Abstract
Monitoring treatment responses in chronic myeloid leukemia (CML) is based on complete blood counts (CBCs) to determine hematologic response, karyotyping of bone marrow metaphase cells to delineate cytogenetic response and quantitative reverse transcription polymerase chain reaction (qPCR) to quantify expression of BCR-ABL1 mRNA (molecular response; MR) in peripheral blood. Fluorescence in situ hybridization (FISH) to identify BCR-ABL1 in interphase nuclei and mutational analysis of the BCR-ABL1 kinase domain (KD) are used in certain clinical circumstances. As most patients treated with tyrosine kinase inhibitors (TKIs) achieve complete cytogenetic responses (CCyRs), qPCR with its increased sensitivity and dynamic range has become the main tool used to monitor CML patients. Landmark analyses of large TKI trials have established MR milestones that identify patients with high risk of failure, are the basis of consensus management guidelines, and have led to a strong push toward qPCR test standardization. Today many laboratories report BCR-ABL1 qPCR results on the international scale (IS), a system based on the conversion of laboratory-specific numerical values to conform to a universal scale. The fact that qPCR is technically demanding and liable to assay variations poses considerable challenges for its routine clinical use. This is important as the prevalence of patients on chronic TKI therapy increases and critical clinical decisions are made based on qPCR results, for example if discontinuation of TKI therapy should be considered. Here we will review the current state of molecular monitoring in CML, focusing on qPCR, the definition of TKI failure and the results of TKI discontinuation studies.
Learning Objectives
Understand how to use and interpret qPCR for BCR-ABL1 to monitor CML patients on therapy
Understand the current state for treatment for remission in CML patients managed with TKIs
Technical notes
As an exhaustive review of the technologic details of qPCR is beyond the scope of this review, we will focus on aspects that are of practical importance and can be a source of misinterpretations. Recommendations for laboratories were published recently.1
Qualitative PCR versus quantitative PCR.
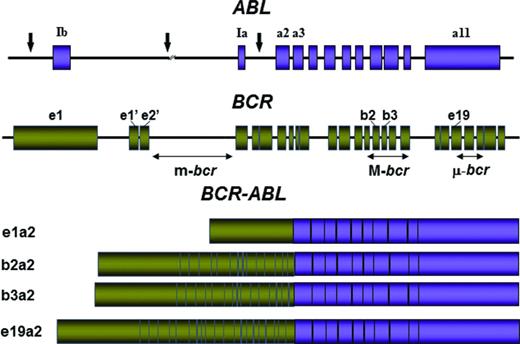
Qualitative qPCR is used to establish or confirm a diagnosis of chronic myeloid leukemia (CML). Most qualitative assays are optimized for detection of all typical BCR-ABL1 transcripts [e1a2; e13a2 (formerly b2a2); e14a2 (formerly b3a2) and e19a2], as well as atypical transcripts such as fusions to a3 (ABL1 exon 3), but not for sensitivity2 (Figure 1). Erroneous use of qualitative assays for monitoring therapy will lead to false-negative results that are interpreted as a profound molecular response (MR). On the other hand, most quantitative assays detect either e1a2 or e13a2 and e14a2, but not atypical transcripts and as such are not suitable to exclude a diagnosis of CML.
Location of the breakpoints in the ABL and BCR genes and structure of the chimeric mRNAs derived from the various breaks. Adapted from Deininger et al.41
Location of the breakpoints in the ABL and BCR genes and structure of the chimeric mRNAs derived from the various breaks. Adapted from Deininger et al.41
Control genes, sample quality and theoretical assay sensitivity.
After much work and discussion several genes are now widely accepted as suitable controls, including ABL1, GUS, and BCR. The expression of the control genes is critically important as it determines the dynamic range of the assay, and as such the maximum possible sensitivity independently of the sensitivity of the BCR-ABL1 detection assay. It is recommended that a sample have at least 10,000 ABL1 or 24,000 GUS copies to pass minimum quality standards,1 although the respective values for BCR have not yet been established. The control gene copies measured in a given sample should be reported routinely to allow for quality assessment. The second critical determinant of qPCR sensitivity is the performance of the assay used to detect BCR-ABL1 itself, which can be optimized to detect a single BCR-ABL1 molecule. The fact that negative qPCR results are dependent on sample quality and test performance has led to calls for abandoning the term complete molecular response (CMR) as it lacks a clear definition.
International scale.
The international scale (IS) is anchored to the average BCR-ABL1 expression in samples from 30 untreated chronic phase CML patients enrolled in the IRIS study,3 which was arbitrarily defined as 100% (Figure 2). Thus, although values on the IS are expressed in percent, they are independent of an individual patient's initial BCR-ABL1 expression. The IS allows for comparison of qPCR values between different laboratories and should be considered standard of care. Expression of qPCR values on the IS requires a laboratory-specific conversion factor that is derived from amplification of standard samples. Only good quality assays are suitable for conversion and significant efforts are necessary to avoid drifting and, if necessary, recalibrate the assay.
Levels of MR (Figure 2).
The first MR level shown to be associated with subsequent outcome was a 3-log reduction of BCR-ABL1 transcripts (MR3.0), which was termed major molecular response (MMR). MR4.0, MR4.5, and MR5.0 refer to 4.0-log, 4.5-log, and 5.0-log transcript reductions expressed on the IS. Good laboratories are able to measure MR4.5 or even MR5.0 using conventional technology; in the future more sensitive technologies, such as droplet digital PCR, may extend the dynamic range beyond the 5.0-log reduction.4 MR4.0 and deeper levels of MR have acquired increasing practical importance for identification of patients eligible for a trial of tyrosine kinase inhibitor (TKI) discontinuation.
Clinical molecular monitoring
Frequency of qPCR monitoring
Measuring BCR-ABL1 expression at diagnosis is not strictly necessary, as the prognostic landmarks of MMR and MR4.0 are based on the IS rather than comparison with the patient's own baseline. However, as emerging evidence suggests that the slope of the initial BCR-ABL1 decline may add prognostic information, we routinely send blood for qPCR in all newly diagnosed patients.5,6 Once TKI therapy has been initiated, qPCR should be done every 3 months until MMR has been achieved and at 3-6 months intervals thereafter.7,8 Clinical judgment is needed to determine the appropriate testing interval. For example, it is good practice to monitor patients with adherence issues every 3 months, even if they have achieved MMR or a deep molecular response. In our experience, most PCR rises in patients who have achieved MMR are due to periods of poor adherence, which explains why BCR-ABL1 kinase mutation testing is almost always negative. In these individuals, qPCR monitors CML as much as patient behavior. Another situation that justifies more frequent monitoring long-term is high Sokal risk chronic-phase or advanced-phase CML. Lastly, testing should be reasonably dense (at least 2-3 times/year) if a discontinuation trial is considered to ascertain that the desired level of response (typically MR4.5 or better) was indeed maintained over the required length of time prior to stopping therapy.9,10
A long-standing debate relates to the use of fluorescence in situ hybridization (FISH) to detect BCR-ABL1 in interphase nuclei from blood or bone marrow white cells. Unlike cytogenetics and qPCR, FISH has never been validated in a prospective clinical study, and there are insufficient data correlating FISH on white blood cell interphases with bone marrow cytogenetics or qPCR. Therefore, FISH is not generally recommended to monitor patients. Justifiable exceptions are patients with a documented CCyR, but no access to qPCR monitoring. In these individuals, a negative FISH result on peripheral blood is highly suggestive of continued CCyR and thus can avoid the invasive procedure of a bone marrow biopsy.
Molecular response and prognosis
Correlations between molecular response and progression free survival (PFS) and overall survival (OS) are typically based on the response achieved after a certain duration of TKI therapy (landmark analysis). The most widely used time points are 3 months (early molecular response) and 12 months. An important issue when considering landmark analyses is that the cohorts become enriched with good risk patients as time progresses, due to the dropout of patients who relapse. Another consideration is that the combination of various levels of molecular response, multiple time points, and several different endpoints leads to a such a large number of possible permutations that results must be controlled for multiple comparisons to avoid identifying false-positive correlations.
Molecular response at 12 months.
MMR at 12 months has been associated with improved PFS and OS in multiple prospective clinical trials of TKIs and has been used as a surrogate endpoint in phase 3 studies of second-generation TKIs.3,11-13 MMR is a somewhat better prognosticator than CCyR, although the difference is small and failed to reach statistical significance in the IRIS study.3 The European Leukemia Net (ELN) considers MMR at 12 months as “optimal” and the range between 0.1% and 1.0% IS as “warning”, implying that CCyR without MMR does not constitute an optimal response.7 According to the National Comprehensive Cancer Network (NCCN) guidelines, cytogenetic monitoring is sufficient to follow patients who have achieved CCyR on a given TKI, implying that CCyR is acceptable as a response long-term8 (Table 1). Thus, at least theoretically, a higher level of residual disease is deemed acceptable in a patient followed with cytogenetics only than in a patient followed by qPCR. Although these discrepancies may have limited practical consequences, they cause confusion and a uniform definition would be desirable. In defense of CCyR as an acceptable therapeutic endpoint, evidence is lacking that a therapeutic intervention to convert CCyR into MMR is beneficial in terms of PFS and OS, although switching from imatinib to nilotinib has been shown to improve deep molecular responses, qualifying more patients for a trial of discontinuation. However, toxicity was also increased in the experimental arm.14 Interestingly, a recent analysis of the German CML IV trial at 8 years of follow-up showed significantly improved OS for patients in MR4.5 compared with patients in CCyR after 4 years on imatinib.15 This is consistent with earlier data on patients who tested negative by the now abandoned nested PCR technique.16 For the time being, CCyR should be considered the general primary objective of TKI therapy, as it has been associated with OS in multiple independent studies.17 This may change as the goals of therapy shift toward treatment-free remission (TFR). Although intuitively deep molecular responses are desirable, they are not a therapy goal in their own right, and the potential toxicities of nilotinib (peripheral arterial occlusive disease) and dasatinib (pulmonary hypertension), as well as their increased costs must be taken into account if a switch is considered.18,19 In our practice, we consider a switch to a second-generation TKI to achieve MR4.0 or MR4.5 only if a trial of TFR is the stated objective, for example in a young woman who desires to become pregnant.
Early molecular response (EMR).
The term EMR is typically used to denote the BCR-ABL1 transcript level after 3 months of therapy, the first routinely obtained assessment of response. Marin et al were the first to show that BCR-ABL1 expression >10% IS is associated with impaired progression-free and overall survival in CP-CML patients treated with imatinib.20 Importantly, in multivariate analysis the 3 month qPCR value superseded all other prognostic factors. Subsequent studies confirmed this association for patients treated with other TKIs in the first and second-line setting, suggesting that this biomarker identifies patients with high-risk biology that cannot be overcome with any TKI.21,22 In clinical practice several important considerations must be taken into account. First, although a change of therapy based on EMR failure is intuitive and may be widely practiced, thus far there are no prospective interventional studies to demonstrate that this is effective. In fact, a recent retrospective study reported improved CCyR and MMR rates after switching to nilotinib or dasatinib compared with continuation of imatinib, but surprisingly this did not translate into improved PFS and OS.23 Secondly, some controversy still exists as to whether it is justifiable to wait for another 3 months before declaring failure. One study found that achieving MMR at 6 months overrides the negative prognostic impact of EMR failure.24 Consistent with this, the ELN recommends obtaining another qPCR test at 6 months,7 whereas current NCCN guidelines mandate intervention.8 This is fairly unproblematic in patients on imatinib, but a more difficult decision in patients who fail a second-generation TKI based on their 3 month BCR-ABL1 level. For most of these patients switching to an alternative second-generation TKI is not a viable long-term strategy, as the rates of CCyR in this scenario are only 20%-30%, and responses are of limited durability.25 Ponatinib, on the other hand, produces more profound and more durable responses but is associated with cardiovascular toxicity.26 Allogeneic stem cell transplant is effective, but also associated with considerable mortality and morbidity. Thus, given the clinical implications of declaring EMR failure it is important to consider the clinical context, including the possibility of noncompliance. Furthermore a BCR-ABL1 level of 11% is statistically no different from 9% and should not be regarded as failure, whereas a patient with a BCR-ABL1 level of 15% at diagnosis but no reduction at 3 months compared with baseline despite continuous therapy, is clearly high risk. This clinically intuitive notion was recently confirmed in a systematic analysis. Patients with EMR failure, but a short halving time (ie, the time required to reduce their individual baseline BCR-ABL1 level by 50%) had superior PFS and OS compared to patients with EMR failure and a long halving time (>76 days).5 Similarly a subset analysis of the German CML IV study found that a half-log reduction of BCR-ABL1 transcripts at 3 months was the most accurate predictor of OS at 5 years.6 Accurate calculation of the halving time requires a precise baseline that allows values above 100%, which is possible only with qPCR assays that use a control gene other than ABL1. This is due to a peculiarity of ABL1-based assays, in which the ABL1 amplicons are derived both from ABL1 and from BCR-ABL1 mRNA transcripts. At high levels of BCR-ABL1 expression, most PCR amplicons are derived from BCR-ABL1 mRNA, which implies that the maximum measurable transcript level is technically equal to 100%.
TKI failure
Recognizing TKI failure.
TKI failure can be primary (ie, failure to achieve a desired response) or secondary (losing a desired response). The ELN and NCCN have established criteria to define primary and secondary TKI failure (Table 1). As most patients are monitored with qPCR, the most common clinical situation is a rise in BCR-ABL1 transcripts. Before initiating a full TKI failure workup one must ascertain adherence, rule out drug interactions that may lower TKI plasma concentrations and exclude a laboratory error. The general recommendation is that increases of BCR-ABL1 in excess of 10-fold warrant further investigation, typically a second test after 4-6 weeks. However, the MR level also needs to be taken into account. Due to the greater assay imprecision at low BCR-ABL1 levels, a 10-fold rise at MR4.5 is less likely to be significant than a 10-fold rise at 1% IS. The interpretation of the results is also dependent on the quality of the PCR assay. In good laboratories, increases of as little as 2 to 3-fold can be significant.27,28 Once lack of adherence and drug interactions have been ruled out, a full resistance workup is indicated that includes bone marrow biopsy and aspirate, metaphase cytogenetics and BCR-ABL1 mutational analysis. Resistance to frontline TKI therapy is a diagnosis with significant prognostic implications, and as such should be made with great care.
Screening for BCR-ABL1 kinase domain mutations.
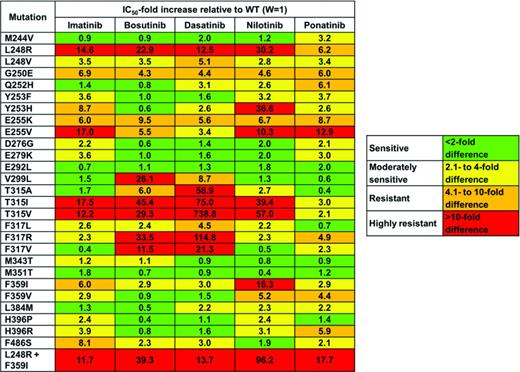
Patients with primary or secondary resistance should be screened for kinase domain mutations. Detection of a kinase domain mutation is an adverse prognostic factor and the specific BCR-ABL1 genotype is used to rationalize TKI selection for salvage (Table 2).29 At the current time Sanger sequencing (SS) is still the most widely used technique for mutation screening. Although SS has a sensitivity of only 15%-20%, it provides a reasonable compromise between sensitivity and specificity. Studies using allele-specific PCR, ligation PCR, Sequenom MASSarray and other sensitive technologies for mutation detection have found that TKI resistant mutants may be present at low level weeks to months before overt relapse.30 Although it is intuitive to believe that early detection of resistance mutants is beneficial to make early and rational adjustments to therapy, prospective studies to prove this point are still lacking. Additionally, it is not yet clear which level of sensitivity provides the optimal balance between sensitivity and specificity, as very sensitive assays may pick up clinically irrelevant spurious signals.31,32 Another level of complexity is compound mutations, ie, 2 or more mutations occurring in the same BCR-ABL1 allele. Compound mutations that include BCR-ABL1T315I confer resistance to all currently approved TKIs, including ponatinib.33 The optimal technology for detection of compound mutations remains to be established as recombination events during PCR amplification can lead to false-positive results when 2 single mutations are present.34 In the future, next generation sequencing may become the technique of choice for BCR-ABL1 mutation screening. However, for the time being, SS remains a perfectly acceptable assay. Importantly, correlations between BCR-ABL1 genotype and clinical phenotype are tight only toward the negative side. Thus, although patients with BCR-ABL1T315I will not respond to imatinib or second-generation TKIs, there is no guarantee that patients with biochemically sensitive mutants will respond, pointing to the importance of BCR-ABL1 kinase-independent mechanisms of resistance.
Treatment-free remission
Several studies have reported that a subset of patients with deep MR on imatinib will maintain their responses after discontinuation of TKI therapy, a state commonly referred to as TFR (for treatment-free remission).9,10,35,36 TFR is increasingly seen as a key endpoint in therapy studies of CML, where demonstrating significant differences in PFS or OS has become difficult due to the effectiveness of standard treatments. TFR as a clinical goal in individual patients is much more popular in Europe and Australia than in the United States, maybe reflecting the lack of any US-led studies in this space.
Requirements for a trial of TFR and indications for restarting TKIs
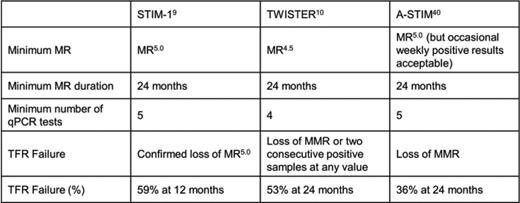
Prospective studies published thus far have focused on patients on imatinib and used deep MR (MR4.5 or better) with a duration of at least 24 months as a requirement for a trial of TFR (Table 3). Additionally, qPCR testing in the 24 months preceding discontinuation had to be reasonably dense (at least 2 tests/year) to ascertain deep MR was continuous during the observation period. The criteria defining TFR failure have evolved from very stringent (confirmed loss of MR5.0 in the STIM trial) to loss of MMR (A-STIM study), which mirrors the increasing confidence in the safety of TFR. It is critical to remember that qPCR monitoring was very dense throughout these studies (typically monthly for the first year), allowing for early detection of molecular positivity. The term “molecular relapse” is frequently used to describe the re-emergence of detectable BCR-ABL1 transcripts or the loss of MMR, but this wording seems less than ideal as it is also used to describe disease relapse on ongoing TKI therapy, a completely different situation. To distinguish between the two scenarios the term “molecular recurrence” may be more suitable to describe patients who lost response after TKI discontinuation.
Success rates of TFR
After discontinuation of imatinib, between 40 and 50% of patients maintain TFR if loss of deep MR is used to define TFR failure, and up to 60% if loss of MMR is used as the criterion defining failure.9,10 Most recurrences occur within the first 3 months after discontinuation, and very few after 6 months. Results from a pilot study in patients with deep MR on second-generation TKIs were similar, although follow-up was shorter.37 All in all these data suggest that about half of CML patients with a deep MR to TKIs will be able to successfully enter TFR. It is interesting to speculate about the proportion of chronic phase CML patients who may eventually enter TFR. Recent updates of the ENESTnd and DASISION studies with 6 and 5 year follow-up reported MR4.5 rates of 40%-50%, although it is unknown how many of these responses will fulfill the durability criteria used in the STIM and TWISTER studies. In an optimistic scenario, approximately 50% of patients may be eligible, and 50% of these are predicted to enter successful TFR. Thus, roughly one-quarter of newly diagnosed chronic phase CML patients may be able to achieve functional cure on TKIs. This is both a very encouraging and a very sobering figure that illustrates both the enormous progress made and the enormous work that still needs to be done.
Predicting successful TFR
Perhaps the most surprising observation in all of the TFR studies is the fact that almost all recurrences occur within the first 6 months, with few at later time points. At face value, one would have suspected that the time to recurrence would correlate with the amount of residual CML. However if this were the case, recurrences should continue to occur over a longer period of time after discontinuation, which is not the case.38 The fact that the TWISTER study with its slightly less stringent entry criteria produced a recurrence rate very similar to the STIM trial and limited data from a discontinuation study in patients on second-generation TKIs also argue against such a model.10,37 The initial suspicion that prior exposure to interferon-α may be beneficial was not confirmed in subsequent studies as was the adverse effect of high Sokal risk at diagnosis. Several studies have found differences in cellular immunity between successful and nonsuccessful TFR patients, but these differences are generally subtle and the jury is out regarding their causal involvement in maintaining TFR.39 What appears to hold in independent studies is that longer exposure to TKIs is associated with a higher rate of successful TFR. From a therapeutic standpoint this is good news as it suggests that TKIs are able to modify the disease biology and to some extent supports the attrition theory, ie, the possibility that long-term TKI therapy may simply exhaust the CML clone.38 As CML progenitor cells, but not CML stem cells, are sensitive to TKIs this could occur if TKIs ever so slightly promoted non self-renewal divisions of CML stem cells, thereby driving the CML clone into extinction.
TFR outside of clinical studies?
Reassuringly, thus far all patients in whom TKIs were discontinued in closely monitored clinical trials have responded upon rechallenge with the same TKI. Most, but not all, of these responses were equally deep as those prior to discontinuation and progression is very rare.9,40 On the other hand, it would be surprising if the risk of TFR was zero, given that TKI suppression of BCR-ABL1 kinase activity reduces genetic instability. Quantifying this risk will require long observation times. Thus far, most TFR patients were treated on clinical protocols, ensuring dense qPCR follow-up and timely recognition of recurrence. In Europe, where many CML patients are followed in university medical centers, discontinuation outside of clinical trials is rather common, although exact numbers are not available. In the US, discontinuation is less popular and usually driven by side effects. A concern with TFR outside of clinical studies is that patients will discontinue TKIs with less than 2 years of a less profound response than used in the pivotal prospective studies. In the US with its highly migratory population, frequently changing health plans and challenging geography this would predictably lead to molecular recurrences being missed and eventually to recurrence of active CML. The increasingly prevalent notion that CML is easy to manage, and in fact, not a real cancer is likely to further aggravate the situation. Therefore, our current practice is to attempt TFR only in the setting of a clinical study or in the case of side effects as additional factors. More work is needed to make TRF a reality for the majority of CML patients, and TKIs alone are unlikely to accomplish this. The ultimate goal of course remains cure. Only time will tell whether we will ever be able to release patients from our care after an “episode of CML”.
Correspondence
Michael W. Deininger, Huntsman Cancer Institute, Rm 4280, 2000 Circle of Hope, Salt Lake City, UT 84112-5550; Phone: +1-801-581-6363; Fax: +1-801-585-0900; e-mail: michael.deininger@hci.utah.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author is on a paid advisory board for Bristol Myers Squibb, Ariad, Novartis, Incyte, and Pfizer; has received research funding from Bristol Myers Squibb, Novartis, Celgene, and Gilead; and has consulted for Bristol Myers Squibb, Ariad; Novartis, Incyte, and Pfizer.
Author notes
Off-label drug use: None disclosed.