Abstract
Daily therapeutic plasma exchange (TPE) transformed the historically fatal prognosis of acquired, anti-ADAMTS13 antibody-mediated thrombotic thrombocytopenic purpura (TTP), leading to the current overall survival rates of 80%-85%. However, relapses occur in ∼40% of patients and refractory disease with fatal outcomes still occurs. In this context, the introduction of rituximab has probably been the second major breakthrough in TTP management. Rituximab is now routinely recommended during the acute phase, typically in patients with a suboptimal response to treatment, or even as frontline therapy, with high response rates. In more severe patients, salvage strategies may include twice-daily TPE, pulses of cyclophosphamide, vincristine, as well as splenectomy in more desperate cases. In this life-threatening disease, relapse prevention represents a major goal. Persistent severe acquired ADAMTS13 deficiency in patients who are otherwise in remission is associated with a high risk of relapse and preemptive treatment with rituximab may be considered in this context. In the coming years, the TTP therapeutic landscape should be enriched by original strategies stemming from clinical experience and new agents that are currently being evaluated in large, ideally international, clinical trials. Promising agents under evaluation include N-acetylcysteine, bortezomib, recombinant ADAMTS13, and inhibitors of the glycoprotein-Ib/IX-von Willebrand factor axis.
Learning Objectives
Patients with acquired TTP who experience a suboptimal response (refractoriness or disease exacerbation) with standard management need a more intensive treatment
Salvage therapies typically include rituximab, and in the more severe cases twice daily plasma exchange, boluses of cyclophosphamide, vincristine, and splenectomy
A persistent severe acquired ADAMTS13 deficiency during TTP remission should prompt consideration of preemptive rituximab to prevent relapses
Thrombotic thrombocytopenic purpura (TTP) is a particular form of thrombotic microangiopathy (TMA), characterized typically by microangiopathic hemolytic anemia, profound peripheral thrombocytopenia and severe deficiency in the von Willebrand factor-cleaving protease ADAMTS13 (A disintegrin and metalloproteinase with thrombospondin-1 motifs; 13rd member of the family). ADAMTS13 deficiency is usually severe (<10%) and results from autoantibodies against ADAMTS13 (acquired TTP) or from biallelic mutations of the encoding gene. In some cases, acquired TTP occurs in association with specific conditions that have to be identified for appropriate management: HIV infection, connective tissue disease, pregnancy, cancer, or treatment with antiplatelet agents.1
The standard treatment of acquired TTP consists mainly of daily therapeutic plasma exchange (TPE) that allows ADAMTS13 repletion and, to a lesser extent, removal of anti-ADAMTS13 antibodies and possibly pro-aggregatory substances. TPE transformed the historically fatal prognosis of TTP, leading to the current overall survival rates of 80%-85%.2 In the last several years, further significant changes have been introduced in the management of acquired TTP. The identification of the central role of anti-ADAMTS13 antibodies in the pathophysiology of TTP,3 which is now considered an autoimmune disease, has led to wider use of immunosuppressive treatments. In this context, the introduction of rituximab has probably been the second major breakthrough in TTP management. However, the current knowledge about the use of rituximab in TTP is based on few studies with a limited number of patients and moderate levels of evidence. Moreover, these studies raised many questions that remain unsolved. Should rituximab be used as frontline therapy, or only in patients with a suboptimal response to TPE? Which is the optimal schedule of rituximab administration? Should rituximab be systematically used as preemptive therapy? Which strategy should be used when rituximab fails to improve ADAMTS13 activity? Lastly, there is no consensus concerning the management of patients with refractory disease and emerging therapies might bring new weapons to our therapeutic arsenal in the future years. These topics are addressed in this review.
Frontline treatment
TTP requires a rapid diagnosis and must be managed as an emergency. The immediate outcome may not be predictable and maximal resuscitative measures may be required. Therefore, our practice is to manage such patients in intensive care units during the first few days after diagnosis until the platelet count reaches 50 × 109/L.
Daily TPE
Daily TPE remains the cornerstone of the current management of TTP.2 TPE must be started as soon as the diagnosis of TTP is established or even suspected. In France, the TPE procedure usually requires 1.5× plasma volume exchange with only plasma for the first procedures, and subsequently a 1.0× plasma volume replacement combined with 0.5×x 4% albumin. TPE is performed daily until organ involvement has resolved and the platelet count has stably recovered (ie, a normal platelet count for at least 2 consecutive days). Whether TPE sessions after remission is achieved should be stopped rapidly or tapered to prevent sudden exacerbations remains a matter of debate.
Steroids
There is a rational basis for the use of steroids in the treatment of acquired TTP given the autoimmune origin of the disease. However, the level of proof concerning steroid efficacy in the treatment of TTP remains quite low. In fact, outcomes were comparable in studies in which steroids were not given in combination with TPE and in studies in which steroids were introduced.2 Moreover, relapses have been observed during treatment with steroids.4
In the report by Bell et al,4 54 patients with a diagnosis of TTP without cerebral involvement were initially treated with high doses of steroids (200 mg of oral prednisone or intravenous prednisolone daily for 5-7 days) and the daily dose was progressively decreased over the following weeks. Thirty patients (55%) responded to steroids in 48-72 hours, whereas the remaining 24 patients did not improve and required TPE.4 This study, which was performed before the current systematic use of TPE in TTP, indicates that the administration of high-dose steroids alone has some efficacy in the treatment of TTP, at least in patients without significant organ involvement. A prospective randomized study on 60 patients compared the effectiveness of standard-dose (1 mg/kg/d) and high-dose methylprednisolone (10 mg/kg/d for 3 days and then 2.5 mg/kg/d) as an adjunctive treatment to TPE in patients with newly diagnosed TTP. After 23 days of treatment, complete remission was not achieved in 23% of patients in the high-dose methylprednisolone group and in 53% of patients of the standard-dose group. Although this study did not answer the question of whether steroids increase the likelihood of remission compared to treatment without steroids, it suggests that high-dose steroid therapy improves the management of TTP.5 Together, these results indicate that steroids might have a place in the management of TTP in association with TPE, provided there is no obvious contra-indication. However, the modality of administration remains debatable.
Antiplatelet agents
The use of antiplatelet agents in TTP is based on the rationale that the main pathological feature of the disease is the presence of platelet thrombi in the microcirculation. Aspirin and dipyridamole are the 2 main compounds considered to be potentially effective for TTP treatment. However, in TTP, the main mechanism of platelet clumping involves the glycoprotein Ib-IX/von Willebrand factor (vWF) axis. This challenges the relevance of aspirin and dipyridamole in TTP treatment because their mechanism of action involves other pathways. This discrepancy may account for the variability in the tolerance and results reported by the clinical trials that evaluated the efficacy of these antiplatelet agents in TTP (for a review, see Coppo and Veyradier1 ).
Immunomodulation with rituximab: a success story
Rituximab was first introduced with encouraging results (Table 1) in the acute phase of autoimmune TTP,6-8 typically in patients with a suboptimal response to conventional first-line treatment (ie, disease exacerbation or refractoriness, as defined by no improvement of the clinical features and/or lack of doubling of the platelet count from baseline after 4 full days of standard treatment). In these trials, TPE was usually continued daily and rituximab was administered immediately after a TPE. These studies were not randomized, usually uncontrolled, retrospective and had many confounding factors. However, they provided the first evidence that rituximab led to remission in most cases and deserved further evaluation.
Reports involving 10 or more patients treated with rituximab in the acute phase of autoimmune TTP

N indicates number of patients; F, female; R, relapsing patients; CR, complete remission; and NA, data not available.
To date, 5 studies (3 retrospective9-11 and 2 prospective7,12 ) have each included >10 adult patients with autoimmune TTP who were treated with rituximab after suboptimal response to standard treatment (Table 1). In the 3 retrospective studies, 39 patients with TTP were treated.9-11 The schedule of administration of rituximab was variable (most frequently 375 mg/m2 weekly, with a variable number of doses). Remission was obtained in 35/39 cases within 7-41 days. Four patients did not respond to treatment and 1 died. Two patients relapsed and responded again to retreatment. In all but 2 cases, ADAMTS13 activity recovered (Table 2).
Reports involving 10 or more patients treated with rituximab in the acute phase of autoimmune TTP: outcome

RFS indicates relapse-free survival; and VZV, varicella zoster virus.
In 2007, Scully et al12 reported on 25 patients who were treated with rituximab for refractory (n = 14) or recurrent disease (n = 11). Rituximab was given at 375 mg/m2/wk for 4 weeks. Remission was achieved in all patients within a median of 11 days (range, 7-21 days). No relapse was observed after a median follow-up of 10 months (range, 1-33 months). At 3 months post-rituximab, 21 patients had a normal ADAMTS13 activity and 23 patients had no detectable inhibitor. In 2012, our group reported the results of a prospective study to evaluate the efficacy of rituximab in patients with TTP and a suboptimal response to TPE.7 Patients empirically received four infusions of rituximab (375 mg/m2) on a tight schedule (ie, within 15 days; on days 1, 4, 8, and 15) because large amounts of rituximab are removed during TPE.13 Rituximab was started on the day of diagnosis of a suboptimal response (day 1), immediately after the TPE session. In the rituximab-treated arm, time to durable remission was significantly shorter and platelet count normalized within 35 days in all 21 survivors, compared to only 78% in the historical group. One patient died despite 2 rituximab infusions. No relapse was reported during the first year, but there were relapses beyond 1 year. Rituximab was associated with rapid and profound peripheral B-cell depletion, greater increases in ADAMTS13 activity, more frequent ADAMTS13 activity recovery and more depletion of anti-ADAMTS13 antibodies than in the group without rituximab. In both studies, rituximab was associated with no significant side effects.
Whether rituximab should be proposed to patients who experience a suboptimal response to standard treatment or as frontline therapy in all patients with acquired TTP is still debated. In 2011, the UK group8 reported a phase II clinical trial that evaluated rituximab as frontline treatment in association with daily TPE in acute acquired TTP. Forty patients received rituximab at a dose of 375 mg/m2/wk for 4 weeks in the first 3 days following the diagnosis of TTP. The median time to sustained platelet count recovery was 12 days. The duration of hospitalization for the patients with less severe disease was shorter compared to the historical group. Relapse occurred in only 10% of patients after a median time of 27 months, whereas 57% of patients relapsed after a median time of 18 months in the historical control group. Rituximab was associated with a more sustained increase in ADAMTS13 activity and a more important decrease in anti-ADAMTS13 antibodies. The tolerance to rituximab was good. These results could therefore encourage the use of rituximab as frontline treatment in acquired TTP. On the other hand, it is important to consider that ∼50% of patients may recover from the TTP episode with standard TPE/steroid-based treatment without the need for rituximab. Consequently, the systematic addition of rituximab in the frontline treatment may lead to overtreatment of a large number of patients. Moreover, it is unclear whether administration of rituximab in the acute phase of disease decreases the incidence of long-term relapses.7,8 The rapid identification of severe acquired ADAMTS13 deficiency in a patient with features of thrombotic microangiopathy (TMA) represents the main limitation of the systematic use of rituximab as frontline treatment in these patients, because not all centers can assess ADAMTS13 activity in an emergency. Moreover, it is also important to rule out other associated conditions that may influence the decision to treat or not with rituximab, such as HIV infection or history of hepatitis B virus infection.14 An additional study from the UK group in 2013 reported that patients treated with rituximab within the first 3 days after diagnosis achieved remission faster than those who received rituximab after a suboptimal response.15 However, the study was not randomized, and the decision-making process leading to the use of rituximab as frontline treatment or only in the case of suboptimal response was not clearly specified. Therefore, one cannot totally exclude that patients with a suboptimal response represented a group of patients with a worse prognosis than the group treated with frontline rituximab, which probably included patients who may have recovered without rituximab.15
In summary, rituximab is now routinely recommended in the acute phase of the disease in patients with a suboptimal response to treatment; its use is associated with remarkably high response rates. In contrast, the value of routinely using rituximab in association with TPE/steroids as frontline therapy remains uncertain.16
Treating the more severe forms
Importantly, rituximab might not be effective for the treatment of unresponsive TTP during the first 2 weeks, with a reported delay in the onset of its effect that may reach 27 days.7
Twice-daily TPE
So far, only 1 study reported the experience of twice-daily TPE in severe forms of TTP.17 Although it was difficult to accurately assess the benefit of an intensified TPE regime because other treatments were often initiated or intensified simultaneously, twice-daily TPE was clearly associated with a definite response in 3/31 patients. In all 3 patients, TTP was associated with acquired severe ADAMTS13 deficiency.17
In our experience, 19 patients with TTP were treated with twice-daily TPE between 2008 and 2014. Twice-daily TPE was started after the first rituximab administration (10 patients) or concomitantly (2 patients). Four patients had twice-daily TPE before rituximab. The median duration of twice-daily TPE treatment was 3 days (2-22 days). In 6 patients (31.6%), additional treatments (mainly, pulses of cyclophosphamide) were performed because of a persistent refractory disease (4 patients) or an exacerbation (2 patients), despite twice-daily TPE. Only 1 patient (5.3%) died. The other 18 achieved a durable complete remission 25.5 days after the first TPE (M. Soucemarianadin et al, manuscript submitted September 2015).
Vincristine and cyclosporine A
Vincristine is used mainly in refractory forms of TTP. In a literature review that involved 56 studies and 105 patients, stable remission was obtained in 73% of patients receiving vincristine as secondary or salvage therapy (ie, >3 days following diagnosis), with adverse events in only 5.7% of patients.18 These studies were retrospective and uncontrolled, but provided the historical evidence that vincristine is an effective salvage therapy in patients with acquired TTP who do not respond optimally to standard treatment. Currently, vincristine is usually not prescribed because rituximab is considered a more attractive (and possibly more effective) strategy.
Cyclosporine A has been reported as an effective treatment in refractory TTP19 and is also used as frontline treatment in association with TPE. The clinical response correlates with improvement in ADAMTS13 activity and suppression of anti-ADAMTS13 antibodies.20 However, a recent randomized study showed no significant difference in the exacerbation rate between patients treated with cyclosporine A and steroids as an adjunct to TPE, questioning the role of cyclosporine A as a frontline therapy in this disease.21
Splenectomy in the acute phase
The concept that the pathophysiology of TTP is immune in nature led to the empiric use of splenectomy in patients with severe refractory disease. This was based on the hypothesis that, similarly to immune thrombocytopenic purpura, spleen could be the preferred home for anti-ADAMTS13 antibody secreting memory B cells and plasmocytes. However, few patients with refractory TTP treated with splenectomy during the acute phase had a successful outcome, which challenges assertions about its efficacy in the treatment of refractory TTP.22 However, in a retrospective study, 15 patients with severe TTP underwent splenectomy 20 days after diagnosis. One patient died the day after splenectomy. The other patients recovered their platelet counts within 13.5 days, along with a rapid improvement in the LDH level. Post-operative complications included thromboembolic events (2 cases) and infections (6 cases). Two patients experienced a disease exacerbation, but subsequently had a favorable outcome.23
Cyclophosphamide
There are few retrospective studies on the efficacy of cyclophosphamide in the treatment of refractory TTP.23 In a retrospective series, 7 patients with refractory, life-threatening TTP received pulses of cyclophosphamide 15 days after TTP diagnosis. In all patients, the platelet count recovered durably within 24 days after the first pulse and ADAMTS13 activity recovered in the following weeks. Four patients experienced manageable infections.23 Cyclophosphamide side effects include bone marrow suppression, infectious complications, decreased fertility and a long-term risk of malignancy, which may be considered as acceptable in the context of refractory, life-threatening TTP.
Summary: which strategy for the more severe patients?
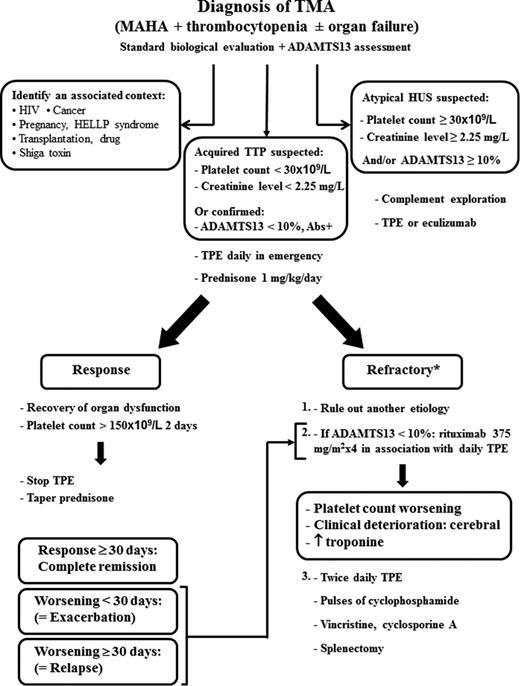
No consensus has been reached on the best approach to treat refractory, life-threatening TTP.24 If a patient does not respond to standard TPE and prednisone, our current strategy is to sequentially increase the intensity of treatment depending on the clinical course (Figure 1) by adding rituximab, followed by twice-daily TPE, pulses of cyclophosphamide and even splenectomy in the more severe cases (Beloncle et al23 and Soucemarianadin, manuscript submitted).
Proposition for the treatment of acute refractory TTP. *As defined by absence of platelet count doubling from baseline after 4 full days of standard treatment. A worsening of the disease was defined as the reappearance of organ dysfunction, thrombocytopenia, and hemolysis after an initial improvement. HUS indicates hemolytic uremic syndrome; Abs, anti-ADAMTS13 antibodies; HIV, human immunodeficiency virus; HELLP, hemolysis, elevated liver enzymes, low platelet count; MAHA, microangiopathic hemolytic anemia.
Proposition for the treatment of acute refractory TTP. *As defined by absence of platelet count doubling from baseline after 4 full days of standard treatment. A worsening of the disease was defined as the reappearance of organ dysfunction, thrombocytopenia, and hemolysis after an initial improvement. HUS indicates hemolytic uremic syndrome; Abs, anti-ADAMTS13 antibodies; HIV, human immunodeficiency virus; HELLP, hemolysis, elevated liver enzymes, low platelet count; MAHA, microangiopathic hemolytic anemia.
Agents that require evaluation
N-acetylcysteine.
N-acetylcysteine (NAC), which is known to reduce mucin multimers, has been proposed as an adjunctive treatment for TTP because vWF multimers have some common structural features with mucin multimers. Indeed, in vitro NAC reduces soluble vWF multimers and rapidly degrades ultra-large vWF multimers. In addition, administration of NAC to ADAMTS13–deficient mice reduces plasma vWF multimers and leads to rapid resolution of thrombi.25 Recently, the use of NAC as adjunctive treatment in a patient with refractory TTP was apparently efficient.26 However, some failures were also reported.27,28 Considering the limited toxicity of NAC, its use as adjunctive treatment in TTP seems interesting and needs to be further evaluated.
Bortezomib.
Bortezomib (Velcade, Millenium Pharmaceuticals), an inhibitor of the 26S unit of the proteasome, is used in the treatment of multiple myeloma and antibody-mediated rejection of solid organs. Recently, it has been proposed for the treatment of refractory TTP, based on the hypothesis that residual anti-ADAMTS13 antibodies produced by plasma cells resistant to the usual immunosuppressive therapies could be eliminated by this drug.28,29 Although single cases suggest that bortezomib is effective, the contribution of other immunosuppressive treatments introduced concomitantly or even the natural history of the disease cannot be excluded. Therefore, this approach needs to be evaluated in prospective clinical trials.
Forthcoming targeted therapies
To reduce the side effects associated with the current therapies (TPE and immunosuppressive drugs) and improve outcomes, less invasive and more targeted strategies are being developed for the treatment of TTP.
Recombinant ADAMTS13.
The key role of ADAMTS13 deficiency in TTP pathophysiology naturally suggests that administration of recombinant ADAMTS13 (rADAMTS13) would be a promising strategy. In vitro, rADAMTS13 addition to the plasma of 2 patients with TTP and congenital ADAMTS13 deficiency led to a dose-dependent normalization of vWF-cleaving activity, resulting in the degradation of vWF multimers.30 rADAMTS13 also prevented TTP development in a ADAMTS13 knockout mouse model.31 These promising experimental data strongly suggest that rADAMTS13 administration could become a valuable therapeutic option in TTP. In line with this statement, a phase I clinical trial is underway to evaluate the safety and pharmacokinetics of rADAMTS13 in children and young adults with congenital TTP (BAX930; https://clinicaltrials.gov/ct2/show/NCT02216084?term=ADAMTS13+baxter&rank=1). However, in patients with acquired TTP, anti-ADAMTS13 autoantibodies could bind to the infused rADAMTS13, inhibit its activity, and accelerate its clearance. This could compromise its therapeutic efficacy. The administration of high doses of rADAMTS13 may overcome this problem. The synthesis of functionally active rADAMTS13 variants that are resistant to the inhibitory effect of autoantibodies may represent an alternative solution that needs to be validated in clinical trials.32
Inhibitors of glycoprotein Ib interaction with vWF.
Recently, compounds that inhibit the interaction between glycoprotein Ib/IX and vWF have been developed and have shown encouraging results in reducing vWF-mediated platelet aggregation in vitro.33 Clinical trials in a limited number of patients have shown promising results.34 However, their possible role as adjuvant therapies in TTP and their safety profiles require further evaluation.
How to prevent relapses?
In 40% of cases, patients with autoimmune TTP experience one or multiple relapses.35-37 Relapses result from severe ADAMTS13 deficiency caused by the persistence or recurrence of anti-ADAMTS13 antibodies.35 Each relapse exposes the patient to a risk of death. Patients are also exposed to complications related to TPE or to intensive care unit hospitalization.38,39 Relapses also raise cost concerns.3 Therefore, the prevention of relapses in TTP represents a major goal. These statements provide a rationale to evaluate the efficacy of rituximab in autoimmune TTP as preemptive therapy for patients in clinical remission, but with persistent severe ADAMTS13 deficiency. A recent study reported 30 such TTP patients who received preemptive infusions of rituximab. Rituximab remarkably reduced the incidence of TTP relapse by diminishing the production of anti-ADAMTS13 antibodies and restoring ADAMTS13 activity, which paralleled B-cell depletion. However, in 30% of these patients, ADAMTS13 recovery was not sustained and further cycles of rituximab were required to maintain detectable ADAMTS13 activity. Moreover, 16% of patients failed to improve ADAMTS13 activity and required other immunomodulatory drugs and/or a splenectomy.37 Adverse effects of rituximab were minimal: 3 patients had mild reactions during infusions, 1 patient had erysipelas, and 1 patient developed asymptomatic hypogammaglobulinemia after multiple infusions of rituximab.
Preemptive rituximab represents a promising strategy that could significantly modify the epidemiology of TTP by dramatically reducing relapses and associated complications. However, multiple infusions of rituximab may expose patients to infections or other long-term complications. On the other hand, persistent untreated severe ADAMTS13 deficiency may expose patients to a potentially sudden and fatal, unanticipated relapse. In line with this statement, the relapse rate is dramatically elevated at 70% in 18 patients to date with persistent chronic severe acquired ADAMTS13 deficiency that we enrolled from 2000 (the year our National registry was established) to 2013 (the year we started to perform rituximab preemptively in most patients). Therefore, it seems likely that almost all patients with persistently severe ADAMTS13 deficiency may eventually relapse (Sadler3 and P.C. and A.F., unpublished data, April 2015), and clearly, preemptive treatment with rituximab may be reasonable.
Future directions: how to further improve the prognosis in TTP?
In recent studies, older age, cerebral involvement and cardiac troponin-I level at diagnosis were consistently associated with a worse prognosis in TTP.40,41 Forthcoming studies will assess whether the initial treatment should be modified based on these prognostic factors.
The diagnosis of TTP in an emergency setting is challenging because the disease is rare, which may lead to a delay in management that can impact the prognosis. Consequently, there is a crucial need to develop educational programs for generalists, emergency department physicians and all other specialists possibly involved in the management of TTP, to improve the diagnosis and the treatment of the disease. In addition, there should also be educational programs for patients about the typical features suggestive of a relapse.
The field of TTP (and of TMA in general) remains an active area of investigation for new therapies and represents a convincing example of the power of translational medicine. In future years, the therapeutic landscape of TTP will be enriched by original strategies stemming from clinical experience and new compounds, through large, ideally international, clinical trials for the benefit of patients.
Correspondence
Paul Coppo, Centre de Référence des Microangiopathies Thrombotiques, Service d'Hématologie, Hôpital Saint-Antoine, Université Pierre et Marie Curie, 184 rue du Faubourg Saint-Antoine, Assistance Publique, Hôpitaux de Paris, 75012 Paris, France; Phone: 00-33-1-49-28-26-21; Fax: 00-33-1-49-28-33-75; e-mail: paul.coppo@aphp.fr.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: Rituximab immunomodulator in autoimmune TTP.