Abstract
When a pregnant or postpartum woman presents with sudden and severe microangiopathic hemolytic anemia (MAHA) and thrombocytopenia, three syndromes that require urgent care must be considered: (1) preeclampsia with severe features/hemolysis, elevated liver function tests, low platelets (PE/HELLP) syndrome; (2) thrombotic thrombocytopenic purpura (TTP); and (3) complement-mediated thrombotic microangiopathy (C-TMA; also referred to as atypical hemolytic–uremic syndrome). The distinction among these three syndromes is often unclear because they share multiple clinical features. Overlap between PE/HELLP syndrome and the other two syndromes is also apparent from the fact that pregnancy can be a trigger for both TTP and C-TMA both before and after delivery and also the increased frequency of PE/HELLP syndrome in women who have recovered from TTP. When diagnostic criteria for PE/HELLP syndrome are present, management of hypertension and delivery is curative. Absence of improvement or actual progression of MAHA, thrombocytopenia, and kidney function abnormalities after delivery requires consideration of TTP and C-TMA. Minimal kidney involvement with severe thrombocytopenia suggests TTP and the need for treatment with plasma exchange; progressive kidney injury (in the absence of a cause for acute tubular necrosis) suggests C-TMA and the need for anti-complement treatment. We describe how we use these criteria to evaluate and manage pregnant/postpartum women with MAHA and thrombocytopenia.
Learning Objectives
To understand the frequency and clinical manifestations of preeclampsia with severe features/hemolysis, elevated liver function tests, low platelets (PE/HELLP) syndrome
To distinguish PE/HELLP syndrome from thrombotic thrombocytopenic purpura and from complement-mediated thrombotic microangiopathy
To learn when to consider plasma exchange treatment for thrombotic thrombocytopenic purpura and anticomplement treatment for complement-mediated thrombotic microangiopathy
Pregnancy and childbirth are typically times of celebration, but pregnant women can become suddenly, severely ill. The intensity of evaluation and management issues is great, because both the woman is young and usually previously healthy and the survival of the infant is at risk. When a pregnant or postpartum woman develops severe microangiopathic hemolytic anemia (MAHA) and thrombocytopenia, three syndromes must be considered: (1) preeclampsia with severe features/hemolysis, elevated liver function tests, low platelets (PE/HELLP) syndrome; (2) thrombotic thrombocytopenic purpura (TTP); and (3) complement-mediated thrombotic microangiopathy (C-TMA; also referred to as atypical hemolytic–uremic syndrome1 ). All three can be life threatening, and all three require their own specific treatment. Although TTP2 and C-TMA1 are uncommon, PE/HELLP syndrome is not. The incidence of PE/HELLP syndrome in the United States is estimated to occur in 1% of pregnancies.3 Worldwide, perinatal conditions, such as preeclampsia, are among the most common causes of disability and death.4
Before we discuss these three syndromes, we must emphasize that other pregnancy-related complications can cause MAHA, thrombocytopenia, and also acute kidney injury (Figure 1). These include sepsis, placental abruption, and postpartum hemorrhage. All of these complications can cause disseminated intravascular coagulation with MAHA and thrombocytopenia, and hypotension with acute tubular necrosis.
A simplified illustration of the most important distinguishing features of MAHA and thrombocytopenia.
A simplified illustration of the most important distinguishing features of MAHA and thrombocytopenia.
Etiologies of PE/HELLP syndrome, TTP, and C-TMA
Although these three syndromes have similar pathologic features of TMA5 and similar clinical features, they are distinct entities with distinct etiologies and pathogenesis.
The etiology of preeclampsia is not well understood. It may be related to abnormal placental function causing increased resistance to placental blood flow, which may be related to the systemic hypertension.6
TTP is a systemic disorder of microvascular thrombosis related to a severe deficiency of ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), most commonly an acquired autoimmune disorder. TTP can also be hereditary, caused by homozygous or compound heterozygous ADAMTS13 mutations.7 Although hereditary TTP is much less common than acquired TTP, the relative frequency of hereditary TTP is increased among women presenting during their first pregnancy.8,9 Pregnancy is a severe risk for women with hereditary TTP10 and requires management with plasma infusion throughout pregnancy.9 Most pregnancies after acquired TTP have successful outcomes.11
C-TMA is a disorder of dysregulation of the alternative complement pathway, most commonly hereditary with heterozygous mutations of genes encoding complement regulatory proteins. It may also be acquired with antibodies to complement factor H, the major regulatory protein of the alternative complement pathway.7
Relative incidence of PE/HELLP syndrome, TTP, and C-TMA
An important issue for the evaluation of a pregnant or postpartum woman with severe MAHA and thrombocytopenia is to appreciate the relative incidence of PE/HELLP syndrome, TTP, and C-TMA. PE/HELLP syndrome is much more common than either TTP or C-TMA (Table 1). The clinical relevance of these data is apparent when they are applied to the Oklahoma University Medical Center (OUMC), a tertiary referral hospital with ∼4000 births per year. Because of referral of women with complications, preeclampsia with severe features occurs in ∼4% of these women (3 women/week). The complete clinical features of HELLP syndrome occur less often, ∼1 woman/2 weeks. In contrast, there have been only 5 woman with TTP occurring during pregnancy or postpartum during the previous 19 years (2 at OUMC and 3 at 3 other Oklahoma City hospitals). For 3 women, it was their initial episode of TTP; for 2, it was a relapsed episode. The initial episodes were diagnosed on the day of delivery, 9 days postpartum, and 45 days postpartum. The relapsed episodes occurred 9 and 29 days postpartum. Therefore, preeclampsia with severe features was an unlikely consideration in 4 of these 5 women in whom TTP occurred 9-45 days postpartum. The incidence of C-TMA associated with pregnancy is not known. Our experience suggests that the incidence of C-TMA may be similar to TTP. The pregnancy-related syndrome that is most clearly recognized as C-TMA is postpartum acute kidney injury (also described as pregnancy-related hemolytic–uremic syndrome), which occurs primarily postpartum.12
Comparison of typical clinical features and specific management of PE/HELLP syndrome, TTP, and C-TMA
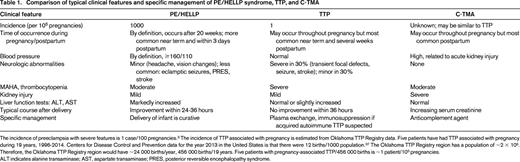
The incidence of preeclampsia with severe features is 1 case/100 pregnancies.3 The incidence of TTP associated with pregnancy is estimated from Oklahoma TTP Registry data. Five patients have had TTP associated with pregnancy during 19 years, 1996-2014. Centers for Disease Control and Prevention data for the year 2013 in the United States is that there were 12 births/1000 population.37 The Oklahoma TTP Registry region has a population of ∼2 × 106. Therefore, the Oklahoma TTP Registry region would have ∼24 000 births/year, 456 000 births/19 years. Five patients with pregnancy-associated TTP/456 000 births is ∼1 patient/105 pregnancies.
ALT indicates alanine transaminase; AST, aspartate transaminase; PRES, posterior reversible encephalopathy syndrome.
Diagnostic criteria for PE/HELLP syndrome, TTP, and C-TMA
These three syndromes cannot be distinguished with confidence by their diagnostic criteria (Table 2). Among these syndromes, PE/HELLP syndrome and eclampsia have the most precise criteria.13,14 Although MAHA is not among the criteria defining preeclampsia with severe features, it commonly occurs together with thrombocytopenia and liver function abnormalities, creating the HELLP syndrome. The American College of Obstetricians and Gynecologists considers the HELLP syndrome to be a part of the clinical spectrum of preeclampsia.13,14 Eclampsia is defined as the occurrence of grand mal seizures in a patient with the diagnostic criteria consistent with preeclampsia. Eclampsia is rare in the United States (5.6 cases/104 pregnancies15 ) and other developed countries; it is not included in our discussion. In developed countries, seizures, as well as other severe neurologic abnormalities, immediately create consideration of TTP in addition to eclampsia.
Diagnostic criteria for preeclampsia with PE/HELLP syndrome, TTP, and C-TMA
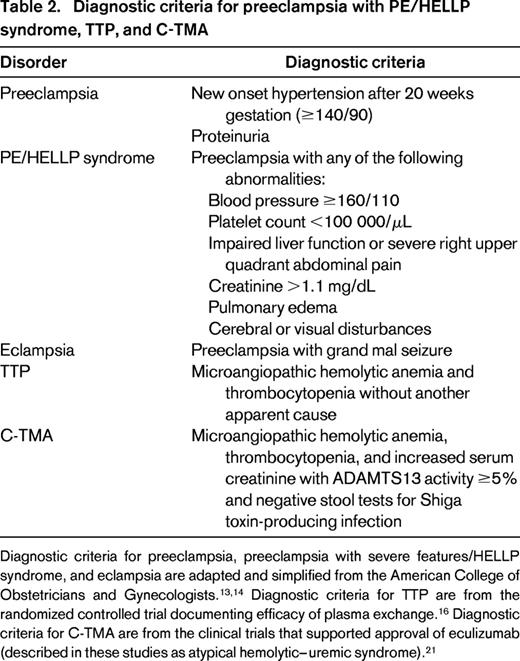
Diagnostic criteria for preeclampsia, preeclampsia with severe features/HELLP syndrome, and eclampsia are adapted and simplified from the American College of Obstetricians and Gynecologists.13,14 Diagnostic criteria for TTP are from the randomized controlled trial documenting efficacy of plasma exchange.16 Diagnostic criteria for C-TMA are from the clinical trials that supported approval of eculizumab (described in these studies as atypical hemolytic–uremic syndrome).21
Long-term outcomes after preeclampsia, TTP, and C-TMA
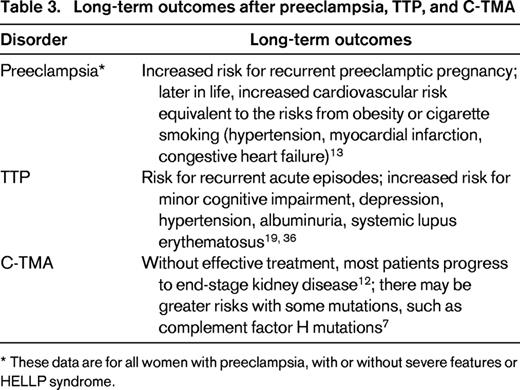
* These data are for all women with preeclampsia, with or without severe features or HELLP syndrome.
Current diagnostic criteria for TTP (Table 2) remain the same as the criteria developed for the randomized clinical trial that documented the efficacy of plasma exchange 24 years ago.16 TTP is defined by severe ADAMTS13 deficiency,7 and ADAMTS13 activity should be measured in all patients in whom the diagnosis of TTP is suspected. However, because the results of ADAMTS13 activity measurements may not be available for several days and also because of potential variability among assays,17,18 the initial diagnosis of TTP and the decision to begin plasma exchange treatment is based on the clinical features. Using these diagnostic criteria, the presence of PE/HELLP syndrome could exclude the diagnosis of TTP by providing an alternative etiology for MAHA and thrombocytopenia. However, the diagnosis of TTP must be considered in addition to PE/HELLP syndrome. The frequency of TTP is increased in women during pregnancy and postpartum because pregnancy can precipitate an acute episode.8,9,11 Both TTP and preeclampsia have similar demographic risk factors, including obesity13,14,19 and a relative increased incidence of black race.2,20 Also, the frequency of preeclampsia and PE/HELLP syndrome are increased among women who have recovered from an acute episode of acquired TTP and who are at risk for recurrent acute episodes.11 Therefore, the diagnosis of TTP must be considered in women with severe preeclampsia who have severe MAHA and thrombocytopenia, although TTP is much less common than PE/HELLP.
The diagnostic criteria for C-TMA (Table 2) were developed in 2011 for the clinical trials that supported the approval of eculizumab, a humanized monoclonal antibody that blocks the generation of C5a and C5b.21 These criteria are not specific because they were developed only to exclude patients with TTP and patients with Shiga toxin-mediated hemolytic–uremic syndrome. Because C-TMA has been defined more recently than either preeclampsia or TTP and because broad awareness of C-TMA has only occurred with the marketing of eculizumab since 2011, much less is known about its incidence and its potential association with pregnancy. However, it appears that the incidence of complement-mediated TMA is increased in pregnancy and postpartum.12 Our interpretation is that women with rapidly progressive postpartum acute kidney injury without an apparent cause for acute tubular necrosis may have a complement-mediated etiology.
The role of genetic testing during the initial evaluation of C-TMA remains limited, although the turnaround time for identification of complement gene mutations is much improved with next-generation sequencing methods. Physicians may not have access to quickly available testing, health insurance plans may deny payment, and approximately one-third of patients with a confident clinical diagnosis of C-TMA do not have an identified complement mutation.21 The greatest value of genetic testing is to provide information about risk for recurrence and for planning long-term management.22,23
Deciding when a pregnant/postpartum woman may have TTP or C-TMA
This is the critical decision. The diagnosis of PE/HELLP syndrome and subsequent obstetric management are clear: control the hypertension and deliver the infant as soon as possible. If PE/HELLP syndrome is present, the hematology and nephrology consultants' responsibility is to determine whether either TTP or C-TMA is also present. However, the diagnoses of TTP and C-TMA are often not clear. If PE/HELLP syndrome is not present, then the diagnosis of TTP or C-TMA is no different from non-pregnant/postpartum women.
Although the occurrence of both TTP and C-TMA appear to be increased in pregnant/postpartum women, we do not consider that both TTP and C-TMA may be occurring simultaneously. We consider them separately and therefore consider intervention with either plasma exchange for TTP or anticomplement treatment for C-TMA. Although case reports and cohort studies suggest that plasma therapy (exchange or infusion) may positively affect the hematologic parameters of C-TMA, renal response is less assured.24 For this reason and also because of risk for complications with plasma exchange,25 eculizumab may be preferred for the treatment of C-TMA. Nonetheless, eculizumab is not universally accepted as first-line therapy for C-TMA, in part because of expense but also because it is not always available. Choosing to use both plasma therapy and anticomplement treatment simultaneously requires the need for additional eculizumab doses and therefore increases the cost of treatment significantly. C-TMA associated with acquired anti-complement factor H antibodies may benefit from plasma exchange, but these antibodies may not be sufficiently common to justify the increased risks and cost.
Table 1 compares the clinical features of PE/HELLP syndrome, TTP, and C-TMA. Figure 1 presents a simplified illustration of their most important distinguishing features. Measurement of ADAMTS13 activity may help the evaluation; a severe deficiency (activity <10%) supports the diagnosis of TTP, whereas normal activity or less severe deficiency is characteristic of preeclampsia26 and complement-mediated TMA.21 Results of ADAMTS13 activity measurements are often not promptly available to help with the initial diagnosis and the patient may be critically ill, leaving little time for observation. However, obtaining plasma for ADAMTS13 activity measurement should be considered at the time of initial evaluation because the results may help to clarify the diagnosis several days later.
The diagnostic issues can be addressed in three clinical situations.
PE/HELLP is present; initial evaluation
The severity of neurologic abnormalities, kidney injury, and thrombocytopenia are important initial observations. Women with PE/HELLP syndrome may be critically ill with severe headache, visual symptoms, and hyperreflexia, but the presence of transient focal abnormalities, such as weakness, numbness, and aphasia, suggest the diagnosis of TTP (not C-TMA). Overt mental status changes are the most common neurologic abnormality of TTP17 ; mental status changes are not part of the neurologic criteria for PE/HELLP syndrome. Women with PE/HELLP syndrome may have increased creatinine and may even meet criteria for acute kidney injury,27 but a continuous increasing serum creatinine or a need for dialysis on initial presentation suggests the diagnosis of C-TMA (not TTP). Although thrombocytopenia always occurs, by definition, in PE/HELLP syndrome, it is typically not severe. In three case series of women with HELLP syndrome, the median nadir platelet counts were 43 000-57 000/μL.28-30 However, the lowest platelet counts in these 3 case series were very low, 6000-15 000/μL.28-30 In contrast, the median platelet count among TTP patients in the Oklahoma Registry was 11 000/μL.17 Our opinion is that platelet counts <20 000/μL suggest the diagnosis of TTP. Serum lactate dehydrogenase (LDH) levels may be very high in all three syndromes.12,17,30 Markedly increased serum transaminase levels suggest PE/HELLP syndrome.30
PE/HELLP syndrome; clinical course after delivery
In most situations, the urgency of delivery in a woman with PE/HELLP syndromes takes precedence over the decision for plasma exchange or anticomplement treatment. Then, if the clinical situation allows, the course in the hours after delivery may clarify the diagnosis. In a study of 30 women with PE/HELLP syndrome, the average nadir platelet count occurred 27 hours after delivery (although not until 69 hours after delivery in one woman). The average time for platelet recovery to >100 000/μL was 3 days after delivery.29 LDH elevation may be slower to recover than thrombocytopenia.31 Increasing platelet counts after delivery, in addition to decreasing LDH and creatinine and resolution of headache and visual symptoms, are evidence against TTP or C-TMA. Persistent, severe thrombocytopenia after delivery, particularly beyond 48-72 hours, suggests the diagnosis of TTP; increasing serum creatinine suggests C-TMA.
PE/HELLP syndrome not present
In these patients, the diagnostic decision is between TTP and C-TMA. The severity of thrombocytopenia, neurologic abnormalities, and kidney injury are also important observations in these patients. Limited data suggest that the platelet count in C-TMA is not typically severely decreased and that overt neurologic abnormalities are rare. Most patients have severe kidney injury requiring dialysis.12 Patients with TTP typically have severe thrombocytopenia and may have overt neurologic abnormalities but rarely have severe kidney injury.17 With either diagnosis, the goal is to continue the pregnancy until an appropriate time for delivery while treatment is initiated. There is no evidence to suggest that either anticomplement treatment32-35 or plasma exchange is harmful for the fetus. There is no evidence to suggest that delivery is beneficial for either TTP or C-TMA. For TTP and particularly C-TMA, the postpartum period appears to have at least as much risk as during pregnancy.
Long-term outcomes of women with preeclampsia, TTP, and C-TMA
Beyond evaluation and management, women who have preeclampsia (with or without severe features and HELLP syndrome), TTP, or C-TMA have significant long-term morbidities. Women who have had preeclampsia have long-term risks of cardiovascular disease comparable with the risks of people with obesity or cigarette smoking.13 Women (and men) who have recovered from acquired (autoimmune) TTP have increased frequency of abnormal kidney function that increases their risk for cardiovascular death.36 They also have increased risk for minor cognitive impairment, severe depression, and systemic lupus erythematosus.19 Although the long-term outcomes of C-TMA in the era of anticomplement treatment are not yet well defined, without effective treatment many patients will develop end-stage kidney disease.12
Correspondence
James N. George, Department of Biostatistics and Epidemiology, College of Public Health, University of Oklahoma Health Sciences Center, Rm CHB 237, P.O. Box 26901, Oklahoma City, OK 73126-0901. Phone: 405-271-4222; e-mail: james-george@ouhsc.edu.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.

