Abstract
Natural killer (NK) cell function is regulated by a fine balance between numerous activating and inhibitory receptors, of which killer-cell immunoglobulin-like receptors (KIRs) are among the most polymorphic and comprehensively studied. KIRs allow NK cells to recognize downregulation or the absence of HLA class I molecules on target cells (known as missing-self), a phenomenon that is commonly observed in virally infected cells or cancer cells. Because KIR and HLA genes are located on different chromosomes, in an allogeneic environment such as after hematopoietic stem cell transplantation, donor NK cells that express an inhibitory KIR for an HLA class I molecule that is absent on recipient targets (KIR/KIR-ligand mismatch), can recognize and react to this missing self and mediate cytotoxicity. Accumulating data indicate that epistatic interactions between KIR and HLA influence outcomes in several clinical conditions. Herein, we discuss the genetic and functional features of KIR/KIR-ligand interactions in hematopoietic stem cell transplantation and how these data can guide donor selection. We will also review clinical studies of adoptive NK cell therapy in leukemia and emerging data on the use of genetically modified NK cells that could broaden the scope of cancer immunotherapy.
Learning Objectives
To understand the influence of various inhibitory and activating KIR receptors on HSCT outcomes
To understand how these effects may vary by donor source and underlying disease
Introduction
Natural killer (NK) cells characterized by a CD3–CD56+ immunophenotype are bone marrow–derived lymphocytes capable of mediating early innate immune responses against virally infected cells or malignant cells.1-7 Because they are the first lymphocytes to reconstitute after hematopoietic stem cell transplantation (HSCT),8-17 NK cells play an important role in mediating the graft-versus-tumor effect.18-28 One of the earliest observations of NK cell alloreactivity was reported in the hybrid resistance model,29,30 which noted that lethally irradiated heterozygous F1 hybrid mice derived from a cross of 2 inbred mouse strains (parent A × parent B) rejected hematopoietic grafts donated by either parent A or parent B.31 Rejection of the parental graft was later shown to be mediated by a subset of recipient NK cells that lacks the appropriate inhibitory receptors to recognize major histocompatibility complex class I (MHC-I) molecules on the donor cells. This observation led to the ingenious “missing self” concept of NK recognition, which postulates that the absence or reduced expression of “self” MHC-I allows a cell to be killed by NK cells.1,2
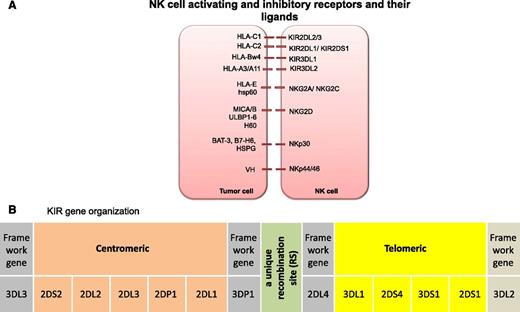
Subsequently, the receptors that recognize MHC-I were identified on NK cells (reviewed by Parham32 and Moretta33 ). Briefly, each mature NK cell expresses a wide array of germ-line–encoded activating and inhibitory receptors.32-49 Inhibitory NK receptors mediate two important functions. They recognize self-HLA class I alleles and contribute to the acquisition of NK function by a dynamic process known as NK cell education or licensing.50 When inhibitory receptors engage with their cognate class I ligands, they deliver inhibitory signals to suppress NK cell activity. If HLA class I antigen expression is sufficiently reduced or modified, as is often observed in virally infected cells or tumor cells (as an immune escape mechanism from T-cell recognition), NK cells can eliminate the abnormal cells.2,51 However, missing-self alone is insufficient to trigger NK cell effector function, because many recipient cells with no ligands (eg, red blood cells) are not lysed. Some level of expression of a stressed ligand is also required. Activating receptors recognize stress proteins expressed on the surface of transformed or abnormal cells33-49 and provide signals for NK cells to kill. Ultimately, NK effector function is dictated by integration of signals received through these activating and inhibitory receptors (Figure 1A).
(A) NK cell activating and inhibitory receptors and their ligands. (B) KIR gene organization. KIR haplotype illustrating centromeric and telomeric KIR gene motifs. BAT-3, HLA-B–associated transcript 3; H60, histocompatibility 60; hsp60, heat shock protein 60; HSPG, heparin sulfate proteoglycans; MIC, MHC class I chain-related gene; VH, viral hemagglutinin; ULBP, UL16 binding protein.83
(A) NK cell activating and inhibitory receptors and their ligands. (B) KIR gene organization. KIR haplotype illustrating centromeric and telomeric KIR gene motifs. BAT-3, HLA-B–associated transcript 3; H60, histocompatibility 60; hsp60, heat shock protein 60; HSPG, heparin sulfate proteoglycans; MIC, MHC class I chain-related gene; VH, viral hemagglutinin; ULBP, UL16 binding protein.83
Killer-cell immunoglobulin-like receptors and their ligands
Among the most comprehensively studied NK cell receptors are the killer-cell immunoglobulin-like receptors (KIRs). KIRs are clonally expressed on the surface of NK cells in a stochastic fashion. Each NK cell can in turn express any possible combination of receptors, leading to the generation of complex NK cell repertoires.52-57
All KIRs are named 2D or 3D, which denotes the number of immunoglobulin-like domains in the molecule. The alphabet following 2D or 3D signifies the length of the cytoplasmic tail, which is either short (S) in activating KIRs or long (L) in inhibitory KIRs.58 The ligands for KIRs are HLA-A, -B, or -C molecules.59 KIR2DL1 recognizes group 2 HLA-C molecules (HLA-C2; alleles with Lys80 residue [eg, Cw2, 4, 5, 6]), KIR2DL2 recognizes group 1 HLA-C molecules (HLA-C1; alleles with an Asn80 residue [eg, Cw1, 3, 7, 8]), and KIR3DL1 recognizes HLA-Bw4 alleles20,55,60-62 (Figure 1A). In vivo63 and in vitro64 studies suggest that KIR3DL2 recognizes HLA-A3 and A11 but this binding occurs only in the presence of the Epstein-Barr virus EBNA3A peptide. In contrast to the inhibitory KIRs, the ligands for many activating KIRs are largely unknown. KIR2DS1 has been shown to interact with HLA-C2 alleles,65-67 whereas KIR2DS2 was recently shown to recognize HLA-A*11.68 The frequencies of KIR alleles vary from population to population, but most individuals have inhibitory KIRs specific for HLA-C1, -C2 or -Bw4 alleles. For instance, in English and white Americans, inhibitory KIR2DL1 (95% to 100%), 2DL2 (43% to 53%), and 2DL3 (85% to 95%) are present in a majority, whereas the genes for the activating KIR2DS1 (35% to 45%) and 2DS2 (45% to 55%) are less commonly present.69
Because KIRs are encoded by a family of genes in the leukocyte receptor complex on chromosome 19q13.4 and segregate independently from the HLA genes,70 in the setting of HSCT, a donor-recipient pair can be HLA-matched and KIR-ligand mismatched at the same time. This generates a situation in which alloreactive donor NK cells can elicit a graft-versus-tumor effect without increasing the risk of graft-versus-host disease (GVHD). A KIR ligand calculator to predict NK cell alloreactivity based on donor and recipient HLA-B and -C typing is available at the Immuno Polymorphism Database Web site (https://www.ebi.ac.uk/ipd/kir/ligand.html).
KIR-ligand mismatch leads to superior outcomes after T-cell deplete haploidentical HSCT in patients with acute myeloid leukemia
In HSCT, KIR-ligand mismatch in the graft-versus-host (GvH) direction occurs when the recipient lacks 1 or more major KIR-ligands (eg, C1, C2, or Bw4). Valiante and Parham56 were the first to predict NK cell alloreactivity in the clinical transplant setting on the basis of the donor and recipient KIR and HLA repertoire. The Perugia group was then the first to report that KIR-ligand mismatch in the GvH direction was associated with a significant reduction in the risk of relapse and improved survival.19,20 In that study, patients with acute myeloid leukemia (AML; n = 57) or acute lymphoblastic leukemia (ALL; n = 35) received granulocyte colony-stimulating factor mobilized, CD34+ selected peripheral blood progenitor cell haploidentical HSCT after conditioning with total body irradiation, thiotepa, fludarabine, and anti-thymocyte globulin. Because the graft was T-cell depleted, no post-HSCT immunosuppression was given. KIR-ligand mismatch correlated positively with NK cell alloreactivity in vitro. Patients who received KIR-ligand mismatched HSCT in the GvH direction had significantly reduced risk of relapse compared with those in the KIR-ligand matched group (probability of relapse at 5 years, 0% vs 75%).20 Furthermore, despite the presence of donor-versus-recipient alloreactive NK cells, no patient in the KIR-ligand mismatched group experienced GVHD or graft rejection compared with 14% and 16%, respectively, in the KIR-ligand matched group.20 Overall survival (OS) was also improved in the KIR-ligand mismatch group (60%) compared with the KIR-ligand match group (5%). Interestingly, these favorable outcomes were observed only in AML patients and not in ALL patients. These findings were later corroborated in a larger cohort of 112 patients with AML who underwent T-cell deplete haploidentical HSCT.21 This study also reported a 52% reduction in the risk of relapse or death in the KIR-ligand mismatched group compared with the KIR-ligand matched group (relative risk [RR], 0.48; 95% confidence interval [CI], 0.29-0.78; P < .001). Moreover, even patients who were transplanted with active disease experienced improved disease-free survival (DFS) if they received a graft from a KIR-ligand mismatched compared with a KIR-ligand matched donor.21 It is noteworthy that the myeloablative regimen used in these studies was highly immunosuppressive, which in conjunction with a T-cell–deplete graft, created a state of intense lymphodepletion in the host, providing an ideal scenario for in vivo persistence and expansion of NK cells.71 Consequently, alloreactive NK cells were detected for up to 7 months in one-third of patients and for up to a year in ∼10% of patients.20 These data led to several retrospective analyses on the effects of KIR-ligand mismatch on outcomes in the HLA-matched unrelated and matched sibling transplant settings (Table 1).23-27,72,73 Although some studies showed improvements in 1 or all aspects of disease outcome, including relapse, DFS, or OS,23-27 others reported a detrimental impact of KIR-ligand mismatch,27,74,75 including greater risk of GVHD and death.76 The conflicting data in the various studies may be explained by a number of factors, most importantly, differences in the degree of T-cell depletion in the graft and the resultant T-cell alloreactivity, which may in turn overcome any potential benefit of NK cell alloreactivity.26,72 Indeed, T cells in the graft have been shown to negatively affect donor-derived NK cell reconstitution and expression of KIRs, thereby impairing NK effector function in vivo.26,72,77 This is exemplified by studies that showed improved outcomes after KIR-ligand mismatched T-cell deplete22,78,79 but not with T-cell replete HSCTs.72,75,80,81
Studies evaluating the impact of donor-recipient KIR-ligand mismatch in related or unrelated donor HSCT
| Reference . | Davies 200225 . | Giebel 200322 . | Bornhäuser 200423 . | Schaffer 200475 . | Hsu 200578 . | Beelen 200580 . | Verheyden 200579 . | Farag 200674 . | Chen 200624 . | Kröger 200673 . | Sun 200727 . | Miller 200776 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 175 | 130 | 118 | 190 | 178 | 374 | 65 | 1571 | 131 | 142 | 378 | 2062 |
| Disease | CML, AML, ALL, others, (other leukemias, MDS, metabolic disease, immune deficiencies, SAA) | ALL, AML, MDS, CML, NHL, HL, MM | AML, CML, MDS | ALL, AML, CML, MDS, NHL, HL, MM | AML, ALL, CML, MDS | AML, CML, MDS | AML, CML, ALL | AML, MDS, CML | AML, CML, ALL, MDS, NHL, other | AML, MDS, CMML, ALL | ALL, AML, MDS, CML | AML, CML, MDS |
| Donor | URD | URD | URD | URD | HLA-matched sibling | Related or URD | HLA-matched sibling | URD | HLA-matched sibling | URD | HLA-matched sibling/ URD | URD |
| TCD (%) | 37 in KIR-ligand compatible group; 29 in KIR-ligand incompatible group | ATG (100) | TCD (20) + ATG (100) | TCD (17) + ATG (100) | 100 | 0 | TCD (52) | 20 | No | ATG | No | — |
| KIR-ligand mismatched (GvH direction) (%) | 35 | 15 | 13 | 12 | 63 | 13 | 68 | 9 | 63 | 70 | 16 | — |
| Proportion of patients with aGVHD (grade 2-4) in KIR-ligand mismatched vs matched groups | NS | NS | NS | NS | NS | NS | NS | NS; aGVHD III to IV higher in KIR-ligand mismatch | NS | NS | NS; aGVHD III to IV higher in KIR-ligand mismatch (CML patients >1 year from diagnosis only) | |
| Risk | HR, 1.64 | RR, 1.58 | ||||||||||
| 95% CI | 1.11–2.42 | 1.13-2.22 | ||||||||||
| P | .001 | .008 | ||||||||||
| Relapse | NS | NS | Higher in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch (myeloid only) | Lower in KIR-ligand mismatch | NS | Higher in KIR-ligand mismatch | NS | NS | Higher in KIR-ligand mismatch | Lower in KIR-ligand mismatch (myeloid early disease only) |
| Risk | HR, 0.41 | RR, 0.24 | HR, 1.56 | RR, 2.98 | RR, 0.54 | |||||||
| 95% CI | 0.18-0.97 | 0.08-0.69 | 0.88–2.75 | 1.17-7.59 | 0.30-0.95 | |||||||
| P | .02 | .04 | < .008 | .04 | .02 | .03 | ||||||
| OS | Lower in KIR-ligand mismatch (myeloid only) | Higher in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch | Higher in KIR-ligand mismatch (myeloid only) | NS | NS | Lower in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch | NS | NS |
| Risk | 13% vs 38% at 5 years | 87% vs 48% at 4.5 years | HR, 2.13 | HR, 0.53 | HR, 1.94 | HR, 2.015 | ||||||
| 95% CI | 0%-26% vs 24%-52% | 1.17-3.90 | 0.3-0.93 | 1.47-2.57 | ||||||||
| P | < .01 | .006 | .01 | .026 | < .001 | .02 |
| Reference . | Davies 200225 . | Giebel 200322 . | Bornhäuser 200423 . | Schaffer 200475 . | Hsu 200578 . | Beelen 200580 . | Verheyden 200579 . | Farag 200674 . | Chen 200624 . | Kröger 200673 . | Sun 200727 . | Miller 200776 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 175 | 130 | 118 | 190 | 178 | 374 | 65 | 1571 | 131 | 142 | 378 | 2062 |
| Disease | CML, AML, ALL, others, (other leukemias, MDS, metabolic disease, immune deficiencies, SAA) | ALL, AML, MDS, CML, NHL, HL, MM | AML, CML, MDS | ALL, AML, CML, MDS, NHL, HL, MM | AML, ALL, CML, MDS | AML, CML, MDS | AML, CML, ALL | AML, MDS, CML | AML, CML, ALL, MDS, NHL, other | AML, MDS, CMML, ALL | ALL, AML, MDS, CML | AML, CML, MDS |
| Donor | URD | URD | URD | URD | HLA-matched sibling | Related or URD | HLA-matched sibling | URD | HLA-matched sibling | URD | HLA-matched sibling/ URD | URD |
| TCD (%) | 37 in KIR-ligand compatible group; 29 in KIR-ligand incompatible group | ATG (100) | TCD (20) + ATG (100) | TCD (17) + ATG (100) | 100 | 0 | TCD (52) | 20 | No | ATG | No | — |
| KIR-ligand mismatched (GvH direction) (%) | 35 | 15 | 13 | 12 | 63 | 13 | 68 | 9 | 63 | 70 | 16 | — |
| Proportion of patients with aGVHD (grade 2-4) in KIR-ligand mismatched vs matched groups | NS | NS | NS | NS | NS | NS | NS | NS; aGVHD III to IV higher in KIR-ligand mismatch | NS | NS | NS; aGVHD III to IV higher in KIR-ligand mismatch (CML patients >1 year from diagnosis only) | |
| Risk | HR, 1.64 | RR, 1.58 | ||||||||||
| 95% CI | 1.11–2.42 | 1.13-2.22 | ||||||||||
| P | .001 | .008 | ||||||||||
| Relapse | NS | NS | Higher in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch (myeloid only) | Lower in KIR-ligand mismatch | NS | Higher in KIR-ligand mismatch | NS | NS | Higher in KIR-ligand mismatch | Lower in KIR-ligand mismatch (myeloid early disease only) |
| Risk | HR, 0.41 | RR, 0.24 | HR, 1.56 | RR, 2.98 | RR, 0.54 | |||||||
| 95% CI | 0.18-0.97 | 0.08-0.69 | 0.88–2.75 | 1.17-7.59 | 0.30-0.95 | |||||||
| P | .02 | .04 | < .008 | .04 | .02 | .03 | ||||||
| OS | Lower in KIR-ligand mismatch (myeloid only) | Higher in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch | Higher in KIR-ligand mismatch (myeloid only) | NS | NS | Lower in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch | NS | NS |
| Risk | 13% vs 38% at 5 years | 87% vs 48% at 4.5 years | HR, 2.13 | HR, 0.53 | HR, 1.94 | HR, 2.015 | ||||||
| 95% CI | 0%-26% vs 24%-52% | 1.17-3.90 | 0.3-0.93 | 1.47-2.57 | ||||||||
| P | < .01 | .006 | .01 | .026 | < .001 | .02 |
aGVHD, acute graft-versus-host disease; ATG, anti-thymocyte globulin; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; HL, Hodgkin lymphoma; HR, hazard ratio; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; NS, nonsignificant; RR, relative risk; SAA, severe aplastic anemia; TCD, T-cell depletion; URD, unrelated donor.
Activating KIRs in donor NK cells are protective against leukemia relapse
In contrast to the well-studied biology of inhibitory KIRs, the function of activating KIRs and their respective ligands has remained largely unexplored. Activating KIRs recognize stress molecules and possibly also HLA molecules or modified HLA molecules, but only the specificity of KIR2DS1 for HLA-C2 alleles has been firmly established.65-67 Although all individuals carry the genes for inhibitory KIR receptors—which indicates that they are necessary for NK cell function—there is striking heterogeneity in the number of inherited activating KIR genes in the normal population.69 Based on the number and distribution of activating KIR genes, individuals can be considered to have 2 broad KIR haplotypes. KIR haplotype A comprises 5 inhibitory genes and the single activating gene KIR2DS4, whereas KIR haplotype B incorporates various combinations of activating genes (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5, KIR3DS1)82 and the inhibitory KIR gene KIR2DL5. The KIR haplotype model proposes that the more activating KIR genes the donor carries, the higher the potential for alloreactivity and the lower the risk of relapse. Indeed, recent analyses by Cooley et al83 in 448 AML patients who received a T-cell-replete unrelated donor HSCT reported that patients transplanted from a donor carrying a higher number of activating KIR genes (haplotype B) have a survival advantage over patients receiving a graft from a haplotype A donor (3-year DFS, 28% vs 17%; P = .003; 3-year OS, 31% vs 20%; P = .007).3 This protection was not observed in adult ALL, suggesting that myeloid leukemia is more susceptible to NK killing, although a recent report showed a benefit of donor haplotype B in relapse protection and survival for pediatric patients with ALL undergoing T-cell-deplete haploidentical HSCT.84
Moreover, KIR haplotype B donors with a higher number of B-specific genes (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5, KIR2DL2, and KIR2DL5), especially if also homozygous for those in the centromeric part of the KIR locus (Cen-B/B), conferred greater protection against relapse after HSCT85,86 (Figure 1B). The risk of relapse was significantly lower if the donor had 2 or more KIR B gene-content motifs (KIR B content score) compared with those with KIR B content score of less than 2, both in the HLA-matched and -mismatched settings,85 supporting the notion that the more activating KIR genes the donor carries, the higher the potential for alloreactivity and protection against relapse.
The influence of specific activating KIR genes on outcomes after HSCT has also been investigated by a number of groups.87-89 A large retrospective study from the Center for International Blood and Marrow Transplant Research included more than 1700 patients with AML (75%) or ALL (25%) who received a 9/10 HLA-matched (48%) or 10/10 HLA-matched (52%) HSCT from unrelated donors.88 In this study, AML (but not ALL) patients whose donors were KIR2DS1 positive had significantly lower risk of relapse (hazard ratio, 0.76; 95% CI, 0.61-0.96; P = .02) and reduced overall mortality (hazard ratio, 0.85; 95% CI, 0.73-1.00; P = .04) compared with those whose donors were KIR2DS1 negative. Similar results have also been reported in the setting of matched sibling donor transplants; patients with AML who received allografts from KIR2DS1-positive donors had a risk of relapse 4 times lower than those who received a graft from KIR2DS1-negative donors.87 Taken together, these studies suggest that selecting donors with KIR haplotype B may have a beneficial effect on outcomes after HSCT for AML and childhood ALL.
NK cell effects in cord blood HSCT
Similarly, several studies have explored the role of KIR-ligand mismatch on outcomes after umbilical cord blood transplantation (CBT) (Table 2). In a study of 218 patients who received a single-unit CBT, Willemze et al28,90 reported that KIR-ligand mismatch between the cord blood unit and the patient was associated with significantly improved DFS (40% vs 55%; P = .005) and OS (31% vs 57%; P = .02). In contrast, 3 studies91-93 failed to show a beneficial effect of KIR-ligand mismatch, whereas 1 study from Minnesota reported a detrimental impact of KIR-ligand mismatch in the setting of double-unit CBT or reduced-intensity conditioned CBT.94 A limitation of those studies is that they take into account only KIR-ligand mismatch without considering the role of activating KIRs or NK licensing. Moreover, they are exclusively based on genetic studies, and they lack functional correlates and a plausible mechanistic basis for the observed effects of KIR genotype on outcome.
Studies assessing the role of KIR-ligand mismatch in cord blood transplantation
| Reference . | Willemze 200928 . | Brunstein 200994 . | Tanaka 201392 . | Garfall 201391 . | Rocha 201693 . | Sekine 201697 . | |
|---|---|---|---|---|---|---|---|
| No. of patients | 218 | 155 | 102 | 357 AML) | 80 | 199 | 204 |
| Conditioning (%) | MA (83) | MA (100) | RIC (100) | MA (62) | MA (27) | MA (100) | MA (72) |
| ATG/ALG (%) | 82 | 40 | 28 | 0 | 100 | 70 | 100 |
| Single-CBT (%) | 100 | 61 | 100 | 100 | 0 | 0 | — |
| KIR-ligand mismatched (GvH direction) (%) | 32 | 26 | 32 | 81 | 44 | 43 | HLA C1/x patients who receive CB unit with HLA-C1-KIR2DL2, KIR2DL3, or KIR2DS2 vs all other |
| aGVHD (2-4) | NS | NS | Higher in KIR-ligand mismatch | NS | NS | NS | NS |
| Risk | 79% vs 57% | ||||||
| 95% CI | 59%-99% vs 44%-70% | ||||||
| P | .01 | ||||||
| TRM | NS | NS | Higher in KIR-ligand mismatch | NS | — | NS | NS |
| Risk | 27% vs 12% at 2 years | ||||||
| 95% CI | 12%-42% vs 5%-19% | ||||||
| P | .03 | ||||||
| Relapse | Lower in KIR-ligand mismatch | NS | NS | NS | NS | NS | Lower in HLA-C1/x recipients who received an HLA-C1-KIR2DL2, KIR2DL3, or KIR2DS2 CB unit |
| Risk | RR, 0.53 | HR, 0.04 | |||||
| 95% CI | 0.28-0.99 | 1.57-31.47 | |||||
| P | .05 | .002 | |||||
| OS | Higher in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch | NS | NS | NS | Higher in HLA-C1/x recipients who received an HLA-C1-KIR2DL2, KIR2DL3, or KIR2DS2 CB unit |
| Risk | RR, 2 | 32% vs 52% at 3 years | HR, 3.46 | ||||
| 95% CI | 1.24-3.22 | 15%-59% vs 47%-67% | 1.46-8.20 | ||||
| P | .004 | .03 | .005 | ||||
| Reference . | Willemze 200928 . | Brunstein 200994 . | Tanaka 201392 . | Garfall 201391 . | Rocha 201693 . | Sekine 201697 . | |
|---|---|---|---|---|---|---|---|
| No. of patients | 218 | 155 | 102 | 357 AML) | 80 | 199 | 204 |
| Conditioning (%) | MA (83) | MA (100) | RIC (100) | MA (62) | MA (27) | MA (100) | MA (72) |
| ATG/ALG (%) | 82 | 40 | 28 | 0 | 100 | 70 | 100 |
| Single-CBT (%) | 100 | 61 | 100 | 100 | 0 | 0 | — |
| KIR-ligand mismatched (GvH direction) (%) | 32 | 26 | 32 | 81 | 44 | 43 | HLA C1/x patients who receive CB unit with HLA-C1-KIR2DL2, KIR2DL3, or KIR2DS2 vs all other |
| aGVHD (2-4) | NS | NS | Higher in KIR-ligand mismatch | NS | NS | NS | NS |
| Risk | 79% vs 57% | ||||||
| 95% CI | 59%-99% vs 44%-70% | ||||||
| P | .01 | ||||||
| TRM | NS | NS | Higher in KIR-ligand mismatch | NS | — | NS | NS |
| Risk | 27% vs 12% at 2 years | ||||||
| 95% CI | 12%-42% vs 5%-19% | ||||||
| P | .03 | ||||||
| Relapse | Lower in KIR-ligand mismatch | NS | NS | NS | NS | NS | Lower in HLA-C1/x recipients who received an HLA-C1-KIR2DL2, KIR2DL3, or KIR2DS2 CB unit |
| Risk | RR, 0.53 | HR, 0.04 | |||||
| 95% CI | 0.28-0.99 | 1.57-31.47 | |||||
| P | .05 | .002 | |||||
| OS | Higher in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch | NS | NS | NS | Higher in HLA-C1/x recipients who received an HLA-C1-KIR2DL2, KIR2DL3, or KIR2DS2 CB unit |
| Risk | RR, 2 | 32% vs 52% at 3 years | HR, 3.46 | ||||
| 95% CI | 1.24-3.22 | 15%-59% vs 47%-67% | 1.46-8.20 | ||||
| P | .004 | .03 | .005 | ||||
ALG, anti-lymphocyte globulin; CB, cord blood; MA, myeloablative; RIC, reduced-intensity conditioning; TRM, treatment-related mortality.
Our group recently studied the impact of NK reconstitution on outcome after CBT. We showed that patients homozygous for HLA-C2 group alleles had a higher 1-year relapse rate and worse survival after CBT than did HLA-C1/C1 or HLA-C1/C2 (HLA-C1/x) patients: 67.8% vs 26.0% (P < .001) and 15.0% vs 52.9% (P < .001), respectively. This inferior outcome was associated with delayed posttransplant recovery of NK cells expressing the HLA-C2–specific KIR2DL1/S1 receptors. Our data support previous studies that show a statistically significant increase in relapse rate in HLA-C2/C2 patients in other transplant settings22,95,96 and suggest that HLA-C2/C2 CBT recipients constitute a high-risk group that may benefit from NK cell-based intervention to accelerate NK cell reconstitution. We also observed that HLA-C1/x patients receiving a CB graft that was licensed (HLA-C1/x positive) and was positive for the activating receptor KIR2DS2 had a lower 1-year relapse rate (6.7% vs 40.1%; P = .002) and superior survival (74.2% vs 41.3%; P = .003) compared with recipients of grafts lacking KIR2DS2 or HLA-C1.98 Thus, we have initiated a clinical trial of CB selection for HLA-C1/x recipients on the basis of HLA-KIR typing and adoptive therapy with CB-derived NK cells for HLA-C2/C2 patients at our center.
Can we select the most appropriate donor based on HLA-KIR genotype?
The degree of HLA match is the single most important factor that determines outcomes after unrelated donor HSCT.99-110 In cytomegalovirus (CMV) –seronegative recipients, the best survival outcomes are seen with a CMV-seronegative donor; however, donor CMV serostatus has no prognostic impact in CMV-seropositive recipients.111 Among several other donor characteristics, including age, sex, parity, and ABO compatibility, increasing donor age is the only factor consistently shown to have a negative impact on survival after transplantation.99-103,112,113 Whereas some studies have reported a lower risk of relapse in female-donor to male-recipient HSCT, this benefit was offset by higher treatment-related mortality in secondary to severe acute or chronic GVHD.115-120 Several maternal and paternal noninherited antigens have also been shown to influence outcomes after HSCT. For instance, using the mother as donor is associated with a lower risk of relapse and treatment-related mortality compared with a paternal graft in the setting of T-cell-deplete haploidentical HSCT,121 but not in the T-cell-replete setting.122 Disparity in some minor histocompatibility antigens (miHAs) has also been reported to influence risk of GVHD and/or relapse.123-126 However, choosing a donor on the basis of miHAs (mismatch except for sex) is not yet practical; more than 50 autosomally encoded miHAs have been identified to date, which when combined with the large extent of variability in HLA genes, makes it likely that one or more immunological disparities will be present in at least 80% of all HSCTs.127
Because many clinical studies support a role for KIR-HLA interactions in HSCT (Table 1), it is likely that KIR immunogenetics will also be included in algorithms of donor selection in the future. KIR genotyping can be performed easily and cheaply along with HLA genotyping at the time of donor screening. Indeed, a number of clinical trials are prospectively investigating the incorporation of KIR genotyping as part of the donor selection criteria. These studies are either observational or interventional, and their approach is dependent on the underlying disease and whether the transplant procedure is performed with or without T-cell depletion. The majority of these studies are based on the following models:
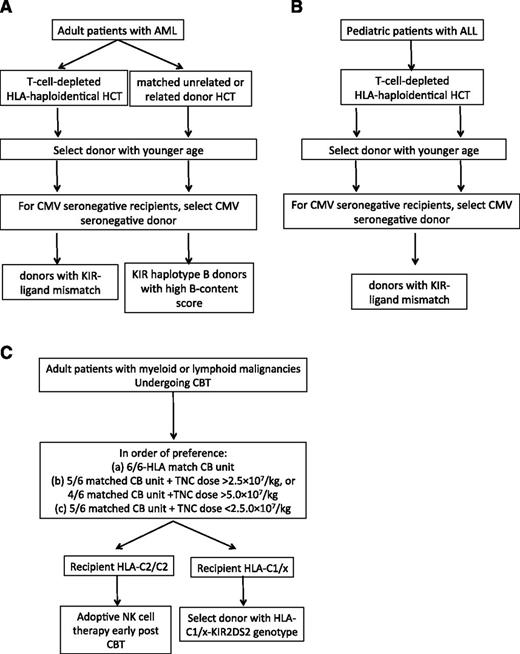
The KIR-ligand model: The data on KIR-ligand mismatch and outcome after HSCT are conflicting (Table 1). Although the majority of studies support a beneficial effect for KIR-ligand mismatch on the risk of relapse in the setting of T-cell-depleted haploidentical HSCT for adult AML and childhood ALL,20,84 a beneficial role for KIR-ligand mismatch on outcomes in other hematologic malignancies has not been convincingly shown. Thus, in adults with AML or children with ALL undergoing a T-cell-deplete haploidentical HSCT, we propose that the selection of a donor with KIR-ligand mismatch may improve outcomes (Figure 2A-B). Several clinical trials (NCT01787474, NCT02646839, NCT01807611, NCT02519114, NCT02508038) are prospectively studying selection of donors on the basis of KIR-ligand mismatch for adult patients with AML or multiple myeloma and children with ALL, AML, myelodysplastic syndrome, or lymphoma in both the T-cell-deplete and T-cell-replete settings.
The KIR haplotype model: This model proposes selection of KIR haplotype B donors with high B-content score in adults with AML undergoing matched unrelated or related donor transplant83,85,87 or children with ALL undergoing T-cell-depleted haploidentical HSCT.84 In situations in which multiple potential donors of similar ages and CMV serostatus are available, we suggest that a donor with high KIR B content and Cen B/B genotype may improve outcomes in adult patients with AML undergoing an HLA-matched unrelated HSCT (Figure 2A). Clinical trial NCT01288222 is prospectively studying this approach.
Selection of cord blood donors based on the combination of licensing and activating KIR genes: After considering HLA match and the total nucleated cell content of the CB unit,129 we propose that the selection of a donor with an HLA-C1-KIR2DS2 genotype may improve outcomes for HLA-C1/x recipients (Figure 2C). The NCT02727803 trial is exploring this approach in adults with myeloid or lymphoid malignancies undergoing CBT.
Proposed algorithm for donor selection based on KIR genotyping in (A) adult AML, (B) childhood ALL, and (C) CBT.
Proposed algorithm for donor selection based on KIR genotyping in (A) adult AML, (B) childhood ALL, and (C) CBT.
NK cell adoptive immunotherapy
Given the potent antitumor efficacy of NK cells, adoptive transfer of NK cells to treat a variety of malignancies has also been explored by several groups.71,130-144 Results of the initial trials using ex vivo activated autologous NK cells were mostly unsatisfactory,130-138 likely because of the inhibition of autologous NK cells by self-HLA molecules. As the impact of KIR-ligand mismatch in the transplant setting became evident, the focus of the trials shifted toward the use of allogeneic NK cells either in combination with HSCT or in a non-HSCT setting (Table 3).71,141-144 Allogeneic NK cells are less likely to be subject to the inhibitory response resulting from NK cell recognition of self-MHC molecules, as seen with autologous NK cells. Moreover, several studies have shown that infusion of haploidentical NK cells to exploit the missing-self concept is safe and can mediate impressive clinical activity in some patients with AML.71,139-144 In those studies, NK cells are activated and expanded ex vivo with a variety of cytokines such as interleukin-2 (IL-2), IL-15, or IL-21 to increase their persistence, enhance their proliferation, and augment their in vivo effector function (Table 3). In a landmark trial of NK cell adoptive therapy in patients with relapsed or refractory AML, Miller et al71 were among the first to show that adoptive transfer of ex vivo–activated haploidentical NK cells after lymphodepleting chemotherapy followed by IL-2 administration to support their in vivo expansion is safe and can result in NK cell persistence for up to 4 weeks without inducing GVHD. Although the clinical responses were modest, a subsequent study that used recombinant IL-2 diphtheria toxin to deplete regulatory T cells (Tregs) (which can impair NK effector function145 ), resulted in enhanced in vivo NK cell expansion with improved leukemia clearance.141
Studies evaluating the impact of donor KIR haplotype or activating KIRs in related or unrelated donor HSCT
| Study . | Kröger 200673 . | Cooley 200983 . | Cooley 201085 . | Stringaris 201087 . | Cooley 201486 . | Oevermann 201484 . |
|---|---|---|---|---|---|---|
| No. of patients | 142 | 448 | 1409 | 246 | 1532 | 85 |
| Disease | AML, ALL, CML, MDS | AML | AML, ALL | AML, ALL, CML, CLL, MDS, NHL | AML | Childhood ALL |
| Donor | URD | URD | URD | HLA-identical sibling | URD | Haploidentical |
| TCD (%) | In vivo 100 (ATG) | 0 | 0 | 100 | 0 | 100 |
| Study group | Donor activating KIRs | Donor genotype B/x | Centromeric B/B donors vs others | Donor KIR B haplotype genes (2DS1, 3DS1, and 2DL5A) | C1/x patient receiving an HSCT from a donor with ≥2 KIR B genes vs other donor | Donor genotype B/x |
| Proportion of patients with aGVHD (grade 2-4) | NS | NS | NS | — | NS | — |
| Relapse | Higher with increasing number of donor activating KIRs | NS | Lower with centromeric B/B donors AML only) | Lower in study group (AML only) | Lower in study group | Lower with genotype B/x donor |
| Risk | HR, 1.37 | RR, 0.34 | HR, 0.24 | RR, 0.70 | RR, 2.82 | |
| 95% CI | 1.1-1.7 | 0.20-0.57 | 0.56-0.87 | 1.37-5.77 | ||
| P | .005 | .001 | .02 | .0018 | .005 | |
| DFS | Lower with increasing number of donor activating KIRs | Higher with genotype B/x donor | Higher with centromeric B/B donors (AML only) | — | Higher in study group | Higher with genotype B/x donor |
| Risk | HR, 1.16 | RR, 0.70 | RR, 0.72 | RR, 0.78 | 50.6% vs 29.5% at 5 years | |
| 95% CI | 0.55-0.88 | 0.55-0.93 | 0.67-0.91 | |||
| P | .04 | .002 | .01 | .0015 | .033 | |
| OS | NS | Higher with genotype B/x donor | Higher with centromeric B/B donors AML only) | NS; (AML only); higher in study group (all patients) | — | — |
| Risk | 31% vs 20% | NR | HR, 0.64 | |||
| 95% CI | 26%-36% vs 13%-27% | 0.43-0.94 | ||||
| P | .007 | .024 |
| Study . | Kröger 200673 . | Cooley 200983 . | Cooley 201085 . | Stringaris 201087 . | Cooley 201486 . | Oevermann 201484 . |
|---|---|---|---|---|---|---|
| No. of patients | 142 | 448 | 1409 | 246 | 1532 | 85 |
| Disease | AML, ALL, CML, MDS | AML | AML, ALL | AML, ALL, CML, CLL, MDS, NHL | AML | Childhood ALL |
| Donor | URD | URD | URD | HLA-identical sibling | URD | Haploidentical |
| TCD (%) | In vivo 100 (ATG) | 0 | 0 | 100 | 0 | 100 |
| Study group | Donor activating KIRs | Donor genotype B/x | Centromeric B/B donors vs others | Donor KIR B haplotype genes (2DS1, 3DS1, and 2DL5A) | C1/x patient receiving an HSCT from a donor with ≥2 KIR B genes vs other donor | Donor genotype B/x |
| Proportion of patients with aGVHD (grade 2-4) | NS | NS | NS | — | NS | — |
| Relapse | Higher with increasing number of donor activating KIRs | NS | Lower with centromeric B/B donors AML only) | Lower in study group (AML only) | Lower in study group | Lower with genotype B/x donor |
| Risk | HR, 1.37 | RR, 0.34 | HR, 0.24 | RR, 0.70 | RR, 2.82 | |
| 95% CI | 1.1-1.7 | 0.20-0.57 | 0.56-0.87 | 1.37-5.77 | ||
| P | .005 | .001 | .02 | .0018 | .005 | |
| DFS | Lower with increasing number of donor activating KIRs | Higher with genotype B/x donor | Higher with centromeric B/B donors (AML only) | — | Higher in study group | Higher with genotype B/x donor |
| Risk | HR, 1.16 | RR, 0.70 | RR, 0.72 | RR, 0.78 | 50.6% vs 29.5% at 5 years | |
| 95% CI | 0.55-0.88 | 0.55-0.93 | 0.67-0.91 | |||
| P | .04 | .002 | .01 | .0015 | .033 | |
| OS | NS | Higher with genotype B/x donor | Higher with centromeric B/B donors AML only) | NS; (AML only); higher in study group (all patients) | — | — |
| Risk | 31% vs 20% | NR | HR, 0.64 | |||
| 95% CI | 26%-36% vs 13%-27% | 0.43-0.94 | ||||
| P | .007 | .024 |
CLL, chronic lymphoid leukemia; NR, not reported.
IL-15, in contrast to IL-2, does not support the expansion of Tregs.146 Thus, IL-15 administration holds promise for specifically boosting NK cell alloreactivity without the undesired stimulation of Tregs. Several groups are evaluating the efficacy of highly potent recombinant cytokines such as an IL-15 superagonist to induce in vivo NK cell activation and expansion (without adoptive therapy) in various malignancies (NCT01885897, NCT01946789, NCT02099539, NCT02384954).
Future directions
Chimeric antigen receptors (CARs) have been used extensively to redirect the specificity of T cells against leukemia with dramatic clinical responses in patients with lymphoid malignancies.147-153 These infusions have been primarily restricted to the autologous setting because activated T cells from an allogeneic source are likely to increase the risk of GVHD. Given their shorter lifespan and potent cytolytic function, mature NK cells provide attractive candidate effector cells to express CARs and provide an excellent source of off-the-shelf cellular therapy for patients with cancer. First, allogeneic NK cells should not cause GVHD, as predicted by observations in murine models,154,155 as well as in patients with leukemia and solid malignancies treated with haploidentical or CB-derived NK cells.19,20,71,141 Second, mature NK cells have a limited life span of a few weeks, allowing for antitumor activity while reducing the probability of long-term adverse events, such as prolonged cytopenias caused by on-target/off-tumor toxicity to normal tissues, or the risk of malignant transformation. Third, unlike T cells, NK cells will also have activity through their native receptors to kill antigen-negative target cells, potentially preventing a mechanism of immune escape. The feasibility of genetically engineering NK cells to express CARs against a number of targets has been shown in the preclinical setting,156-164 and 2 clinical studies (both targeting CD19+ malignancies using a retroviral transduced anti-CD19-BB-ζ NK-CAR) are testing the safety and efficacy of this approach in the clinic (NCT00995137 and NCT01974479).
An alternative strategy under investigation to redirect NK cytotoxicity toward tumor cells is to create either bispecific or trispecific antibodies (BiKE, TriKE).165-171 BiKEs are constructed by joining a single-chain variable fragment (Fv) against CD16 and a single-chain Fv against a tumor-associated antigen (BiKE), or 2 tumor-associated antigens (TriKE), such as CD19, CD22, CD33, CD30, or EpCAM.165-170 To enhance NK cell expansion and survival in vivo, investigators have developed a novel TriKE that also includes a modified recombinant human IL-15 cross-linker sandwiched between single-chain Fv against CD16 and the target antigen of interest.171 It will be interesting to observe whether these strategies alone or in combination with HSCT can provide durable clinical responses and immediate access to treatment with the development of off-the-shelf products.
Summary
Significant advances have been made in understanding the role of NK cell activity after HSCT. Although it is well recognized that the antitumor activity of NK cells is intensified in the setting of HLA-mismatched HSCT, the benefit of selecting a donor on the basis of KIR genotyping has not yet been established. A number of institutions have established local guidelines to assist in the selection of donors with NK cell alloreactivity predicted by HLA or KIR genotype; however, the definition and models of alloreactivity used by these centers are not standardized, underscoring the importance of well-designed, prospective studies of donor selection based on KIR-HLA immunogenetics.
We suggest that NK cells and KIR immunogenetics are likely to gain increasing importance to clinicians in the selection of the best donors for transplant, along with HLA matching, CMV status, blood group, age, and sex. Furthermore, the identification of optimal donors on the basis of KIR genotype may be an important next step for the design of successful NK cell-based interventions and the development of the next generation of engineered NK cells for immunotherapy of cancer.
Correspondence
Katayoun Rezvani, Department of Stem Cell Transplantation, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston, TX 77030; e-mail: krezvani@mdanderson.org.
References
Competing Interests
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.