hodgkin lymphoma, AML, and MDS peak in older adults and yet transplant is infrequently offered to patients ≥60 years. Oran and Weisdorf estimated among patients ≥65 years with AML, 2.4% underwent HLA typing and 0.8% pursued allografts.8 Similarly, Yao et al estimated the utilization rate for unrelated allografts for all hematologic malignancies was 44%, 29%, and 8% among patients aged 20 to 44, 45 to 64, and 65 to 74 years, respectively, after adjusting for greater expected exclusions in older adults due to health impairments.9
Age as a transplant prognostic factor in older adults
The importance of biologic age as a prognostic factor has been thoroughly documented and yet poorly addressed. McClune et al summarized reduced intensity allogeneic transplant for AML in remission and MDS, showing similar OS in younger and older age patients.10 Sorror et al reported on 372 patients aged 60 to 75 years enrolled on nonmyeloablative approach before matched-related and matched-unrelated donors. Outcomes did not differ by age group.11 A European study summarized 2-year survival after autologous HCT for MM showing 85% to 86% for patients in different age categories from <40 years through 60 to 64 years of age, and only slightly lower at 82.9% and 80.2% for patients 65 to 69 years and >70 years, respectively.7 Most investigators have concluded age should not be a “barrier to transplant.”
Older age still confers greater risks of nonrelapse mortality (NRM). In a study of allogeneic transplant for AML in first complete remission from 2000 to 2004, NRM was 10% for patients aged 21 to 30 years of age, 16% in patients 31 to 40 years of age, and 24% in patients 41 to 50 years of age.12 Autologous transplant for diffuse large B-cell lymphoma showed 1-year NRM of 8.7% for patients ≥60 years compared with 4.7% patients <60 years.13 Finally, Auner et al described 2.4% day-100 day death rate after autograft for myeloma (95% confidence interval [CI], 1.9-3.1) in patients ≥70 years compared with 1.2% in patients 40 to 49 years and 0.7% for those <40 years of age.7
Acceptable, if not favorable, outcomes have been reported in a recent series of older adults after allogeneic transplant. Muffly et al observed a 38% 2-year survival in a registry study of patients ≥70 years for any malignant indication, most commonly for AML and MDS. A multi-institutional trial of reduced intensity fludarabine and busulfan described encouraging low rates of 2-year NRM of 15% and 2-year survival of 48% for AML in first remission.14 Sorror et al reported favorable 5-year OS of 35% after nonmyeloablative allogeneic for prospectively enrolled patients ≥60 years.11
The more important and unanswered question is to understand the risks and benefits of transplant vs nontransplant approaches rather than a simple comparison of older vs younger transplanted recipients. Prospective studies addressing the value of allogeneic or autologous HCT relative to nontransplant approaches have generally restricted eligibility to patients under 60 or 65 years of age.15 One European study of AML in first complete remission in patients ≥60 years is ongoing, randomizing to matched-related or -unrelated donor transplant vs consolidation (#NCT00766779). Versluis et al explored transplant and nontransplant outcomes for AML in first remission in patients ≥60 years after induction therapy. Among 97 patients receiving allogeneic reduced-intensity transplant, 5-year OS after HCT was 35% compared with 26% for those receiving others forms of consolidation therapy. Adjusting for time to transplant, allogeneic HCT was associated with better survival (hazard ratio [HR], 0.71; 95% CI, 0.53-0.95; P = .017) relative to other remission or no remission therapy.16 All of the above studies are hampered by a lack of thorough data on patient health prior to transplant, as well as posttransplant quality of life and function, leaving an uncomfortable void on appropriate patient selection and generalizability.
Physiologic age
“Physiologic age” or “functional age” incorporates approaches to address vulnerability or reserve; loss of reserve strongly correlates with worse health and interferes with life expectancy. The standard transplant evaluation of biologic age and physician-rated performance status (PS) works well to accept young fit patients and to exclude older adults, displaying grossly impaired function or severe comorbidity. For the growing proportion of transplant patients who are 50 to 79 years of age without overt health limitations, physiologic age enables more complete characterization of reserve.
Clinical measurement of physiologic age before transplant
Comorbidity
The most recognized and established determinant of physiologic age in transplant has been comorbidity. The HCT-comorbidity index (HCT-CI) advanced by Sorror et al, revised the Charlson Comorbidity Index by adding and reweighting comorbid conditions.17 The presence of certain comorbid diseases (eg, cardiac conditions, pulmonary impairments, prior solid tumor malignancy, renal dysfunction, etc) derived from history and objective testing were assigned points and lack of scored conditions resulted in a score of 0. The HCT-CI scale has been well validated to predict survival and NRM after allogeneic and autologous transplant. In a validation study inclusive of all ages, patients undergoing allogeneic transplant had 1-year NRM of 17%, 21%, and 26% for HCT-CI scores of 0, 1-2, and 3, respectively (P < .001) and worse survival for higher HCT-CI.18 HCT-CI scores ≥5 exacted the most adverse effect on NRM, although few patients entered transplant with such a high burden. For autologous transplant, pretransplant HCT-CI of ≥3 produced a 50% increase in NRM (HR, 1.49; 95% CI, 1.2-1.85; P = .000) and worse survival (HR, 1.37; 95% CI, 1.23-1.52; P < .0001). High HCT-CI does not equate to failure; OS after allografting at 3 years was ∼30% even with HCT-CI scores of ≥5 and day-100 mortality for myeloma autografts was 3% for HCT-CI scores of ≥3. Adjusting for comorbidity does not negate adverse effects of advancing age.18-20 Thus, risk-stratification by the HCT-CI is invaluable but additive to the effects of biologic age.
Physiologic age
Functional status and geriatric assessment
Functional impairments and frailty may develop in older age without comorbidity. Functional status forms a central foundation in portraying physiologic health through self-report and performance-based testing. Self- or patient-reported function hold surprisingly high value because this offers insight into both function and environmental adaption to limitation. Self-report limitations are vital to judging life expectancy; the ability to manage finances or walk several blocks has similar prognostic effects on long-term survival as cigarette use or heart failure.21 A limitation can emerge due to a severe functional deficit, or more often a combination of vulnerabilities that may not be readily apparent. Performance or objective functional status isolate and quantify function often through simple bedside tests, such as the number of times a person can rise from a chair (ie, timed up and go). More extensive testing, such as a 6-minute walk test or provocative cardiopulmonary testing will better define various levels of functional capability.22
Geriatric assessment (GA) consists of a multidimensional tool to evaluate numerous health domains of interest in older adults and have found widespread application in cancer.23 Table 1 depicts typical domains in a GA and common instruments employed, recognizing that no standard GA exists. Some domains in GA do not directly measure physiologic age in a biologic sense yet hold value in the global picture of age-related health. Less robust social support or polypharmacy (ie, taking many medications) track with older age and affect long-term survival.24
GA domains and frequently used tools
| Domains . | Common and recommended tools . |
|---|---|
| Comorbidity | HCT-CI |
| Charlson CI | |
| Cumulative illness rating scale | |
| Function | |
| Patient report | PS, ADL, IADL, falls, and exhaustion |
| Performance-based | PS, 4-m walk, timed up and go, 6-min walk test, grip strength, and short physical performance battery |
| Social support | ISSS |
| MOS social support | |
| Cognition | MMS, Montreal cognitive assessment, Mini-Cog, BOMC |
| Psychological | GDS |
| HADS | |
| MHI-17 | |
| Nutritional status | Body mass index, weight loss |
| Polypharmacy | “Beers Criteria,” >5 medications or >8 medications |
| Domains . | Common and recommended tools . |
|---|---|
| Comorbidity | HCT-CI |
| Charlson CI | |
| Cumulative illness rating scale | |
| Function | |
| Patient report | PS, ADL, IADL, falls, and exhaustion |
| Performance-based | PS, 4-m walk, timed up and go, 6-min walk test, grip strength, and short physical performance battery |
| Social support | ISSS |
| MOS social support | |
| Cognition | MMS, Montreal cognitive assessment, Mini-Cog, BOMC |
| Psychological | GDS |
| HADS | |
| MHI-17 | |
| Nutritional status | Body mass index, weight loss |
| Polypharmacy | “Beers Criteria,” >5 medications or >8 medications |
ADL, activities of daily living; BOMC, Blessed orientation-memory concentration; GDS, geriatric depression scale; HADS, hospital anxiety and depression scale; IADL, instrumental ADL; ISSS, illness-specific subscales of social support; MHI-17, mental health inventory-17; MMS, mini-mental state examination; MOS, medical outcomes study.
Pretransplant limitations by physiologic age
Our group prospectively applied GA in 166 patients ≥50 years prior to allogeneic transplant for any disease or donor source.25 Frailty, following the research definition developed by the Hopkins group requiring abnormalities in 3 of 5 domains covering grip strength, walk speed, self-report exhaustion, weight loss, and physical activity was present in 25% of patients. Functional impairments interfering with basic activities by the Katz ADL survey were rare. However, by Lawton IADL, which evaluates the ability to live independently (eg, manage medications and finances) revealed a lack of independence or disability in at least 1 area for 40% of patients. For comparison, surveys of 70-year-olds living in the community show <10% prevalence of IADL impairments.26 The Short-Form 36 quality-of-life instrument documented 45% of patients describing physical functional impairments and 59% meeting criteria for limitations in emotional health, defined as 1 standard deviation below the median from population normative data.
Self-report physical function and mental health did not differ in the younger vs older age categories in this cohort of patients ≥50 years. Likewise, comorbidity was not higher with older age. Sorror et al also showed no difference in comorbid burden for patients 60 to 64 years of age, 65 to 69 years, or ≥70 or similar.11 This contradicts the expected increase in prevalence of comorbidity and functional impairments due to older age. Thus, the paradox is that older patients have clinical findings of age-related impairments beyond that expected for their biologic age (ie, a phenotype of accelerated aging) prior to transplant, yet the oldest patients are still more cautiously selected for transplant relative to younger patients.
The MD Anderson Cancer Center (MDACC) examined 50 patients ≥60 years before allogeneic HCT undergoing a more extensive GA and confirmed a high prevalence of limitations.27 Twenty-two percent met the frailty criteria described earlier and 84% exhibited at least 1 abnormality in the 5-point frailty index, mirroring the 78% found in the Chicago study. The Short Performance Physical Battery uncovered deficits in 18%, 28% met criteria for polypharmacy (ie, 9 or more medications), and 36% were found to have nutritional impairment by the Mini-Nutritional Assessment. Finally, Olin et al reported an abstract on GA before autologous or allogeneic transplant in patients ≥50 years and reported 42% of patients with at least 1 IADL limitation.28 By the Mental Health Inventory-5, 36% of patients rates indicated having anxiety or depression, although only 11% met criteria by the HCT-CI scale for a psychiatric disturbance. Both the Chicago and MDACC studies showed physician-rated PS offered little insight into which patients would have vulnerability by GA.
Cognitive function presents one of the most important yet difficult domains because screening tests require time to administer and are still insensitive. In the MDACC experience, 8 of 50 patients (16%) displayed cognitive impairment by an executive function cognitive screen. We reviewed 27 consecutive patients ≥60 years undergoing complete neuropsychological testing before allogeneic HCT (unpublished data). The screening test, the Mini-Mental Status examination, suggested cognitive impairment in only 10%. However, a complete neuropsychological battery found 67% of patients harbored a limitation by at least 1 test, which was most commonly delayed recall. Future studies addressing the frequency and risk factors for cognitive impairment (eg, delirium, dementia) after transplant are needed.
The findings of more frailty and physical impairments than expected relative to population controls in older adults already selected to undergo transplant, supports the hypothesis that disease and disease treatment produces a phenotype of accelerated aging. The mechanisms for this advanced age phenotype requires an investigation into the role of prior intensive treatment or baseline impairments at the time of diagnosis.
Physiologic age to risk stratify for transplant
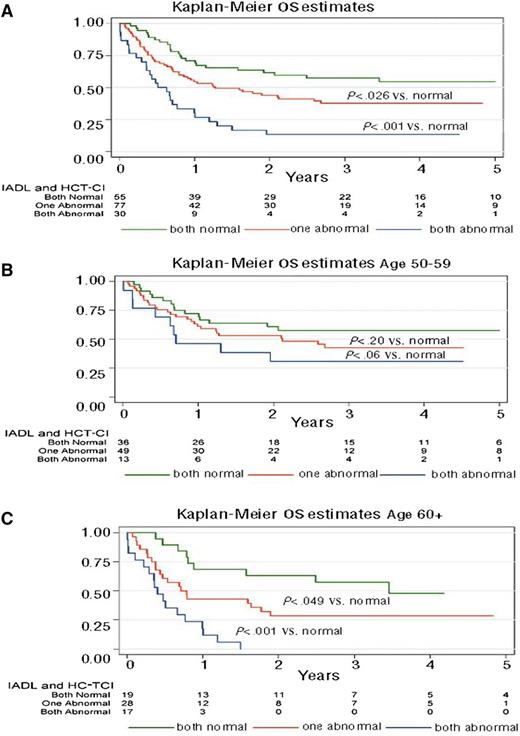
We reviewed the prognostic effects of GA for patients ≥50 years prior to allogeneic transplant in 203 patients of all disease and donor types.20 Age ≥60 years, a high HCT-CI comorbidity score, and myeloablative regimens correlated with higher mortality. Adjusted for these clinical factors and disease status, functional impairment by IADL, slow walk speed, and low self-report emotional health were associated with worse survival. The prognostic value of limitations by GA was magnified in patients ≥60 years relative to patients 50 to 59 years of age. A simple 3-point scoring system was generated from these data: 1 point for high comorbidity and 1 point for functional impairment by IADL is shown in Figure 3. Among 17 evaluable patients ≥60 years with both high comorbidity and functional impairment, there were no survivors at 2 years after HCT. The effect was less pronounced in the 50- to 59-year-old age group, supporting the role of GA particularly in patients ≥60 years prior to allografting.
OS for patients with no impairments (score = 0), high HCT-CI comorbidity or IADL impairment (score = 1), or both limitations (score = 2) in all patients (A), patients 50 to 59 years (B), and patients ≥60 years (C). Reprinted from Muffly et al20 with permission.
OS for patients with no impairments (score = 0), high HCT-CI comorbidity or IADL impairment (score = 1), or both limitations (score = 2) in all patients (A), patients 50 to 59 years (B), and patients ≥60 years (C). Reprinted from Muffly et al20 with permission.
The role of other domains in GA will require validation such as social support, polypharmacy, and nutrition, although we know in general that better social support enhances outcomes.29 Larger studies inclusive of autografts are needed to clarify the prevalence of GA-documented abnormalities and the association with posttransplant outcomes.
Physiologic age and biomarkers
Biomarkers are another way to characterize physiologic age and they illustrate how knowledge of aging can be applied in the transplant setting. C-reactive protein before transplant in adults is a powerful prognostic factor, independent of age and comorbidity, for worse survival through higher NRM.30,31 Biomarkers that more directly address molecular and cellular features of aging require investigation such as P16INK4a and telomere length. For example, a recent study showing shorter telomere length in donors but not patients was associated with shorter survival for aplastic anemia submitted to unrelated-donor allogeneic transplant.32 Rosko et al recently found that autologous transplants for myeloma increased in P16INK4a expression after transplant, consistent with accelerated T-cell aging or senescence induced by transplant.33
Physiologic age and transplant candidacy in older adults
Summary of selecting older patients for transplant
Decision making for HCT always demands individualization, and the lack of well-designed studies characterizing the risks and benefits in older adults of HCT vs non-HCT approaches precludes definitive recommendations. Considering transplant-related morbidity and mortality by physiologic age informs transplant candidacy; theoretically for fit older patients, we should consider transplant indications similar to younger adults, whereas in older adults lacking adequate reserve, transplant should be avoided. The intermediate group poses the greatest challenge but also opportunities.
Diseases, transplant regimens, center experience, expected transplant benefit, and patient intangibles (eg, motivation) all will factor into decisions in when and how best to pursue transplant. Each center should review their experience in older adults by graft type and available measures of physiologic age (eg, comorbid burden, PS).
Our approach
We perform a GA for all patients ≥50 years prior to transplant to screen for abnormalities. The GA is modeled after the Cancer and Aging Research Group tool except for the addition of the HCT-CI.34 Based on our experience and similar data in AML as well as geriatric oncology, patients manifesting functional limitations in IADL should be offered allogeneic transplant cautiously, especially with higher biologic age (eg, ≥60 years) and/or high comorbidity.35 Reinforcing the importance and utility of basic questions of self-reported function, Palumbo et al demonstrated in a large cohort of nontransplant myeloma patients, the prognostic value of function by ADLs and IADLs.36
The author believes any clinical concern for cognitive impairments should be formally evaluated by a cognitive screen (Table 1) and referred if the results are abnormal. For AML induction therapy, older adults manifesting cognitive impairment by GA fared poorly.37
Patients with Karnofsky Performance State scale <70% for autografting, <80% for allografts, HCT-CI scores of 5 or more, and an HCT-CI of 3 or more combined with an IADL impairment are considered high risk, and require thorough evaluation and consensus agreement before pursuing transplant.
Many patients are categorized as intermediate risk-heightened but not prohibitive risks of morbidity and NRM. We need to exercise caution, yet at the same time decide early, because delays may prevent transplant due to disease progression and/or debilitation from treatment. The lower absolute rates of NRM for autografts challenges developing strict exclusions and many older adults with impairments will still successfully receive autologous transplant especially with melphalan-based conditioning for MM.
Transplant optimization in older adults
Nonspecific and simple interventions may not have a high yield. A study by the Blood and Marrow Transplant Clinical Trials Network randomizing 711 patients including a self-directed exercise program and self-administered stress management program showed no benefit in adult patients, although the population was not enriched for older age or those with limitations.38 For allogeneic transplant, the application of reduced-intensity or nonmyeloablative regimens and appropriate graft-versus-host disease prophylaxis, remains an essential component in older adults based on available data and center experience.
GA-guided interventions
At our center, patients ≥60 years planned for allogeneic HCT, ≥70 years before autologous HCT, or ≥50 years where physiologic age concerns exist (eg, high comorbidity, poor PS) are seen after completion of the GA by a multidisciplinary team to better address limitations and recommend interventions. Table 2 pairs suggested interventions to impairments in specific domains. This list is not exhaustive. The author advocates the US Preventive Task Force conclusion: “…we can only truly optimize the care of all older adults by affecting multiple aspects of health, from multiple perspectives/disciplines, over a span of aging that includes many possible functional trajectories.”39(p2163) This may require creating teams with expertise, including geriatrics.40 Promoting and enrolling on clinical trials study transplant for older adults incorporating pretransplant testing of physiologic age and posttransplant analysis of functional trajectories is crucial to advance the field.
Considerations to optimize vulnerable HCT patients
| Domain impaired . | Intervention . |
|---|---|
| Significant comorbid conditions | Subspecialty consultation and management in context of transplant and disease |
| Impaired function | Structured prehabilitation, encourage and teach patient appropriate activity through transplant. Home assessment aligned with patient limitations |
| Limited social support | Pretransplant family meeting, assign “Team Captain,” and request secondary caregivers |
| Cognitive impairment | Delirium precautions, medication avoidance, and encourage greater presence of family support |
| Depression or anxiety | Recognize problem, cognitive ± medication management, and assess expected adherence post-HCT |
| Weight loss | Exclude concurrent medical problems, add supplements, and develop nutritional plan for transplant |
| Polypharmacy | Hold medications. Re-evaluate day 30 to 100 post-HCT |
| Any impairment | Adjust preparative regimen, donor source, and/or escalate posttransplant follow-up frequency. Assess posttransplant and modify intervention as needed. Enlist caregiver in optimization plan |
| Domain impaired . | Intervention . |
|---|---|
| Significant comorbid conditions | Subspecialty consultation and management in context of transplant and disease |
| Impaired function | Structured prehabilitation, encourage and teach patient appropriate activity through transplant. Home assessment aligned with patient limitations |
| Limited social support | Pretransplant family meeting, assign “Team Captain,” and request secondary caregivers |
| Cognitive impairment | Delirium precautions, medication avoidance, and encourage greater presence of family support |
| Depression or anxiety | Recognize problem, cognitive ± medication management, and assess expected adherence post-HCT |
| Weight loss | Exclude concurrent medical problems, add supplements, and develop nutritional plan for transplant |
| Polypharmacy | Hold medications. Re-evaluate day 30 to 100 post-HCT |
| Any impairment | Adjust preparative regimen, donor source, and/or escalate posttransplant follow-up frequency. Assess posttransplant and modify intervention as needed. Enlist caregiver in optimization plan |
Correspondence
Andrew S. Artz, Section of Hematology/Oncology, Department of Medicine, University of Chicago, 5841 S. Maryland Ave, MC 2115, Chicago, IL 60637; e-mail: aartz@medicine.bsd.uchicago.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Miltenyi.
Author notes
Off-label drug use: None disclosed.