Abstract
The non-Hodgkin lymphomas (NHLs) occurring in children and adolescents and young adults (AYA) are characterized by various age-related differences in tumor biology and survival. Children generally present with high-grade lymphomas, such as Burkitt lymphoma, diffuse large B-cell lymphoma, lymphoblastic lymphoma, and anaplastic large cell lymphoma, whereas low-grade histologic subtypes, such as follicular lymphoma, occur more frequently with increasing age. Treatment outcome for children with NHL is generally superior to that observed in adults. Factors contributing to this discrepancy include psychosocial factors, patient factors, and differences in tumor biology and therapy. These factors will be reviewed, with particular attention to the biological features of diffuse large B-cell lymphoma and anaplastic large cell lymphoma and corresponding therapeutic challenges. Novel targeting agents have been developed, which have been shown to be active in some patients. There is clearly a need for treatment protocols with eligibility criteria that cover the full span of the pediatric and AYA age range and that incorporate detailed molecular characterization of the tumors.
Learning Objectives
To describe age-related biological differences in NHL occurring in the pediatric and AYA population (ages 1-39)
To describe current treatment approaches for NHL in children and adults, and to identify ongoing challenges in determining optimal treatment approach for the AYA population
Introduction
Define NHL with demographics (SEER)
The non-Hodgkin lymphomas (NHLs) are characterized by various age-related differences in clinical presentation, biology, and outcome.1,2 Children younger than 16 years of age are defined as the “pediatric” group, whereas patients whose age is within the 16 to 39 range, are considered the “adolescent and young adult (AYA)” group.3,4 The frequency of newly diagnosed NHL increases with increasing age. The annual frequency in the US (SEER database) for those in the pediatric, AYA, and older adult (>40) age groups is 0.5 to 1.2 per 100 000, 1.8 to 7.2 per 100 000, and 10.5 to 116.4 per 100 000, respectively.3 The treatment outcomes for those with newly diagnosed NHL in the pediatric age group are generally better than that for the AYA and older age groups.3-8 There are various reasons for the age-associated discrepant treatment results, and these challenges must be carefully considered as initiatives are developed to improve outcomes. Some of these challenges pertain to sociologic and psychosocial aspects of the individual age groups, whereas others are more directly related to the patient’s general medical condition and specific biological NHL subtype.3,4
Sociological and Psychosocial challenges.
Those in the pediatric age group generally have a parent or guardian advocating for their care, and in cases where this is lacking, the multidisciplinary medical team (social worker, child life, nursing, pharmacy, and medical staff) in the local children’s hospital often makes extra efforts to ensure compliance with the chemotherapy plan and delivery of appropriate supportive care for this vulnerable population. Those in the AYA group face some unique challenges as they transition to relative independence. Risk factors that may affect the AYA age group include difficulty to secure insurance, inconsistent medical care with delay in diagnosis, poor compliance with medication, low rates of enrollment in clinical trials, and less structured supportive care.3 In this regard, the AYA population may in some cases also be considered at potentially higher risk or more vulnerable.3,9 Programs to help address these needs have been implemented by the National Cancer Institute and the American Society of Clinical Oncology.3 The most promising new treatment approaches will not be successful if the psychosocial infrastructure is not securely in place to deliver them.
Patient factors.
There are certain age-associated patient/host factors that can influence treatment outcome. The International Prognostic Index (IPI), which includes the patient’s performance status, has been shown to have significant prognostic value for adults with NHL.10 Although components of the IPI, such as lactate dehydrogenase (LDH), may have prognostic significance in children, performance status is not routinely used in planning therapy for those with newly diagnosed NHL in the pediatric age group. However, there are some inherited immunodeficiency conditions, such as ataxia-telangiectasia and X-linked lymphoproliferative syndrome, where specific treatment modifications need to be incorporated.
Age-related pharmacokinetic challenges must be considered in treatment planning. In this regard, drug clearance may vary with age and the maximum tolerated dose for many cancer agents is higher in younger patients.11 An example of this is the inability of older patients with aggressive NHL to tolerate dose-intensification with CHOEP in NHL-B2, whereas the addition of etoposide improved outcomes in younger patients.12,13 Moreover, drug-drug interactions may influence clearance and metabolism of certain chemotherapy agents.14
Tumor subtype and biology.
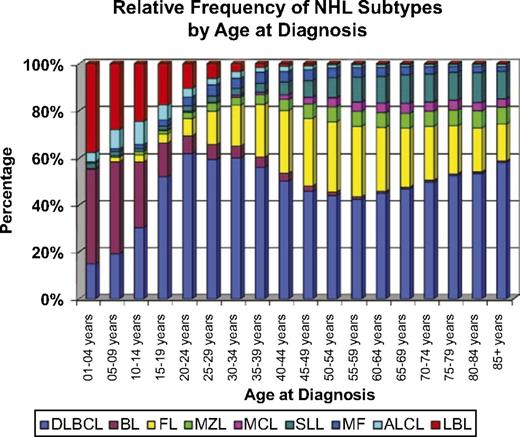
The spectrum of NHL subtypes varies with age (Figure 1).4 The NHL subtypes occurring in children younger than 16 years of age are primarily high-grade tumors comprising BL, DLBCL, lymphoblastic lymphoma (LL), and ALCL.15 Low-grade B-cell lymphomas, such as FL and marginal zone B-cell lymphomas (MZBCL), are much less common, but increase in frequency with increasing age.1,4,15 Among histologic subtypes, biological differences between age groups have been described, which have significant impact on treatment approach and outcome.16-20
ALCL, anaplastic large-cell lymphoma; BL, Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; LBL, lymphoblastic lymphoma; MCL, mantle cell lymphoma; MF, mycosis fungoides; MZL, marginal zone lymphoma; SLL, small lymphocytic lymphoma. Reprinted with permission from Jaglowski et al.4
ALCL, anaplastic large-cell lymphoma; BL, Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; LBL, lymphoblastic lymphoma; MCL, mantle cell lymphoma; MF, mycosis fungoides; MZL, marginal zone lymphoma; SLL, small lymphocytic lymphoma. Reprinted with permission from Jaglowski et al.4
The remainder of this review will deal with age-associated tumor biology and treatment outcome differences, with an emphasis on DLBCL and ALCL, where the greatest challenges lie.
High-grade mature B-cell lymphomas
Diffuse large B-cell lymphoma
Biology.
The DLBCLs are mature B-cell neoplasms characterized by the expression of surface immunoglobulin (sIg) and B cell–associated surface markers (CD19, CD20, CD22, and CD79a).15 There are 2 main categories of DLBCL, which include the germinal center (GCB) and activated B-cell (ABC) subtypes. This biological distinction among patients with DLBCL appears to vary with age. Younger patients are more likely to have GCB disease than adults (age 0-9 years 95% GCB, 10-19 years 80% GCB, adults 42% GCB, χ2 2-sided P value < .0001).21,22 GCB DLBCL has been consistently associated with improved outcomes over non-GCB disease.22,23
BCL2 expression generally increases with age and is associated with inferior outcomes in DLBCL.24 The adverse risk associated with the ABC subtype is likely in part secondary to the upregulation of downstream targets of NF-κB, which include BCL-2 and cyclin D2.4
Various genetic abnormalities have been detected in cases of DLBCL. 8q24 MYC rearrangements may be present and have been associated with a poorer prognosis in some studies.20 Other reported abnormalities include the t(14;18)(q32;q21) and 3q27 BCL6 rearrangements; however, these are uncommon in pediatric cases.2,25 IRF4 oncogene rearrangements, which have been identified in a small subset of B-NHLs with a distinct immunophenotype from the GCB and ABC subtypes of DLBCL, occur more commonly in those younger than 18 years of age.20,26
Recurrent translocations involving BCL-6, BCL-2, and MYC are of particular interest because of the associated adverse risk. Double-hit lymphomas (DHLs), which are associated with a very poor prognosis in adults, are DLBCLs that contain translocations involving any 2 of these translocations (MYC/BCL2, MYC/BCL6, MYC/BCL2/BCL6). DHLs are associated with dismal outcomes and are largely restricted to adults with GCB-DLBCL as DHLs are incredibly rare in the pediatric population.20,27,28 The spectrum of oncogenic mechanisms and therapeutic targets for the DLBCLs, which vary according to the molecular subtype (ABC, GCB, and PMLBCL [primary mediastinal large B-cell lymphoma]), have been extensively reviewed (Table 1).19
Non-Hodgkin lymphoma: oncogenic mechanisms and therapeutic target
| Lymphoma Subtype . | Pathways altered . | Targeted Agents . | Clinical trials . | Age of patients eligible (years) . |
|---|---|---|---|---|
| DLBCL | Programmed death-1 | Pembrolizumab | NCT02541565 | 18+ |
| CD79b | Polatuzumab vedotin | NCT01992653 | 60-80 | |
| Programmed death-1 plus BCR activation | Nivolumab plus ibrutinib | NCT02329847 | 18+ | |
| CD20 | Obinutuzmab | NCT02670317 | 18-60 | |
| BCL-2 | Venetoclax | NCT02055820 | 18+ | |
| NK cell activation | Lenalidomide | NCT00670358 | 18-120 | |
| CD30 | Brentuximab vedotin | NCT01925612 | 18+ | |
| GCB | BCL-2 translocation | |||
| EZH2 mutations | CUDC-907 | NCT01742988 | 18+ | |
| PTEN deletions | ||||
| nonGCB | NF-κB activation | Bortezomib | NCT01848132 | 18-70 |
| CARD11 mutations | ||||
| BCR mutations | Ibrutinib | NCT02219737 | 18+ | |
| MYD88 mutations | Ibrutinib | NCT02219737 | 18+ | |
| DHL | MYC aberrations | CUDC-907 | NCT02674750 | 18+ |
| PMLBCL | Programmed death-1 | Pembrolizumab | NCT02576990 | 18+ |
| NF-κB activation | ||||
| CD30 | Brentuximab vedotin | NCT02423291 | 18+ | |
| JAK2 mutations | Ruxolitinib | NCT01965119 | 19-80 | |
| REL amplifications | ||||
| ALCL | ||||
| ALK+ | CD30 | Brentuximab vedotin | NCT01777152 | 18+ |
| ALK fusion protein | Crizotinib | NCT01979536 | 0-21 | |
| ALK– | CD30 | Brentuximab vedotin | NCT01777152 | 18+ |
| Lymphoma Subtype . | Pathways altered . | Targeted Agents . | Clinical trials . | Age of patients eligible (years) . |
|---|---|---|---|---|
| DLBCL | Programmed death-1 | Pembrolizumab | NCT02541565 | 18+ |
| CD79b | Polatuzumab vedotin | NCT01992653 | 60-80 | |
| Programmed death-1 plus BCR activation | Nivolumab plus ibrutinib | NCT02329847 | 18+ | |
| CD20 | Obinutuzmab | NCT02670317 | 18-60 | |
| BCL-2 | Venetoclax | NCT02055820 | 18+ | |
| NK cell activation | Lenalidomide | NCT00670358 | 18-120 | |
| CD30 | Brentuximab vedotin | NCT01925612 | 18+ | |
| GCB | BCL-2 translocation | |||
| EZH2 mutations | CUDC-907 | NCT01742988 | 18+ | |
| PTEN deletions | ||||
| nonGCB | NF-κB activation | Bortezomib | NCT01848132 | 18-70 |
| CARD11 mutations | ||||
| BCR mutations | Ibrutinib | NCT02219737 | 18+ | |
| MYD88 mutations | Ibrutinib | NCT02219737 | 18+ | |
| DHL | MYC aberrations | CUDC-907 | NCT02674750 | 18+ |
| PMLBCL | Programmed death-1 | Pembrolizumab | NCT02576990 | 18+ |
| NF-κB activation | ||||
| CD30 | Brentuximab vedotin | NCT02423291 | 18+ | |
| JAK2 mutations | Ruxolitinib | NCT01965119 | 19-80 | |
| REL amplifications | ||||
| ALCL | ||||
| ALK+ | CD30 | Brentuximab vedotin | NCT01777152 | 18+ |
| ALK fusion protein | Crizotinib | NCT01979536 | 0-21 | |
| ALK– | CD30 | Brentuximab vedotin | NCT01777152 | 18+ |
The BFM (Berlin-Frankfurt-Meunster) study of pediatric DLBCL confirmed that the majority of cases have a GCB phenotype. They also reported a stronger association with CD10 and BCL-6 expression in children compared with adult cases; however, there did not appear to be age-related differences in expression of BCL-2 or MUM-1.4,25 The International FAB (French-American-British) study also demonstrated a predominance of the GCB phenotype among cases of pediatric DLBCL.21 They reported that pediatric cases have a higher proliferative rate and greater MYC expression, but are generally not associated with BCL-2 overexpression.21 The superior outcome for children with DLBCL had been thought to be secondary to the predominance of the GCB subtype in children; however, there does not appear to be adverse risk associated with the ABC phenotype in the pediatric population.20 The inability to demonstrate an associated adverse risk for ABC cases in children may simply be a consequence of the small number of ABC cases in this age group, or may reflect age-related differences in treatment.
Treatment
Pediatric.
Over the past 15 to 20 years, most children with DLBCL have been treated with regimens designed for BL, resulting in what appear to be superior outcomes compared with CHOP-based approaches used in earlier treatment eras. For example, a 4-year event-free survival (EFS) of 92% was reported for children with DLBCL (excluding those with primary mediastinal large B-cell lymphoma, PMLBCL) compared with 93% for those with BL treated with the very successful LMB-96 regimen for children with BL and other high-grade mature B-cell lymphomas (Group B, age range: 2.5-20.5 years).29-31 A similar observation was made in the BFM mature B-cell study in which children with DLBCL were treated with a contemporary regimen designed for those with BL (all patients <18 years of age).32 Efforts to further improve outcomes for those with DLBCL have featured the incorporation of rituximab into frontline therapy. The COG performed a pilot study in which 6 doses of rituximab were incorporated into an LMB-96 backbone.33,34 The feasibility results of this study led to an international B-NHL protocol for children with high-grade mature B-cell lymphomas (including DLBCL and BL), which featured a rituximab randomization for those with high-risk disease (stage III with LDH > 2X ULN, stage IV, and Burkitt leukemia; NCT01516580). The first interim analysis indicated a survival advantage for those receiving rituximab, so the randomization was stopped.35 The BFM reported preliminary results on a trial that incorporated a rituximab window into a BFM backbone designed for children with high-grade mature B-cell NHL (BL and DLBCL); the outcome results for this trial are pending (all patients <19 years of age).36
The optimal treatment of the PMLBCL subtype, which is associated with a poorer outcome than other DLBCLs in children, has yet to be defined. In the LMB-96 trial, which featured a BL-like approach, the 5-year EFS for the stage III PMLBCL was 66% (age range, 12.5-19.7 years; median, 15.7 years)—a result that was significantly less than other stage III DLBCLs treated on the same regimen (5-year EFS, 85%; P < .001).37 In contrast to what has been observed in children, adults with PMLBCL have a better prognosis than adults with other DLBCL subtypes.19 The highly successful DA-EPOCH-R regimen used in adults with PMLBCL38 (ages 19-52 years; median, 30 years) is currently being studied in children in an international trial (NCT01516580).
Adult.
The most significant improvements in outcomes for adults with DLBCL has been achieved with the addition of rituximab (R) to CHOP as reported by Coiffier, in older adults.39 The MinT trial demonstrated an improvement in progression-free survival (PFS) (6-year 80% vs 64%) and overall survival (OS) (6-year 90% vs 80%) with the addition of rituximab to 6 cycles of CHOP in younger adults 18 to 60 years old with stage II-IV disease.40 The RICOVER-60 trial confirmed Coiffier’s results in patients aged 61 to 80 years and demonstrated that 6 cycles were equivalent to 8 cycles.41 The LNH03-6B study further defined that every 3-week therapy was equivalent to dose-dense therapy in the era of rituximab.42 The addition of rituximab to CHOP induction therapy has also largely obviated the need for autologous stem cell transplant consolidation for patients in CR1.43 Finally, the attenuated immunochemotherapy regimen R-mini-CHOP suggests that the benefits of rituximab can be extended to the elderly and frail.44
Adults with DHL are often treated with DA-EPOCH-R, with consideration of autologous stem cell consolidation in CR1,45 though this approach has not been validated in prospective studies.
Outcomes for adults with PMBLC using DA-EPOCH-R without radiation are outstanding38 —other regimens have also been shown to be active in PMBLC.46
In contrast to the pediatric regimens, R-CHOP-21 does not routinely include central nervous system (CNS) prophylaxis. CNS prophylaxis is reserved for adults with increased risk for CNS disease (NCCN guidelines). The optimal CNS prophylaxis regimen remains controversial, but recent data favor systemic high-dose methotrexate over intrathecal therapy.47-49
How does the field move beyond R-CHOP-21? DA-EPOCH-R is currently being compared with R-CHOP in DLBCL based on phase 2 studies (CALGB 50303),50-52 but its use off-study currently remains a category 2B recommendation by the NCCN. Several new agents are also being tested and may help to improve patient outcomes. These include the novel anti-CD20 obinutuzumab and the BCL-2 inhibitor venetoclax with CHOP (NCT02055820), lenalidomide in combination with DA-EPOCH-R or R-CHOP (NCT02213913, NCT00670358), ibrutinib with obinutuzumab, and CHOP (NCT02670317) and brentuximab vedotin with R-CHOP.
AYA Recommendations.
Most AYA patients ages 16 to 21 years with DLBCL referred to a children’s hospital, will be treated with a BL-based regimen (± rituximab). Regimens featuring rituximab, such as R-CHOP and DA-EPOCH-R, have excellent results in an older population with no direct comparisons of the adult R-CHOP/DA-EPOCH-R with pediatric Burkitt–based approaches in the AYA population. Considerations for older AYA patients (21-39 years) who do not chose to participate in a clinical trial include risk adapted adult regimens discussed before.
Burkitt lymphoma
Biology.
Burkitt lymphoma is a mature B-cell lymphoma expressing surface immunoglobulin (sIg) and a spectrum of surface B-cell markers (CD19, CD20, CD22): CD10, BCL6, CD38, CD77, and CD43.15 These lymphomas are also characterized by the presence of a reciprocal translocation involving the c-myc gene and one of the immunoglobulin genes. The t(8;14) is considered the classical translocation and involves the heavy-chain Ig gene on chromosome 14, and the t(8;22) and t(2;8) translocations are considered the variant translocations involving the light-chain immunoglobulin genes. Other genetic changes that have been observed in BL include 13q gains or losses, 11q gains or losses, and mutations in ID3 and/or TCF3.20 Gains and losses in 13q have been associated with a poorer prognosis. Gains or losses in 11q have been associated with MYC negative BL. ID3 and or TCF3 mutations have been associated with sporadic BL but not DLBCL.20
A study of cytogenetic abnormalities in adults and pediatric patients found no age-associated difference within the spectrum of cytogenetic abnormalities; however, there were age-related differences with respect to prognosis. In this cytogenetic study, abnormalities in chromosome 22q and 13q appeared to be associated with adverse risk in the pediatric population, whereas abnormalities in chromosome 17 was associated with adverse risk in adults.53 Gene expression profiling and comparative genomic hybridization profiling have not shown significant differences between adult and pediatric BL.20
Gene profiling studies of BL demonstrate molecular features that distinguish it from the DLBCLs.54 In the study by Dave et al, those patients with morphologic DLBCL with an expression profile more consistent with BL (mBL) had a poorer outcome when treated with CHOP as compared with those treated with more aggressive regimens.20,54
Treatment
Pediatric.
The CCG trial comparing the alkylator-based COMP regimen with the multiagent acute lymphoblastic leukemia (ALL)-like regimen demonstrated a superior outcome for children with BL treated with the COMP regimen.55 Since that time, further improvements have been achieved by intensification of therapy including the addition of HDMTX (high-dose methotrexate), cytarabine, etoposide, and aggressive CNS prophylaxis/treatment. One of the most widely used regimens was the LMB-89 regimen, which featured 3 arms (Groups A, B, and C) of therapy based on risk.30 The current criteria for these group designations are as follows: Group A comprises those with completely resected limited-stage disease, Group C includes those with CNS involvement and/or ≥25% lymphoma blasts in the bone marrow, and Group B includes those not meeting the criteria for either Group A or C. Outcomes with this regimen were excellent. A subsequent international trial demonstrated that reductions could safely be made in the Group B arm (age range, 2.5-20.5 years) but resulted in worse outcome in the Group C arm (all patients <20 years of age).29,31 The BFM designed a study featuring a rituximab window and BFM backbone designed for children with BL and DLBCL; the outcome results for this trial are pending.36 A current international COG/SFOP (French Society of Pediatric Oncology) trial for children with BL and DLBCL demonstrated a survival advantage for those higher-risk (stage III with LDH > 2X ULN, stage IV and Burkitt leukemia) patients (all <18 years of age) who were randomly selected to receive rituximab (NCT01516580).35
Adult.
The approaches for treating adults with BL largely mirror the approaches implemented in pediatrics, with excellent results for most patients.56-58 Examples include the CODOX/IVAC regimen57 R-Hyper-CVAD regimen,52,56 and DA-EPOCH-R.59 The very successful pediatric LMB-based approach has also been shown to be very active in adults.58 In this regard, Soussain et al reported a 3-year OS rate in a retrospective analysis of 65 adults (17-65 years of age; median, 26 years; mean, 30 years) with BL/Burkitt leukemia treated with LMB pediatric protocols.58 All regimens include a dose-intense schema and intrathecal therapy.
AYA recommendations.
There is little controversy regarding the optimal approach to treating patients with BL in the AYA group because the regimens used in both children and adults are quite similar. The therapeutic options for AYA with BL include currently open clinical trials or the aforementioned published regimens, with a consideration for the addition of rituximab in some cases. In this regard, higher-risk patients (stage III with LDH > 2X ULN, stage IV, and Burkitt leukemia) have been shown to benefit from the addition of rituximab (NCT01516580)35 . Although DA-EPOCH-R has been quite active in adults with BL, it is unclear whether this approach would be successful in cases of CNS+Burkitt leukemia.
Anaplastic large-cell lymphoma
Biology.
Anaplastic large-cell lymphoma (ALCL) was first described by Stein as a pleomorphic large-cell lymphoma with anaplastic morphology and expressing CD30.15,60 The ALCLs are primarily of T-cell immunophenotype, although null-type and B-lineage cases have rarely been reported. The t(2;5) translocation is commonly associated with this lymphoma and features a fusion gene comprising NPM and ALK61 ; however, variant translocations involving ALK and another partner gene may occur in some cases. The protein product can be detected with immunohistochemistry stains for ALK in both cytoplasm and nucleus for cases containing the t(2;5) and in the cytoplasm alone in cases with the variant ALK translocations. Effectors downstream of ALK, including RAS/ERK, PI3K/AKT, and JAK3/STAT3 pathways, may be potential therapeutic targets.20
The frequency of genetic abnormalities varies with age in ALCL. Most pediatric cases of ALCL are ALK-positive. Among adults, ALK-positive patients are typically younger (median age 28-34 years, range 6-77 years) than ALK-negative patients (median age 58 years, range 22-94 years).16,62 Emerging prognostic markers such as DUSP22 (median age 54 years, range 36-76 years) and TP63 (median age 48 years, range 30-73 years) are largely restricted to adult populations and are rarely seen in pediatric patients. Regarding impact on prognosis, the 30% of ALK- negative patients that had DUSP22 rearrangements had a 5-year OS of 90%, in contrast to the 8% of ALK-negative patients with TP63 rearrangements who had a 5-year OS of 17%. “Triple-negative” ALCL patients had an intermediate prognosis with a 5-year OS of 42%.16 Studies have suggested that ALK-negative DUSP22-positive ALCL may be morphologically distinct from other ALK-negative ALCL.63
The prognosis for children with ALCL is superior to that observed in adults, which may be driven by the differences in biology described here before. Among adults, the outcome for those with ALK- positive ALCL is superior to that for those with ALK-negative disease.17,64 In fact, the outcome for adults with ALK-positive ALCL approaches that described for children with ALCL (usually ALK-positive), suggesting that biology is a key prognostic factor independent of age in ALCL.
Treatment
Pediatric.
Varied approaches have been used in the management of pediatric ALCL and range from CHOP-based approaches to those designed for the treatment of BL, with fairly comparable treatment results. The 2 most recently used regimens are the anthracycline-based APO regimen65 (doxorubicin, vincristine, prednisone) given every 21 days for approximately 1 year, and the BFM Burkitt-like backbone approach used in the ALCL99 European trial (age range, 4 months to 19.5 years; median, 11 years).66 The ALCL99 approach has a total cumulative anthracyline dosage less than that in the APO regimen; however, it is more intensive with increased risk for fever and neutropenia. A 3-year EFS of ∼70% was achieved with both regimens. Encouraging results from the study of single-agent vinblastine67 in patients with relapsed ALCL prompted its inclusion in 2 multicenter randomized trials in an effort to improve outcome. The ALCL99 trial randomized the use of vinblastine in higher-risk patients in the context of a previously reported and successful BFM Burkitt-like backbone (eligibility, age <22 years).68 Concurrently, the COG randomized the use of vinblastine for advanced-stage patient in the context of an APO backbone (age range 0.7-20 years).69 Both studies failed to show an improvement in outcome, with the addition of vinblastine into the backbone regimens.68,69
Factors associated with adverse risk include ALK antibody status, minimal disseminated disease (MDD), and minimal residual disease (MRD), and may be used to risk stratify-therapy.20,70
Targeted therapeutic approaches have been shown to be active in adults and children with relapsed ALCL. The antibody drug conjugate, brentuximab vedotin, which targets CD30, has demonstrated safety and activity in phase 1 and 2 trials.71,72 Crizotinib, a small-molecule inhibitor of ALK, has also been shown to be active and well tolerated in both children and adults.73 In this regard, both brentuximab vedotin and crizotinib have both been incorporated into the current frontline COG trial for children with newly diagnosed ALCL (NCT01979536).
Adult.
Current adult NCCN guidelines recommend multiagent anthracycline-based regimens for ALCL (NCCN version 2.2016). NHL-B1 suggests that CHOEP is superior to CHOP (either every 2 or 3 weeks) in patients aged 18 to 60 years with normal LDH with aggressive lymphomas, with an improvement in 5-year EFS from 55% to 61% (every 2 weeks vs every 3 weeks) to 69% (P = .004).12 NHL-B2 suggests that CHOP-14 is superior to CHOP-21 (5-year EFS 33% vs 44%, P = .003) in patients with aggressive lymphomas aged 61 to 75 years and that the addition of etoposide is too toxic in older patients.13 Though ALCL patients represented a minority of patients on these studies (9.4% and 3.5%, respectively), these studies examined the role of etoposide and dose intensification in the prerituximab era across aggressive lymphomas and provide the foundation for the NCCN recommendations in ALCL. NHL B-1 and B-2 only included routine CNS prophylaxis for patients with lymphoblastic disease.
Adults with ALK-positive disease are believed to have better outcomes than those with ALK-negative disease when treated with CHOP-like regimens (5-year PFS 70% in ALK+ vs 49% in ALK– disease).16,62 DA-EPOCH may be a promising approach to both ALK+ and ALK– disease, with a reported 5-year PFS of 80% and 71%, respectively, though reported numbers were small and DUSP22 and TP63 status were unknown and unbalances may have influenced outcomes (age range 19-68 years, median 38 years).74
The French reported favorable outcomes with consolidative autologous stem cell transplant in CR1 in patients with ALCL, though 7 of 15 patients were ALK+.75 The Nordic NLG-T-01 trial suggested that consolidative autologous stem cell transplant in CR1 may be beneficial for patients with ALK– disease, but again numbers were small (31 patients) and biological subtypes were unknown.76 Others have reported similar results in single-arm studies, suggesting a benefit for autologous stem cell transplant in CR1.77-80 In contrast, a Cochrane review suggested that consolidative autologous stem cell transplant in CR1 did not benefit patients with aggressive NHL but further, ideally randomized, studies are needed to clarify this point.81 Future studies will need to stratify patients with emerging prognostic variables such as DUSP22 and TP63.
Fueled by encouraging results in advanced disease, brentuximab vedotin and crizotinib are being studied in frontline trials in ALCL.82 As a single agent, brentuximab vedotin produced an 86% objective response rate (ORR) among 58 patients with relapsed or refractory ALCL enrolled in the study (number of patients treated by ALK status: ALK–, n = 42 (72%) and ALK+, n = 16 [28%]), with a median duration of response of >1 year.72 Similarly, crizotinib has produced ORR of 90% in patients with relapsed or refractory ALK+ ALCL with a 2-year PFS of 64%.83,84 Ongoing studies in adult ALCL include the substitution of brentuximab vedotin for vincristine on the CHOP backbone as firstline therapy in CD30+ mature T-cell lymphomas (ECHELON-2) (NCT01777152).
AYA recommendations.
There are no data suggesting a clear advantage for any single regimen in the treatment of ALCL, regardless of age. Nevertheless, the risks and benefits of different regimens should be considered. Therapeutic options for AYA patients with newly diagnosed ALCL include open clinical trials featuring novel targeting agents. Alternatively, other aforementioned regimens can be considered.
Lymphoblastic lymphoma
Biology.
Lymphoblastic lymphomas (LLs) comprise both precursor-T and precursor-B phenotypes.15 Among the pediatric age group, the majority have a precursor-T immunophenotype. They are characterized by the expression of various surface markers as detected by immunohistochemistry or flow cytometry. The cytogenetic features of T-lymphoblastic lymphoma are less well described than other NHL subtypes in children. Chromosomal abnormalities have been described in T-ALL and T-LL which involve the TCR genes and result in the juxtaposition of an oncogene with the regulatory regions of the TCR genes. Examples of oncogenes involved in these translocations in T-LL include TAL1, LMO2, LYL1, HOXA9, TLX1, and TLX3.20 Among children with T-LL, Notch 1 mutations have been associated with a better prognosis, whereas LOH6q (loss of heterozygosity at 6q) is associated with a poorer prognosis.85 A poorer prognosis has also been associated with absence of biallelic TRG deletion (ABD) and PTEN mutations.20
Although T-ALL and T-LL share some of the same cytogenetic and molecular abnormalities, genomic studies featuring RNA-expression profiling and whole-exome sequencing analysis have suggested some differences between T-ALL and T-LL.20,86 Additional next-generation sequencing studies are clearly needed to more comprehensively compare these entities. There are very limited data on the biology of B-LL. Molecular characterization of these tumors is needed both for clarifying the pathogenic mechanisms compared with B-ALL and, more importantly, to identify molecular lesions for which novel targeting agents can be used as has been done in B-ALL.
Treatment
Pediatric.
Current strategies for the treatment of LL have largely been derived from successful approaches to the treatment of T-ALL.87-91 These regimens generally comprise induction (vincristine, daunorubicin, asparaginase, and a corticosteroid), consolidation, continuation, and reinduction/reintensification phases. With contemporary treatment protocols, a 5-year EFS of 80% to 85% can be achieved for children with advanced-stage LL. Several trials have demonstrated that prophylactic cranial irradiation can be safely eliminated without compromising outcome.88-90 Two randomized trials in the United States demonstrated that HDMTX can be safely eliminated for most children with LL, as long as sufficient intrathecal chemotherapy is administered.90,92
There are no clinical features that have been shown to have reliable prognostic significance. The measurement of minimal disseminated disease (MDD) in the bone marrow at diagnosis has been shown to have prognostic significance.93 Current trials are including this risk factor in trial design with some form of intensification for those with increased MDD. Some children with B-LL have been treated on the same protocol/regimen as that used for T-LL (eg, COG A5971),90 and not modified based on biological features as is done with B-ALL—the consequence of limited biological data for B-LL. Other current protocols are phenotype-specific and therefore include patients with T-LL and T-ALL on the same protocol, which is distinct from the protocol designed to include both B-LL and B-ALL.
Adult.
The GMALL studies for adults with LL mirror to some degree the ALL-like approach used in children, featuring induction, consolidation, and re-intensification phases, delivered over 6 to 12 months.94 In contrast, most contemporary pediatric regimens are given over 2 to 2.5 years. MD Anderson Cancer Center also implemented the hyper-CVAD regimen for adults with LL, and reported estimated 3-year PFS and OS rates of 66% and 70%, respectively (age range 17-59 years, median 28 years).95 Other trials for adults with LL have incorporated an intensification phase with hematopoietic stem cell transplant support,96 an approach that is not used in pediatric regimens. The treatment outcome for adults is inferior to that achieved in children.4 The reasons for this are not entirely clear and may be secondary to as yet unidentified biological differences in lymphoma pathology or differences in tolerance of intensive ALL-based therapy. Adults with B-LL are often treated with regimens used for B-ALL and may incorporate novel agents.
AYA recommendations.
There are no clear data indicating that one treatment approach is superior to others. Among patients who are not eligible for or who do not chose to enroll in a contemporary clinical trial, previously published approaches as listed before can be considered. The risk and benefits of previously published approaches will help determine the optimal approach individual AYA patients.
Follicular lymphoma
Biology.
The follicular lymphomas (FLs) are mature low-grade B-cell neoplasms characterized by expression of sIg, CD10, CD20, CD22, CD23, CD79a, BCL-6. and BCL-2; the t(14;18) translocation is present in some cases.15 More recently, “pediatric subtype” has been recognized in the World Health Organization classification.97 Although this is a clonal B-cell process as indicated by IgR, the tumors are typically BCL-2– on immunohistochemistry stain and lack the t(14;18). This pediatric subtype is generally associated with limited stage and excellent outcome with conservative approaches. Although most children with FL have the “pediatric” subtype, some present with advanced-stage disease and are found to have the classical adult type. Conversely, some adults have been found to have the pediatric subtype and have excellent outcomes as observed in children.98
Treatment
Pediatric.
Historically, most children with FL have received some type of conservative management approach, which generally includes surgical resection and chemotherapy, although some have been treated successfully with surgery alone.99,100 In this regard, most children with completely resected “pediatric” FL (BCL-2– and lacking the t(14;18)) are currently treated with close observation without chemotherapy or radiotherapy. Additional data for this “watchful waiting” approach are needed.100
Adult.
The approaches to treating adults with FL are quite varied and include complete resection, low-dose chemotherapy, immunotherapy including rituximab, intensification strategies such as hematopoietic stem cell transplant, and other modalities such as involved field radiation therapy. These approaches have been reviewed in detail.101 Of note, adults with the “pediatric type” of FL do well with conservative management, as is the case in children.98
AYA recommendations.
The most important determinant to treatment approach is whether the patient has the “pediatric” subtype of FL. If they do, conservative approaches with complete surgical resection of limited-stage disease and watchful waiting are reasonable. If the pathology indicates a nonpediatric FL, any of the adult approaches can be considered depending on presenting risk factors.
Conclusions
There is clearly a need for trials that span the complete age range of the pediatric and AYA groups (ie, ages 1-39 years). This will permit more accurate age-related comparisons regarding outcome and toxicity. Moreover, these trials should incorporate sophisticated molecular studies (eg, next-generation sequencing) of the lymphoma biopsy samples. This will be particularly important for the DLBCL and ALCL histologic subtypes, where striking age-associated biological differences have already been discovered that have both prognostic and therapeutic design implications. Another important challenge is the refinement of a multidisciplinary approach to diagnosis. Currently, most clinicians attempt to make the diagnosis of NHL with the least invasive approach such as interventional radiological–directed fine-needle biopsy; however, this strategy often does not provide enough tissue for more comprehensive biological characterization of the tumors. This challenge must be addressed in the context of a research trial and may necessitate re-biopsy.
The use of Web sites that review the availability of open clinical trials for AYA patients with NHL should provide AYA patients with better access to novel treatment approaches. It has been suggested that trained navigators would be important advocates for some AYA patients seeking help in reviewing Web site options and in dealing with barriers to contemporary therapy.3 The rapidly increasing knowledge of the complex molecular pathology of NHL in the pediatric and AYA populations, coupled with the rapidly expanding armamentarium of novel biological and targeting agents, will require some degree of subspecialization within the pediatric and adult medical oncology communities.
Acknowledgments
Supported in part by the American Lebanese Syrian Associated Charities (ALSAC) and National Institutes of Health, National Cancer Institute grant CA 21765.
Correspondence
John T. Sandlund, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: john.sandlund@stjude.org.
References
Competing Interests
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.