Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is biologically distinct from its B lymphoblastic (B-ALL) counterpart and shows different kinetic patterns of disease response. Although very similar regimens are used to treat T-ALL and B-ALL, distinctions in response to different elements of therapy have been observed. Similar to B-ALL, the key prognostic determinant in T-ALL is minimal residual disease (MRD) response. Unlike B-ALL, other factors including age, white blood cell count at diagnosis, and genetics of the ALL blasts are not independently prognostic when MRD response is included. Recent insights into T-ALL biology, using modern genomic techniques, have identified a number of recurrent lesions that can be grouped into several targetable pathways, including Notch, Jak/Stat, PI3K/Akt/mTOR, and MAPK. With contemporary chemotherapy, outcomes for de novo T-ALL have steadily improved and now approach those observed in B-ALL, with approximately 85% 5-year event-free survival. Unfortunately, salvage has remained poor, with less than 25% event-free and overall survival rates for relapsed disease. Thus, current efforts are focused on preventing relapse by augmenting therapy for high-risk patients, sparing toxicity in favorable subsets and developing new approaches for the treatment of recurrent disease.
Learning Objectives
Identify unique clinical features of T-ALL that distinguish it from B-lymphoblastic leukemia
Evaluate advances in treatment that have led to outcome improvements in frontline disease
Assess new developments in the biology of T-ALL and potential implications for future therapy
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) represents approximately 12% to 15% of all newly diagnosed ALL cases in pediatric patients and is noteworthy for its unique clinical and biological features. Although historically, outcomes for T-ALL were inferior to those of B lymphoblastic leukemia (B-ALL), with recent advances in therapy, event-free survival (EFS) rates have been steadily improving and now exceed 85% in many contemporary clinical trials (Table 1).1-6 Cure, however, has not come without a cost, as intensive therapy is required. Further, recurrent disease is very difficult to salvage, and relatively few new drugs have been developed for children with resistant disease.
Recent T-ALL trial outcomes
| Study . | Years of accrual . | Population receiving CRT . | Induction steroid . | EFS or DFS; OS . |
|---|---|---|---|---|
| COG AALL04346 | 2007-2014 (n = 1895) | Intermediate and high-risk and CNS disease | Prednisone | 89.3% DFS (4 y) |
| UKALL 20032,5 | 2003-2011 (n = 388) | CNS disease only | Dexamethasone | 81.2% EFS (5 y); 86.4% OS (5 y) |
| DFCI 05-0013,4 | 2005-2010 (n = 97) | All T-ALL patients | Prednisone | 83% EFS (4 y); 89% OS (4 y) |
| AIEOP-BFM ALL 20001 | 2000-2006 (n = 280 PGR) | All T-ALL patients* | Dexamethasone vs prednisone | 87.8% EFS DEX (5 y); 91.4% OS DEX (5 y); 79.2% EFS PRED (5 y); 82.6% OS PRED (5 y) |
| Study . | Years of accrual . | Population receiving CRT . | Induction steroid . | EFS or DFS; OS . |
|---|---|---|---|---|
| COG AALL04346 | 2007-2014 (n = 1895) | Intermediate and high-risk and CNS disease | Prednisone | 89.3% DFS (4 y) |
| UKALL 20032,5 | 2003-2011 (n = 388) | CNS disease only | Dexamethasone | 81.2% EFS (5 y); 86.4% OS (5 y) |
| DFCI 05-0013,4 | 2005-2010 (n = 97) | All T-ALL patients | Prednisone | 83% EFS (4 y); 89% OS (4 y) |
| AIEOP-BFM ALL 20001 | 2000-2006 (n = 280 PGR) | All T-ALL patients* | Dexamethasone vs prednisone | 87.8% EFS DEX (5 y); 91.4% OS DEX (5 y); 79.2% EFS PRED (5 y); 82.6% OS PRED (5 y) |
DEX, dexamethasone; PGR, prednisone good response; PRED, prednisone.
Except AIEOP CNS negative; non-HR T-ALL with WBC < 100 × 109/L.
Prognostic factors and risk classification differ in T-ALL compared with its B-lineage counterpart.7 Several of the clinical variables used to classify risk in patients with B-ALL, including age and presenting white blood cell count (WBC), are not independently prognostic in T-ALL. Many chromosomal translocations leading to aberrant transcription factor expression have been identified in T-ALL; however, the prognostic significance of these findings within the context of contemporary therapy is unclear, and blast cytogenetics features are also not presently used for risk stratification.8-10
Similar to B-ALL, the key prognostic determinant in T-ALL is minimal residual disease (MRD) response at the end of induction and at a second point at the end of the consolidation treatment phase, or its equivalent. The kinetic pattern of MRD response differs from that in B-ALL, with a slower pattern of disease regression in T-lineage disease. Although MRD negativity at the end of induction is favorable, patients with T-ALL who are MRD-positive at the end of induction but achieve MRD negativity by the end of consolidation (week 12) have very favorable outcomes (7-year EFS, 80.6% ± 2.3%) with conventional chemotherapy.11 Another distinctive feature of T-ALL is its immunophenotypic diversity, as exemplified by the recently described subtype of early T-cell precursor (ETP) ALL, in which blasts are characterized by lack of expression of CD1a and CD8, weak CD5 expression, and expression of 1 or more myeloid or stem cell markers.12 This review summarizes recent developments in the treatment and biology of T-ALL in pediatric patients.
Treatment approaches for T-ALL
Patients with newly diagnosed T-ALL are typically treated with risk-based multiagent chemotherapy regimens for 2 to 3 years, with or without cranial radiation therapy (CRT). Although there are differences between the treatment regimens studied in a number of international consortia, overall outcomes are similar. The most significant predictor of outcome is end-of-consolidation (or the equivalent point) MRD, and treatment allocation is commonly directed by MRD response. The current risk classification schema for T-ALL used within the Children’s Oncology Group (COG) is shown in Table 2. Varying strategies for central nervous system (CNS)-directed therapy are currently used, ranging from the elimination of CRT in all patients to selected application in higher-risk subsets. Given the dismal prognosis for recurrent disease, there have been ongoing efforts to optimize the use of conventional agents, including dexamethasone, asparaginase, intrathecal chemotherapy, and methotrexate (MTX), and some treating groups are also investigating new agents in the frontline setting. The current COG phase 3 trial for T-ALL uses dexamethasone exclusively and increases the number of doses of asparaginase compared with prior studies based on the success of this approach in UKALL trials. The use of CRT is limited to the 10% to 15% of patients with either CNS disease or persistent MRD positivity, and patients are randomly assigned to the addition of bortezomib during the induction and delayed intensification phases of therapy. Allogeneic hematopoietic cell transplantation is not routinely used in first remission but is considered for patients with persistent MRD at later times in treatment.
Risk group definitions
| Risk . | COG T-ALL risk group definitions . |
|---|---|
| Standard risk | M1 day 29 marrow; Day 29 MRD* <0.01%; CNS 1; no testicular disease; no steroid pretreatment |
| Intermediate risk | M1 or M2 day 29 marrow; day 29 MRD ≥ 0.01%; end-of-consolidation MRD < 0.1%; any CNS and testicular disease status; any steroid pretreatment status |
| Very high risk | M3 day 29 marrow or end-of-consolidation MRD ≥0.1% |
| Risk . | COG T-ALL risk group definitions . |
|---|---|
| Standard risk | M1 day 29 marrow; Day 29 MRD* <0.01%; CNS 1; no testicular disease; no steroid pretreatment |
| Intermediate risk | M1 or M2 day 29 marrow; day 29 MRD ≥ 0.01%; end-of-consolidation MRD < 0.1%; any CNS and testicular disease status; any steroid pretreatment status |
| Very high risk | M3 day 29 marrow or end-of-consolidation MRD ≥0.1% |
M1 marrow, less than 5% blasts; M2 marrow, 5-25% blasts; M3 marrow, more than 25% blasts by morphology; CNS1, less than 5 WBC/µL and no blasts in the CSF.
Central flow cytometry-based MRD.
Drugs of Interest for T-ALL
| Target . | Type of drug . | Agents . |
|---|---|---|
| Notch49 | γ-secretase inhibitors | RO4929097, BMS906024, PF03084014, LY3039478, MK0752 |
| Monoclonal antibodies (anti-Notch1, anti-Notch2, anti-DLL4) | OMP52M51, OMP59R5, REGN421 | |
| Soluble notch proteins | Preclinical only | |
| Mastermind inhibiting peptides | Preclinical only | |
| PI3K/Akt/mTOR51 | PI3K inhibitors | BYL719, idelasib, GSK2636771, BKM120, BAY80-6946, IPI145, TGR1202, AMG319, SAR260301 |
| Rapalogs (mTOR inhibitors) | Sirolimus, everolimus, temsirolimus, ridaforolimus | |
| PI3K/mTOR inhibitors | BEZ235, GDC0980, VS5584, SAR245409 | |
| Akt inhibitors | MK2206, GSK2110183 | |
| mTORC1/2 inhibitors | OSI027, DS-3078a, CC223 | |
| Jak/Stat61 | Jak1/2 | Ruxolitinib, momelotinib |
| Jak 2 inhibitors | Fedratinib, pacritinib, BB594 | |
| Stat inhibitors | C1889, pimozide, S31201, STA21 | |
| MAPK62 | MEK inhibitors | Trametinib, pimsertib, cobimetinib, selumetinib |
| Farnesyl transferase inhibitors | Tipifarnib | |
| Cell cycle Machinery63 | CDK4/6 inhibitors | Palbociclib, ribociclib, abemaciclib |
| Pan-CDK inhibitors | Flavopiridol, dinaciclib, AT7519 | |
| Proteasome57 | Proteasome inhibitors | Bortezomib, carfilzomib, ixazomib |
| Deubiquinating enzymes | Preclinical only | |
| Neddylation inhibitors | MLN49243 | |
| E3 ubiquitin ligase inhibitors | Preclinical only | |
| Epigenetic58 | HDAC inhibitors | Vorinostat, romidepsin, SAHA |
| DNA methyltransferase inhibitors | 5-azacitidine, decitabine | |
| IDH1/2 inhibitors | AGI6780, AGI5198, AG221 | |
| BRD4 inhibitors | JQ1, OTX015 | |
| DOT1L inhibitors | EPZ004777, EPZ5676 | |
| Immunotherapy59 | Monoclonal antibodies | Daratumomab, basiliximab, alemtuzumab |
| BiTEs, CARs | Preclinical only |
| Target . | Type of drug . | Agents . |
|---|---|---|
| Notch49 | γ-secretase inhibitors | RO4929097, BMS906024, PF03084014, LY3039478, MK0752 |
| Monoclonal antibodies (anti-Notch1, anti-Notch2, anti-DLL4) | OMP52M51, OMP59R5, REGN421 | |
| Soluble notch proteins | Preclinical only | |
| Mastermind inhibiting peptides | Preclinical only | |
| PI3K/Akt/mTOR51 | PI3K inhibitors | BYL719, idelasib, GSK2636771, BKM120, BAY80-6946, IPI145, TGR1202, AMG319, SAR260301 |
| Rapalogs (mTOR inhibitors) | Sirolimus, everolimus, temsirolimus, ridaforolimus | |
| PI3K/mTOR inhibitors | BEZ235, GDC0980, VS5584, SAR245409 | |
| Akt inhibitors | MK2206, GSK2110183 | |
| mTORC1/2 inhibitors | OSI027, DS-3078a, CC223 | |
| Jak/Stat61 | Jak1/2 | Ruxolitinib, momelotinib |
| Jak 2 inhibitors | Fedratinib, pacritinib, BB594 | |
| Stat inhibitors | C1889, pimozide, S31201, STA21 | |
| MAPK62 | MEK inhibitors | Trametinib, pimsertib, cobimetinib, selumetinib |
| Farnesyl transferase inhibitors | Tipifarnib | |
| Cell cycle Machinery63 | CDK4/6 inhibitors | Palbociclib, ribociclib, abemaciclib |
| Pan-CDK inhibitors | Flavopiridol, dinaciclib, AT7519 | |
| Proteasome57 | Proteasome inhibitors | Bortezomib, carfilzomib, ixazomib |
| Deubiquinating enzymes | Preclinical only | |
| Neddylation inhibitors | MLN49243 | |
| E3 ubiquitin ligase inhibitors | Preclinical only | |
| Epigenetic58 | HDAC inhibitors | Vorinostat, romidepsin, SAHA |
| DNA methyltransferase inhibitors | 5-azacitidine, decitabine | |
| IDH1/2 inhibitors | AGI6780, AGI5198, AG221 | |
| BRD4 inhibitors | JQ1, OTX015 | |
| DOT1L inhibitors | EPZ004777, EPZ5676 | |
| Immunotherapy59 | Monoclonal antibodies | Daratumomab, basiliximab, alemtuzumab |
| BiTEs, CARs | Preclinical only |
Recent clinical observations
Dexamethasone reduces rates of relapse
Several recent clinical trials in B- and T-lineage ALL have compared dexamethasone vs prednisone during the induction phase of treatment.1,13-16 The rationale for using dexamethasone includes greater potency and CNS penetration, which is appealing in T-ALL, given the higher rates of CNS disease. This has been counterbalanced, however, by higher rates of infectious toxicity with dexamethasone-based treatment regimens.17 Several recent trials have supported the use of dexamethasone in T-ALL.1,5,15 On the UK ALL97 and ALL97/99 protocols, patients randomly assigned to receive dexamethasone vs prednisone had half the risk of an isolated CNS relapse.18 Excellent outcomes were observed in patients with T-ALL on the successor UKALL 2003 trial with reported 5-year EFS of 81.2% and a 3.5% risk for isolated CNS relapse.2,5 All patients on this trial received dexamethasone as the sole corticosteroid formulation throughout therapy, and toxicity was acceptable using a dexamethasone dose of 6 mg/m2/day (maximum dose, 10 mg) for 28 days during induction. Additional support for dexamethasone in T-ALL comes from the AEIOP-BFM 2000 trial, in which replacement of prednisone (60 mg/m2/day) with dexamethasone (10 mg/m2/day) during induction in patients with T-ALL and prednisone good responses resulted in a one third reduction in relapse rates from 17% to 7% (hazard ratio, 0.4) and significant improvements in both EFS (dexamethasone, 87.8% ± 2.8% vs, prednisone 79.2% ± 3.4%; P=.037) and OS (dexamethasone, 91.4% ± 2.4% vs prednisone, 82.6% ± 3.2%; P=.036).1 Notably, however, on this trial, the benefits of dexamethasone were counterbalanced by higher rates of toxicity and treatment-related mortality, especially among patients aged 10 years or older. The survival benefits of dexamethasone were also not observed in prednisone poor responders. In an ongoing phase 3 trial for T-ALL, COG is presently using dexamethasone exclusively, and several other treating consortia have adopted a similar approach in T-ALL, with careful monitoring for infectious toxicity.
CNS radiotherapy is not needed for most patients with T-ALL
Given the higher rates of neurocognitive sequelae, endocrinopathies, and secondary malignancies associated with CRT, there have been concerted efforts to minimize its use in pediatric ALL treatment protocols. Several studies have now shown that CRT can be successfully eliminated from regimens that contain intensive systemic and intrathecal chemotherapy in some patients with T-ALL.19,20 The European Organization for the Research and Treatment of Cancer has eliminated CRT in all patients with T-ALL, including those with CNS disease, in its recent studies and has adopted regimens with an intensified schedule of high-dose MTX (HD MTX) and triple intrathecal chemotherapy (MTX, cytarabine, and hydrocortisone). With this approach, isolated and overall CNS relapse rates of 5.3% to 8.5% were observed on study 58951.21 St. Jude Children’s Research Hospital reported CNS relapses in 6 (7.9%) of 76 patients with T-ALL with therapy that included intensified intrathecal chemotherapy and asparaginase, HD MTX, and dexamethasone with elimination of CRT for all patients; a rate of CNS relapse that was equivalent to that observed on the predecessor study, in which CRT was used in selected high-risk patients with T-ALL.22 Excellent outcomes with 5-year EFS of 81.2% and low rates of isolated CNS relapse (3.5%) were also reported for T-ALL on the UKALL 2003 trial with a regimen that did not include either HD MTX or prophylactic CRT.2 Although the COG has previously administered CRT to the vast majority of patients with T-ALL, the current phase 3 trial that is underway reserves CRT for the 10% to 15% of patients with CNS disease at diagnosis or persistent end-of-consolidation MRD within a regimen that includes both dexamethasone and HD MTX. Other treating groups have also selectively recommended CRT for these high-risk subsets, while eliminating its use in other populations. There is still heterogeneity in approaches to CNS-directed therapy in T-ALL, and this, coupled with the challenges in treating relapsed disease, makes definitive conclusions about the optimal strategy a challenge. However, current data do suggest CRT can be safely eliminated for most patients with T-ALL.
Response-based T-ALL therapy is successful for the majority of patients with ETP ALL
T-ALL can be divided into categories on the basis of the corresponding stage of intrathymic differentiation. The European Group for the Immunologic Classification of Leukemia proposed a breakdown of T-ALL into pro-T (cCD3+, sCD3−, CD1a−, CD2+, CD5−, CD7+, CD34−), pre-T/immature (cCD3+, sCD3−, CD1a−, CD2+, CD5+, CD7+, CD34−), cortical T (cCD3+, sCD3+/−, CD1a+, CD2+, CD5+, CD7+, CD34−), and mature-T (cCD3+, sCD3+, CD1a− CD2+, CD5+, CD7+, CD34−).23 The prognostic value of the maturational stage of the dominant clone differs between studies. Early studies suggested cortical T-ALL has the best prognosis and early stages have worse outcomes, but developmental stage loses prognostic significance with modern chemotherapy and MRD-based risk stratification.
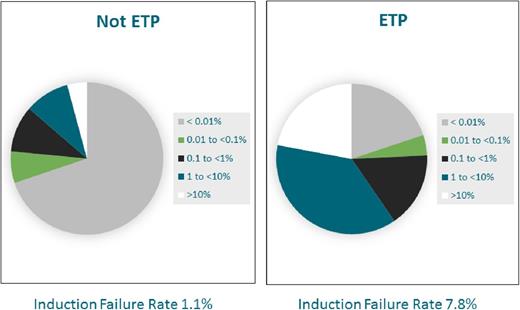
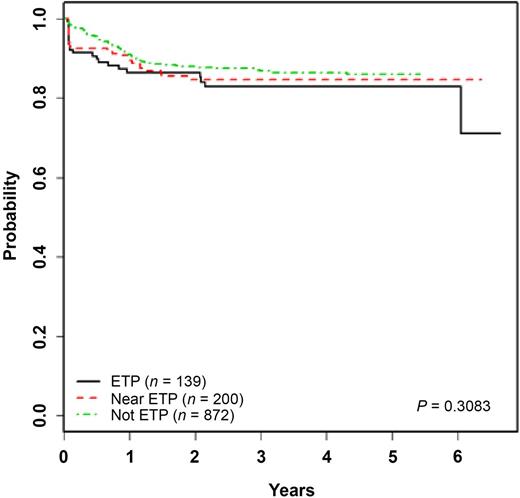
ETP ALL is a recently defined T-ALL subtype characterized by a unique immunophenotype (cCD3+, sCD3−, CD1a−, CD2+, CD5 dim [< 75%+], CD7+, and positivity for stem cell and/or myeloid markers, including HLA-DR, CD13, CD33, CD34, or CD117), representing approximately 15% of cases. Although initial reports demonstrated unfavorable outcomes,12,24,25 other groups have reported outcomes similar to those observed in non-ETP T-ALL.26,27 The kinetic pattern of disease response in ETP ALL is distinctive, with significantly higher end induction MRD burdens and rates of induction failure (>25% blasts by morphology at end induction) (Figure 1).27,28 The COG AALL0434 treatment study accrued 1144 patients from 2007 to 2014, 11.3% of whom were categorized as ETP ALL by flow cytometry.27 Induction failure rates were 7.8% in patients with ETP ALL compared with 1.1% in patients with T-ALL without ETP, and 81.4% of patients with ETP ALL had end-induction MRD higher than 0.01% by flow cytometry. Yet, despite this, outcomes were comparable to non-ETP T-ALL when using contemporary response-based treatment regimens with 5-year EFS and OS rates for ETP (87.0% and 93.0%, data cutoff 29 April 2014), near-ETP (ETP but with elevated CD5; 84.4% and 91.6%), and non-ETP ALL patients (86.9% and 92.0%), respectively (Figure 2).27 Similar findings have been reported among the 16% of patients with T-ALL categorized as ETP on the UKALL 2003 trial.26 Patients with ETP ALL on that trial had an intermediate outcome that was nonsignificantly inferior to that in other patients with T-ALL without the ETP phenotype. Five-year EFS was 76.7% (ETP) vs 84.6% (non-ETP), and OS was 82.4% (ETP) vs 90.9% (non-ETP; P = .1). ETP ALL is a very unique subtype of T-ALL with similarities to T-myeloid leukemia and a lower frequency of classical T-ALL genetic alterations. Genomic profiling may provide insights for refinement in therapy in the future, but current data suggest treatment with a contemporary response-based T-ALL regimen is successful for the majority of patients.
Day 29 (end induction) flow cytometry–based MRD response in ETP vs non-ETP ALL. The distribution of central flow cytometry–based MRD response at the end of induction in children and adolescents treated on COG study AALL0434 without ETP ALL are compared with MRD responses in the ETP ALL subset.
Day 29 (end induction) flow cytometry–based MRD response in ETP vs non-ETP ALL. The distribution of central flow cytometry–based MRD response at the end of induction in children and adolescents treated on COG study AALL0434 without ETP ALL are compared with MRD responses in the ETP ALL subset.
EFS in ETP vs non-ETP ALL. Five-year EFS probabilities for patients treated on COG trial AALL0434 are depicted for ETP, near ETP, and T-ALL without an ETP phenotype (data cutoff 19 November 2014).
EFS in ETP vs non-ETP ALL. Five-year EFS probabilities for patients treated on COG trial AALL0434 are depicted for ETP, near ETP, and T-ALL without an ETP phenotype (data cutoff 19 November 2014).
A regimen containing escalating MTX with asparaginase vs high-dose MTX was superior in T-ALL
HD MTX (5 g/m2/dose) has been a successful element of ALL protocols for decades. The effect of HD MTX on particular subgroups of patients with ALL, however, has been an area of ongoing investigation. The COG recently reported superior outcomes in a regimen containing dexamethasone during induction and HD MTX in interim maintenance in children younger than 10 years with newly diagnosed B-ALL,14 and the Pediatric Oncology Group study 9404 also showed a benefit for HD MTX in T-ALL.29 The recently completed COG AALL0434 trial for T-ALL randomized patients aged 1 to 31 years to therapy that included HD MTX vs escalating MTX plus 2 doses of pegylated (PEG)-asparaginase in the interim maintenance phase of therapy, as well as a randomization to nelarabine.6 Although the results of the nelarabine randomization remain blinded, 4-year disease-free survival (DFS) rates were 89.3% ± 1.5% overall and 92.5% ± 1.8% for the escalating MTX regimen vs 86.1% ± 2.4% for the HD MTX regimen (P = .0173). Of note, the timing of administration of prophylactic CRT was different between the groups and was delivered at week 4 in the escalating MTX group vs week 26 in the HD MTX group. Further, 2 additional doses of PEG-asparaginase were administered in the escalating MTX group. Although all these differences in the randomized regimens could have also contributed to the superiority of the escalating MTX group, these results further highlight unique differences in response to elements of therapy in T- vs B-lineage ALL.
Nelarabine can be safely administered in newly diagnosed T-ALL
Given the dismal salvage rates for recurrent T-ALL, efforts have been underway to optimize frontline treatment strategies for high-risk patients. Nelarabine is a purine nucleoside analog prodrug of AraG, which is cytotoxic to T-lymphoblasts at micromolar concentrations. Nelarabine has demonstrated promising single-agent activity in T-ALL, with a 55% response rate in relapsed/refractory T-ALL, providing the rationale for investigating the use of this agent in higher-risk frontline T-ALL.30 On the COG AALL0434 study, patients were randomly assigned to receive 5-day courses of nelarabine in 6 cassettes in the combination with an augmented BFM regimen with single interim maintenance and delayed intensification phases of treatment. During the initial safety phase, 94 patients received this therapy with no heightened risk for neurological or other toxicities.31 Although any outcome effect is still being determined, administration of nelarabine cassettes (650 mg/m2/day for 5 days) was feasible within the context of augmented BFM-based chemotherapy and establishes a paradigm for moving T-cell–directed therapies into the frontline.
Relapsed disease
Most T-ALL disease recurrences occur within 2 years of diagnosis, and relapsed disease remains very difficult to salvage, with survival rates lower than 25%.32 At this time, hematopoietic cell transplantation is the only curative treatment, but successful remission reinduction is a prerequisite, and this has remained another significant challenge. Historical reinduction remission rates in T-ALL are estimated to be 30% to 40%. On the COG AALL01P2 trial, only 2 of 7 patients with relapsed T-ALL achieved a second remission (CR2) with an intensive 3-block reinduction regimen.33
There has been great enthusiasm for investigating novel agents in recurrent disease (Table 3). The promising single-agent activity of nelarabine in relapsed T-ALL prompted combination studies with cyclophosphamide and etoposide. Remission rates of 44% were achieved in pediatric patients.34 The COG recently completed the AALL07P1 trial, in which the proteasome inhibitor bortezomib was combined with a 4-drug reinduction platform in B- and T-lineage relapsed ALL. Results for patients with T-ALL were promising, with CR2 rates of 68%.35 This provided the rationale for investigating bortezomib in the current COG frontline phase 3 T-ALL trial. As more insight into the biology of T-ALL is gained, several other new therapies are being pursued. In contrast to the dismal outcomes and early recurrence patterns that are most commonly observed in T-ALL, a subset of patients appears to develop late marrow relapses, which are associated with more favorable outcomes. Studies have demonstrated unique molecular profiles at the time of disease recurrence in these cases that differ from those observed at diagnosis, suggesting these late recurrences may be second leukemias.36
Recent discoveries in the biology of T-ALL
T-ALL genomics
Unlike many lesions found in patients with B-ALL and other hematologic malignancies, the majority of genetic alterations that have been identified do not independently predict outcome in T-ALL.37 At this time, response to treatment, primarily by assessment of MRD, is the strongest predictor of outcome for patients with T-ALL. Nevertheless, the majority of relapses occur in patients predicted to do well based on a favorable MRD response. Thus, new insights into T-ALL biology are needed to improve risk stratification and alter therapy.
T-ALL arises from malignant transformation of T-cell precursors. The spectrum of genetic abnormalities in T-ALL is heterogeneous and diverse.38,39 Despite this significant heterogeneity, most lesions can be classified into 1 of 2 categories: chromosomal translocations that are associated with distinct gene-expression signatures, and mutations and deletions that affect signaling and/or the cell cycle. Approximately 50% of blasts of patients with T-ALL have identified chromosomal translocations that can be grouped on the basis of biology.39 One subgroup of chromosomal abnormalities includes rearrangements of proto-oncogenes to the T-cell receptor (TCR), leading to overexpression of the proto-oncogene. These proto-oncogenes include TLX1 (HOX11), MEF2C, HOXA, LMO1, LMO2, TAL1, and TAL1.40 Some of these are more common than others, with rearrangements of LMO1, LM02, or TAL1 to TCRD occurring in ∼9% of patients, TLX1 to TCRD or TCRB in ∼10% of patients, and HOXA to TCRB in ∼5% of patients.38,39 A second subgroup includes rearrangements of 2 transcription factor genes, resulting in an abnormal fusion transcription factor protein. These include PCIALM-MLLT10, STIL-TAL1, TLX3-BCL11B, and NUP214-ABL1, which affect ∼8%, ∼20%, ∼15%, and less than 5% of patients, respectively.41 Moreover, a large number of rare fusions have been identified that affect a very small subset of patients such as EML-ABL1 and SET-NUP214.41 Finally, ∼5% to 10% of patients with T-ALL have MLL gene rearrangements. None of these fusions has been shown to predict outcome consistently and independently from end-of-consolidation MRD.
A large number of mutations and deletions have been described in T-ALL by several independent groups, and although they are heterogeneous, these also can be grouped according to biologic mechanism. The TARGET group recently confirmed the heterogeneity of lesions in T-ALL through extensive genomic analysis of 264 cases of T-ALL, including whole-exome sequencing, copy number analysis, and RNA sequencing of tumor RNA. They identified more than 170 potential genes that are oncogenic drivers.42 Many of these were known, including NOTCH1, PHF6, FBXW7, USP7, PTHEN, DNM2, and BCL11B, and others were novel in T-ALL, including CCND3, MYB, CTCF, MED12, SMARCA4, CREBBP, and USP9X. Many of these alternations can be grouped on the basis of dysregulated signaling pathways, including Notch, PI3K/Akt/mTOR, MAPK, and Jak/Stat.
The most commonly mutated genes in T-ALL are CDKN2A/B (65%-70% of cases).40 Other cell cycle and tumor suppressor genes commonly mutated in T-ALL include CDKN1B (p27kip1) and RB1. Other common mutations in T-ALL include mutations in WT1 (10%-15% of T-ALL), which are typically associated with rearrangements in TLX1, TLX3, and HOXA,40 and inactivating mutations in PHF6 (15%-30% of T-ALL), which only occur in males and commonly associate with TLX1 and TLX3.40 Biallelic TCRy deletion is a recently described alteration that may portend poor outcome, but additional studies are needed to validate prognostic significance.43
Differences in mutational spectrum and biologic pathway dysregulation do appear to vary according to the maturational stage of the dominant clone of T-ALL blasts. ETP ALL, for example, has a different genomic profile than non-ETP T-ALL. Most genomic abnormalities in ETP ALL can be categorized in 3 groups: hematopoietic development (IKZF1, ETV6, RUNX1, GATA3, EP300), MAPK, and cytokine receptor signaling (NRAS, KRAS, IL7R, JAK1, JAK3, PTPN11, NF1, SH2B3) and chromatin-modifying genes (EED, EZH2, SUZ12, and SETD2).44
Clonal evolution
Approximately 20% of patients with T-ALL will relapse, and the prognosis for relapsed T-ALL is poor. Genome-wide analyses comparing the genetic makeup of major and minor leukemic clones present at diagnosis with those at relapse are providing important insights into the biology of relapsed ALL. Relapsed clones tend to be enriched with genes that infer resistance to conventional cytotoxic chemotherapy, including NT5C2 and MSH6; alter a number of signaling pathways, such as MAPK and Jak/Stat; are involved in epigenetic modification; or function in cell cycle regulation.44,45 Often the dominant clone at relapse was a minor clone at diagnosis, harboring genetic alterations that made it chemo refractory. In contrast, in some cases, the accumulation of genetic alterations leading to relapse occurs after initiation of treatment. Understanding clonal evolution is important, as therapy may be tailored to prevent the emergence of drug-resistant clones or target clones at relapse. For example, Tzoneva and colleagues demonstrated that 19% of relapsed T-ALL blasts have mutations in NT5C2, which lead to resistance to nucleoside analogs, including mercaptopurine and thioguanine.46 The majority of T-ALL relapses occur during maintenance, and mercaptopurine is one of the key agents used in maintenance. If a patient was found to have a minor clone with NT5C2m before relapse, in theory, an alternative maintenance regimen less dependent on mercaptopurine could be used to prevent relapse. Jones and colleagues recently established that MAPK alterations are more common at relapse than diagnosis.47 Moreover, they demonstrated that these MAPK alternations lead to corticosteroid resistance and that MAPK inhibitors can restore corticosteroid sensitivity in preclinical models. These data suggest MAPK inhibition may be therapeutically relevant in relapsed ALL.
Notch signaling
Constitutive activation of Notch signaling is the most common abnormality in T-ALL.48 Notch is an evolutionally conserved signaling pathway that has important roles in hematopoiesis, angiogenesis, cell proliferation, apoptosis, and T-cell development. Notch signaling is mediated through a pathway of 4 transmembrane Notch receptors (Notch1-Notch4).49 After ligand binding, Notch receptors are cleaved twice: first by a metalloprotease and subsequently by γ-secretase. After cleavage, the intracellular domain of Notch translocates into the nucleus and activates transcription of a key number of proteins. Notch signaling can be activated in T-ALL by mutations in NOTCH1 (>50% of cases), FBXW7 (15%), or chromosomal translocation t(7;9)(q34;q34.3) (<1%), which juxtaposes NOTCH1 and TCRB.49 Notch signaling can also be activated secondary to alterations in other signaling pathways, including PI3K/Akt/mTOR and c-myc.49 Conversely, activated Notch signaling regulates PI3K/Akt/mTOR, c-myc, and NF-kβ.49 Notch mutations are more common in mature T-ALLs and are relatively rare in ETP ALL.44
As Notch signaling is commonly dysregulated in T-ALL, a large number of preclinical studies and clinical trials have investigated the efficacy of targeting Notch in T-ALL, most commonly with γ-secretase inhibitors (GSIs). GSIs block the second cleavage of Notch, preventing its ability to activate transcription.48 Preclinical studies of GSIs demonstrated single-agent activity and synergy with a number of cytotoxic chemotherapeutics. Unfortunately, they have not translated successfully into the clinic. Early trials delivered GSIs as monotherapy at high doses for short duration, which led to significant gastrointestinal toxicity from goblet cell hyperplasia.49 Preclinical studies suggest that alternate doses and schedules may mitigate GI toxicity.49 Moreover, Real and colleagues demonstrated that corticosteroids can ameliorate GSI-induced intestinal toxicity through transcriptional upregulation of cyclin D2, which prevented goblet cell accumulation from GSIs.50 Targeting Notch may also reverse corticosteroid resistance in T-ALL through induction of glucocorticoid receptor expression, making a combination of corticosteroids and Notch inhibition particularly attractive.50 Despite a compelling biologic rationale and the ability to avoid GI toxicity, the use of GSIs for T-ALL has stalled based on the lack of efficacy of GSIs in more common adult solid tumors. Alternative approaches in various stages of preclinical and clinical development include monoclonal antibodies against Notch ligand or receptors, soluble Notch proteins that block Notch signaling, and Mastermind-inhibiting peptides that block the interaction of Mastermind-like1 with Notch.49
PI3K/Akt/mTOR signaling
PI3K/Akt/mTOR signaling is frequently activated in T-ALL. Most commonly, this occurs from inactivation of PTEN, either from PTEN mutations or deletions or defects in other signaling pathways that alter PTEN transcription or translation.51 PTEN loss results in activation of Akt. In addition, PI3K/Akt/mTOR can be activated directly by mutations in AKT1, PI3KCA, PI3KR1, and IL7R, or indirectly from abnormalities in Jak/Stat, Notch, or MAPK.51 Targeting the PI3K/Akt/mTOR signaling pathway has been investigated extensively in preclinical models of T-ALL.51 Initial studies focused on targeting mTOR with rapalogs, demonstrating single-agent efficacy and synergistic benefit when combined with conventional cytotoxics.51 mTOR inhibitors have also been shown to reverse corticosteroid resistance in T-ALL.51 No signaling inhibitor is likely to provide significant benefit as monotherapy in ALL. Thus, it is essential to understand the effects of combination therapy. Treatment with mTOR inhibitors leads to compensatory upregulation of feedback loops in the PI3K/Akt/mTOR signaling pathway, which reduces long-term efficacy.51 This has led to the development of multiple additional drugs targeting the PI3K/Akt/mTOR signaling pathway, including PI3K/mTOR dual inhibitors, mTORC1/2 dual inhibitors, PI3K inhibitors, and Akt inhibitors, and has also led to studies combining mTOR inhibitors with drugs that inhibit other pathways, including Notch and Jak/Stat.51 The majority of these studies have demonstrated increased efficacy over targeting mTOR alone. According to the preclinical efficacy data, a number of early phase trials are currently opened or have recently been completed that test PI3K/Akt/mTOR inhibitors alone or in combination with conventional cytotoxics in a number of hematologic malignancies. Despite the inclusion of patients with relapsed or refractory T-ALL in many of these trials, the rarity of this population has led to slow accrual and a paucity of published results.
Jak/Stat and MAPK signaling
Jak/Stat pathway alterations are also common in T-ALL, especially in the ETP phenotype.52 These alterations can be from activating mutations in a number of genes, including JAK1, IL7Ra, JAK3, STAT5, deletions in PTPN2, or TEL-JAK2 fusions.53 Jak/Stat signaling can also be activated by aberrant signaling through PI3K/Akt/mTOR. Recent data suggest that targeting Jak/Stat may be attractive in ETP ALL in particular, as the pathway is commonly dysregulated in ETP ALL and significant single-agent efficacy was seen in preclinical models of ETP ALL, using the JAK1/2 inhibitor ruxolitinib.54 Although less common than the other pathways mentioned, a reasonable percentage of patients with T-ALL have MAPK alterations, including KRAS, NRAS, FLT3, and BRAF mutations.38 Similar to Jak/Stat alternations, MAPK alternations are more common in ETP ALL than non-ETP ALL. A number of studies have demonstrated that MAPK mutations are enriched in relapsed clones in patients with a number of malignancies, including B-ALL; however, published studies of clonal evolution in T-ALL are scant.55 MEK inhibitors and farnesyl transferase inhibitors have demonstrated some efficacy in preclinical studies of T-ALL with MAPK alterations.38,39 Other MAPK inhibitors, including FLT3 and RAF inhibitors, have not been studied.
D-Type cyclins and cyclin-dependent kinases
D-type cyclins (cyclin D1, D2, and D3) are important in hematopoiesis and leukemogenesis.48 D-type cyclins bind to and activate cyclin-dependent kinases 4 and 6 (CDK4/6), forming cyclin D–CDK complexes that promote cell cycle progression through phosphorylation of the retinoblastoma protein and interactions with cell cycle inhibitors, such as p16 and p15.48 Cyclin D3, in particular, has been demonstrated to be important in the development and expansion of normal T lymphocytes. Cyclin D3 is commonly overexpressed in T-ALL and is essential for disease initiation and progression.56 Accordingly, drugs that target D-type cyclins and/or cyclin-dependent kinases are under investigation. Early data demonstrate they are active in preclinical models of T-ALL,56 leading to integration in early-phase clinical trials.
Proteasome inhibition
The ubiquitin-proteasome pathway (UPP) is the principle mechanism of protein degradation in cells.57 The UPP is a key driver in regulating the cell cycle, proliferation, and apoptosis. Important regulatory proteins affected by inhibition of the UPP system include NF-κβ, p53, Bax, p27, and p21. In nonproliferative cells, the inhibitor protein Iκβ sequesters NF-κβ in the cytoplasm.57 Under stress, Iκβ is ubiquinated and targeted for degradation, leaving NF-κβ free to dimerize and translocate to the nucleus, where it acts as a transcription factor. Constitutive activation of NF-κβ has been shown to occur in numerous hematologic malignancies, including T-ALL.57 In T-ALL, abnormal activation can occur directly from NF-κβ gene amplification or NF-κβ chromosomal rearrangements, or indirectly as a consequence of Notch or Akt activation.57 On the basis of these data, targeting the proteasome has been of considerable interest in T-ALL. A number of proteasome inhibitors are in different stages of preclinical and clinical development. The best-studied agent is bortezomib, which has been shown to have single-agent activity in T-ALL, to synergize with conventional cytotoxics, and to reverse corticosteroid resistance.57 Moreover, it was found to be effective in early-phase clinical trials in relapsed B- and T-ALL, leading to its incorporation into a randomized phase 3 trial in de novo T-ALL through COG.35
Epigenetic changes
A number of recent genomic studies have identified recurrent lesions in genes involved in DNA methylation (DNMT3A, DNMT3B, TET1, IDH1, IDH2), histone methylation (EZH2, SUZ12, MLL1, MLL2, DOT1L, SETD2, EED, JARID2, UTX, JMJD3, NSD2), and histone acetylation (CREBBP, EP300, HDAC7, HDAC5, NCOA3) in T-ALL.58 Moreover, epigenetic changes may correlate with poor outcome and chemoresistance. For example, low levels of global DNA methylation may portend high risk and poor outcome in T-ALL.58 In B-ALL, studies comparing diagnostic and relapsed blasts have identified relapse-specific alterations in DNA-promoter methylation and histone modification that lead to chemotherapy resistance and can be reversed with treatment with epigenetic agents in preclinical models.58 Studies are needed to determine whether the same alterations are relevant in T-ALL. Based on the high frequency of epigenetic alterations in T-ALL, a number of epigenetic modifying agents have been studied in preclinical models, including DNA methyltransferase inhibitors, HDAC inhibitors, IDH1 and IDH2 mutant inhibitors, BRD4 inhibitors, and DOT1L inhibitors.58
Immunotherapy
During the last few years, immunotherapeutic approaches using monoclonal antibodies, bispecific T-cell–engaging (BiTEs) antibodies, and chimeric antigen receptor (CAR)-modified T cells have transformed the treatment of hematologic malignancies, including B-ALL.59 Despite the development of effective therapies using all of these approaches for B-ALL, few immunotherapies have been developed or investigated in T-ALL. Two principle problems exist with immunotherapies for T-ALL. The first is identifying a target unique to T-ALL blasts that is not present on normal T lymphocytes, as the toxicity of prolonged T-cell depletion is not trivial. This is potentially less of an issue with monoclonal antibodies than BiTEs or CAR T cells. Nevertheless, the only monoclonal antibody studied in T-ALL in clinical trials is alemtuzumab (Campath, anti-CD52), and those trials ended because of infectious toxicity.59 Alternative monoclonals that may warrant investigation include daratumumab (anti-CD38) and basiliximab (anti-CD25), as the majority of T-ALL blasts express both of these surface markers, but neither of these has been studied extensively in T-ALL (Brent Wood, personal communication, unpublished data). An approach to reduce toxicity with CAR T cells for T-ALL is the development of “off-switches” such as suicide genes that could eliminate the immunotherapy and allow for T cell recovery. The second issue hindering the development of BiTEs and CARs for T-cell malignancies is fratricide, meaning the T-cell product attacks and destroys itself. Despite this theoretical risk, preclinical studies using a CD5-directed CAR T cell for T-ALL had only limited fratricide and had sufficient proliferative capacity to be effective in mouse models.60
Conclusions
With recent advances in treatment, including refinement in corticosteroid, asparaginase, and CNS-directed therapy, outcomes for T-ALL have improved significantly and now approach those observed in B-lineage disease. The therapy needed to achieve cure, however, is intensive, with risks for acute and late toxicities, and there has been limited success in treating relapsed disease. In recent years, there have been remarkable discoveries in underlying T-ALL biology, which are envisioned to lead to further advances in therapy in the near future.
Correspondence
Elizabeth A. Raetz, University of Utah, Primary Children’s Hospital, 100 N. Mario Capecchi Dr, Salt Lake City, UT 84113; e-mail: elizabeth.raetz@hci.utah.edu.
References
Competing Interests
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.