Abstract
The sideroblastic anemias are a heterogeneous group of inherited and acquired disorders characterized by the presence of ring sideroblasts in the bone marrow. X-linked sideroblastic anemia (XLSA) is caused by germline mutations in ALAS2. Hemizygous males have a hypochromic microcytic anemia, which is generally mild to moderate and is caused by defective heme synthesis and ineffective erythropoiesis. XLSA is a typical iron-loading anemia; although most patients are responsive to pyridoxine, treatment of iron overload is also important in the management of these patients. Autosomal recessive sideroblastic anemia attributable to mutations in SLC25A38, a member of the mitochondrial carrier family, is a severe disease: patients present in infancy with microcytic anemia, which soon becomes transfusion dependent. Conservative therapy includes regular red cell transfusion and iron chelation, whereas allogenic stem cell transplantation represents the only curative treatment. Refractory anemia with ring sideroblasts (RARS) is a myelodysplastic syndrome characterized mainly by anemia attributable to ineffective erythropoiesis. The clinical course of RARS is generally indolent, but there is a tendency to worsening of anemia over time, so that most patients become transfusion dependent in the long run. More than 90% of these patients carry somatic mutations in SF3B1, a gene encoding a core component of the RNA splicing machinery. These mutations cause misrecognition of 3′ splice sites in downstream genes, resulting in truncated gene products and/or decreased expression attributable to nonsense-mediated RNA decay; this explains the multifactorial pathogenesis of RARS. Variants of RARS include refractory cytopenia with multilineage dysplasia and ring sideroblasts, and RARS associated with marked thrombocytosis; these variants involve additional genetic lesions. Inhibitors of molecules of the transforming growth factor-β superfamily have been shown recently to target ineffective erythropoiesis and ameliorate anemia both in animal models of myelodysplastic syndrome and in RARS patients.
Learning Objectives
To learn the 2 genes most commonly responsible for congenital sideroblastic anemia
To understand that X-linked sideroblastic anemia is an iron-loading anemia and that treatment of iron overload is at least as important as pyridoxine administration in the management of these patients
To understand that SLC25A38-mutant autosomal recessive sideroblastic anemia is a severe disease whose only curative treatment is allogenic hematopoietic stem cell transplantation
To describe the somatic mutations of RNA splicing machinery that are found in refractory anemia with ring sideroblasts and related myeloid neoplasms
To understand that SF3B1-mutant myelodysplastic syndrome represents a distinct entity
To describe the novel drugs that target ineffective erythropoiesis in patients with refractory anemia with ring sideroblasts
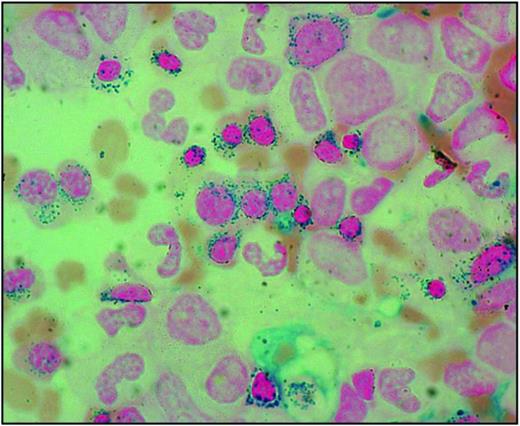
The sideroblastic anemias are a heterogeneous group of disorders characterized by anemia of varying severity and the presence of ring sideroblasts in the bone marrow.1 Ring sideroblasts are erythroblasts with iron-loaded mitochondria, which are visualized by Prussian blue staining (the so-called Perls reaction) as a perinuclear ring of blue granules (Figure 1).2
Bone marrow smear from a patient with RARS. Perls staining shows that most erythroblasts have positive granules disposed in a ring surrounding the nucleus, that is, the typical pattern of ring sideroblasts. Magnification, 1250.
Bone marrow smear from a patient with RARS. Perls staining shows that most erythroblasts have positive granules disposed in a ring surrounding the nucleus, that is, the typical pattern of ring sideroblasts. Magnification, 1250.
Classification of sideroblastic anemias
The sideroblastic anemias include both inherited and acquired conditions; the main disorders are reported in Table 1.
Classification of inherited and acquired sideroblastic anemias
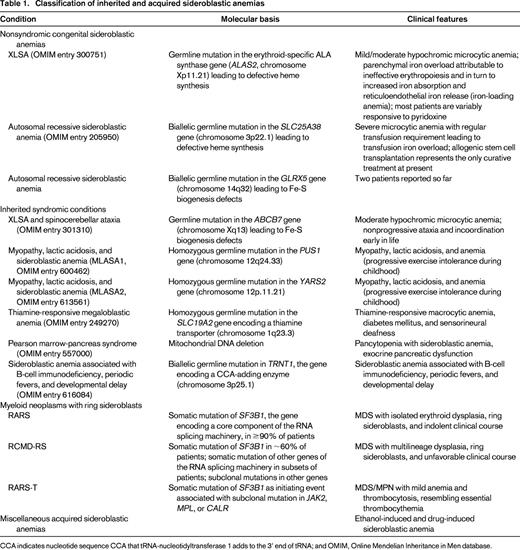
CCA indicates nucleotide sequence CCA that tRNA-nucleotidyltransferase 1 adds to the 3' end of tRNA; and OMIM, Online Mendelian Inheritance in Men database.
Congenital sideroblastic anemias include nonsyndromic and syndromic conditions; for an in-depth analysis of the molecular genetics and pathophysiology of these disorders, see a previous review article in this book from 2011.3 The 2 most common congenital sideroblastic anemias are X-linked sideroblastic anemia (XLSA) attributable to germline mutations in ALAS2 and the autosomal recessive sideroblastic anemia attributable to mutations in SLC25A38.
Acquired sideroblastic anemias include conditions that range from clonal disorders (myeloid neoplasms) to ethanol-induced and drug-induced sideroblastic anemia.
XLSA attributable to germline mutation in ALAS2 and defective erythroid-specific ALA synthase activity
Numerous ALAS2 mutations have been detected in families with XLSA, most of them being missense mutations.3 Mutations in a GATA transcription factor-binding site located in a transcriptional enhancer element in intron 1 of the ALAS2 gene have also been reported.4,5 Most ALAS2 mutations responsible for sideroblastic anemia are partial loss-of-function alleles affecting heme biosynthesis. A deleterious ALAS2 mutation causing X-linked macrocytic dyserythropoietic anemia in females was reported recently.6
Variable phenotypic expression and penetrance of XLSA
The phenotypic expression of XLSA is highly variable, presumably because the different mutations have diverse effects on ALAS2 enzymatic activity. In addition, within a single familial tree, the penetrance of XLSA is variable; hemizygous males with ALAS2 mutation without the phenotype of XLSA may occur, and these individuals are likely to go unnoticed.7 Hemizygous males with XLSA may present in the first 2 decades of life with symptoms of anemia or later with either manifestations of anemia and/or those of parenchymal iron overload.8
Anemia is generally mild to moderate and is rarely transfusion dependent: it is typically microcytic [mean corpuscular volume (MCV) between 60 and 70 fL] and hypochromic [mean corpuscular hemoglobin (MCH) <27 pg] and is characterized by elevated values for red cell distribution width (RDW >14%). Perls staining of the bone marrow aspirate allows the detection of ring sideroblasts. However, for a conclusive diagnosis of XLSA, the identification of the ALAS2 mutation is now required: molecular diagnosis is of fundamental importance for genetic counseling.
XLSA as a typical iron-loading anemia that may become clinically manifest with complications of iron overload in middle-aged men
The vast majority of patients with XLSA have evidence of parenchymal iron overload in the absence of blood transfusion requirement. This feature is also typical of patients with congenital dyserythropoietic anemia and those with β-thalassemia intermedia. In these conditions, iron loading is independent of the degree of anemia, although it is closely related to the patient's age and the degree of ineffective erythropoiesis.9 Expanded but ineffective erythropoiesis leads to increased iron absorption and reticuloendothelial iron release, presumably through suppression of hepcidin production.10 Whether this suppression is mediated by erythroferrone alone11 or, more likely, also by additional factors remains to be established.
Complications of iron overload are typically the first manifestation of disease in middle-aged men with mild anemia. These patients may have marginally low hemoglobin levels, so that it is only the low MCV volume that should alert the physician about a possible diagnosis of XLSA. Coinheritance of the HFE (C282Y) allele, whose frequency is ∼0.05 in populations of Caucasian ancestry, represents a risk factor for worsening parenchymal iron overload in XLSA.8
Late-onset XLSA and age-related clonal hematopoiesis (clonal hematopoiesis of indeterminate potential): a link between inherited and somatic mutations
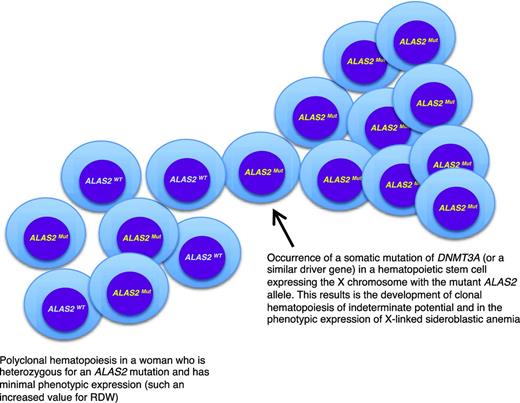
A peculiar clinical issue is late-onset XLSA, that is, a condition that becomes clinically manifest with symptoms of anemia in elderly individuals. In a few cases, this may simply reflect the fact that the anemia is so mild that diagnosis is made only late in life.12 Late-onset XLSA also has been described in heterozygous women who preferentially express the mutant allele in erythroid cells.12,13 Most females heterozygous for an ALAS2 mutation have only minor red cell abnormalities, such an increased RDW attributable to the dimorphic red cell population. However, lyonization may be unbalanced on a genetic basis and/or skewed X-chromosome inactivation may develop late in life, thereby leading to the preferential expression of the mutant allele. Recent studies14,15 have shown that age-related clonal hematopoiesis (also defined as clonal hematopoiesis of indeterminate potential without hematologic abnormalities)16 is a relatively common condition in elderly individuals that is associated with the acquisition of somatic mutations in various genes, most frequently in DNMT3A, ASXL1, and TET2. As shown in Figure 2, in a woman who is heterozygous for an ALAS2 mutation, the development of age-related clonal hematopoiesis may lead (with 50% probability) to the exclusive expression of the mutant ALAS2 allele and the onset of anemia mimicking an acquired condition, such as myelodysplastic syndrome (MDS). Interestingly, the anemia of late-onset XLSA is characterized by borderline MCV values (∼80 fL): this possibility should be considered in the diagnostic workup of an MDS.
A link between inherited and somatic mutations in the pathogenesis of sideroblastic anemia. Schematic representation of the process that may lead to the phenotypic expression of XLSA in a woman who is heterozygous for an ALAS2 mutation. ALAS2WT indicates wild-type allele; ALAS2Mut, mutant allele. The development of age-associated clonal hematopoiesis—in this example caused by the occurrence of a somatic mutation of DNMT3A—may lead to the selective expansion of a hematopoietic stem cell that expresses the X chromosome carrying the mutant ALAS2 allele. This would in turn lead to the phenotypic expression of XLSA, as happens in hemizygous males. The interaction between XLSA and clonal hematopoiesis may alter some features of XLSA, such as the MCV, which can be borderline (∼80 fL) in patients with clonal hematopoiesis rather than markedly decreased (60-70 fL) as it is in typical hemizygous males.
A link between inherited and somatic mutations in the pathogenesis of sideroblastic anemia. Schematic representation of the process that may lead to the phenotypic expression of XLSA in a woman who is heterozygous for an ALAS2 mutation. ALAS2WT indicates wild-type allele; ALAS2Mut, mutant allele. The development of age-associated clonal hematopoiesis—in this example caused by the occurrence of a somatic mutation of DNMT3A—may lead to the selective expansion of a hematopoietic stem cell that expresses the X chromosome carrying the mutant ALAS2 allele. This would in turn lead to the phenotypic expression of XLSA, as happens in hemizygous males. The interaction between XLSA and clonal hematopoiesis may alter some features of XLSA, such as the MCV, which can be borderline (∼80 fL) in patients with clonal hematopoiesis rather than markedly decreased (60-70 fL) as it is in typical hemizygous males.
Management of XLSA
Management of XLSA involves treatment of anemia, prevention and treatment of iron overload, and genetic counseling. The responsiveness of patients with XLSA to oral pyridoxine supplementation varies considerably: in terms of amelioration of anemia, the response is primarily influenced by ALAS2 mutation type and may be absent, partial, or complete.17 Overall, the majority of patients with XLSA are to some extent responsive to pyridoxine, and every patient should initially be treated with pyridoxine at a dose of 75 to 150 mg/d. In responsive patients, this treatment ameliorates or normalizes the hemoglobin level, whereas MCV, MCH, and RDW remain somewhat abnormal. Once a response has been achieved, the lowest dose of pyridoxine needed to maintain an adequate hemoglobin level should be determined: in fact, doses >100 mg/d for prolonged periods of time may cause a sensory neuropathy in some patients. Dosages up to 300 mg/d should be used only in unresponsive or partially responsive patients, paying specific attention to the issue of sensorial neuropathy.
Response to pyridoxine supplementation is affected by body iron status, and iron overload may suppress pyridoxine responsiveness.8 Patients with iron overload can safely undergo mild phlebotomy programs under pyridoxine supplementation: venesections (∼250-350 mL each or 4-5 mL/kg body weight) can be performed every 2 weeks without any significant decline in hemoglobin level as long as there is parenchymal iron overload.
Autosomal recessive sideroblastic anemia attributable to mutations in SLC25A38
SLC25A38 encodes the erythroid-specific mitochondrial carrier protein that is important for the biosynthesis of heme in eukaryotes. In 2009, biallelic germline mutations in SLC25A38 were shown to be responsible for autosomal recessive pyridoxine-refractory sideroblastic anemia in Canadian families.18 These observations have been confirmed in 2 subsequent studies, indicating that mutations in the SLC25A38 gene cause severe, nonsyndromic, sideroblastic anemia in many populations.19,20 The study by Bergmann et al19 provides information about the prevalence of the different types of congenital sideroblastic anemias. These researchers systematically analyzed a cohort of 60 previously unreported patients, searching for ALAS2, SLC25A38, PUS1, GLRX5, and ABCB7 mutations. Twelve probands had biallelic mutations in SLC25A38, whereas 7 had ALAS2 mutations and 1 had a novel homozygous null PUS1 mutation.
The sideroblastic anemia attributable to mutations in SLC25A38 is microcytic, hypochromic, and severe: nearly all the patients reported so far developed a regular need for transfusion. Sequencing of SLC25A38 is now needed for diagnosis. The clinical course of this congenital anemia is very similar to that of thalassemia major, and conservative therapy includes regular red cell transfusion and iron chelation therapy. As in thalassemia major, allogenic stem cell transplantation represents the only curative therapy at present and should therefore be considered in young patients with this disorder.
Clonal acquired sideroblastic anemias (myeloid neoplasms with ring sideroblasts)
The most common acquired sideroblastic anemia is refractory anemia with ring sideroblasts (RARS) as defined as in the World Health Organization classification of MDSs.21 Variants of this condition include refractory cytopenia with multilineage dysplasia and ring sideroblasts (RCMD-RS) and RARS associated with marked thrombocytosis (RARS-T). This latter condition combines the myelodysplastic features of RARS, thrombocytosis (platelet count ≥450 × 109/L) and the presence of large atypical megakaryocytes in the bone marrow similar to those observed in myeloproliferative neoplasms (MPNs)22,23 ; RARS-T is classified currently within the MDS/MPN.
The genetic basis of clonal acquired sideroblastic anemias is summarized in Table 1 and analyzed below.
Somatic mutations of RNA splicing machinery in myeloid neoplasms
The molecular basis of RARS and related disorders was deciphered in 2011 by Papaemmanuil et al24 and Yoshida et al.25 The investigators of the Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium identified somatically acquired point mutations in SF3B1 in the protein-coding exons in 6 of 8 patients with RARS.24 All mutations clustered in exons 12 to 16 of the gene and appeared to be heterozygous substitutions; SF3B1 (K700E) accounted for >50% of the variants observed. Follow-up revealed SF3B1 mutations in ∼20% of patients with MDSs, with a particularly high frequency among patients whose disease was characterized by ring sideroblasts. Yoshida et al25 performed whole exome sequencing in 29 patients with various subtypes of MDS and found somatic mutations in genes of the RNA splicing machinery, including SF3B1, SRSF2, U2AF1, and ZRSR2. Follow-up in a large patient population showed that RNA splicing mutations were frequent in patients with MDS and MDS/MPN and that SF3B1 mutations were associated closely with ring sideroblasts.25
In a subsequent study, we screened the coding exons of SF3B1 in patients with myeloid neoplasms and found somatic mutations in ∼30% of patients with MDS and 20% of patients with MDS/MPN.26 A significantly higher mutation prevalence was noticed in the “sideroblastic” categories of MDS and MDS/MPN, including RARS, RCMD-RS, and RARS-T. The close relationship between SF3B1 mutations and ring sideroblasts in myeloid neoplasms has been confirmed in all subsequent studies.27 This is consistent with a causal relationship and makes SF3B1 the first gene to be associated strongly with a specific morphological feature in myeloid neoplasms.
RARS, RCMD-RS, and RARS-T share the SF3B1 mutation but differ in the so-called subclonal or co-occurring mutations. RCMD-RS frequently has co-occurring mutations in genes of other biologic pathways, such as DNA methylation. In addition, a fraction of RCMD-RS can be associated with somatic mutations of other genes of RNA splicing machinery, such as SRSF2. RARS-T results from a combination of an SF3B1 mutation, as an initiating event, and subclonal mutations in genes such as JAK2, MPL, or CALR, which cause myeloproliferative features.23
Somatic mutation of RNA splicing machinery as a novel paradigm of disease pathophysiology
Somatic mutations of RNA splicing machinery, particularly SF3B1 mutations, frequently represent the initiating genetic event in MDSs and related myeloid neoplasms. However, it is still unclear how these mutations cause clonal proliferation of hematopoietic stem cells, myelodysplasia, and ineffective hematopoiesis, although recent studies have considerably advanced the field. Kim et al28 have shown that SRSF2 mutations alter the recognition of specific exonic splicing enhancer motifs to drive recurrent missplicing of key hematopoietic regulators, including EZH2. Shirai et al29 found that mutant U2AF1 alters downstream gene isoform expression, particularly in RNA processing genes, ribosomal genes, and recurrently mutated MDS and acute myeloid leukemia (AML)-associated genes.
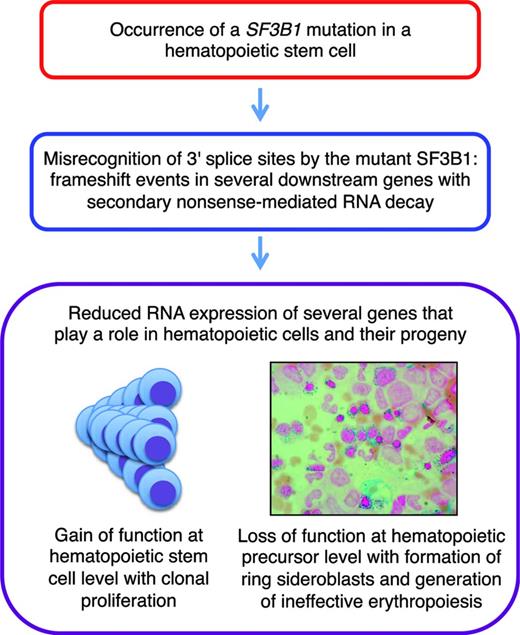
We recently did a comprehensive analysis of aberrant RNA splicing in CD34-positive cells and circulating granulocytes from patients with MDSs.30 We found hundreds of splicing events significantly enriched in SF3B1-mutated cases, most of which were caused by misrecognition of 3′ splice sites; ∼50% of these altered 3′ splice sites resulted in a frame shift. More recently, we found that these abnormal transcripts are degraded by nonsense-mediated RNA decay. These observations indicate that SF3B1 mutations cause deleterious effects in many downstream genes simultaneously, particularly in genes involved in heme biosynthesis, cell cycle progression, and DNA repair, leading to reduced expression of these genes. A schematic representation is reported in Figure 3.
A recent study by McKerrell et al31 using ultra-deep sequencing in unselected individuals showed that mutations in SF3B1 and SRSF2 were identified selectively in individuals aged ≥70 years; this suggests that selection pressure related to the aging of the hematopoietic system and the bone marrow environment may contribute to the expansions of clones harboring these gene mutations.
The molecular mechanisms underlying iron accumulation in the mitochondria of immature red cells from patients with RARS is still unclear.27 A reduced expression of ABCB7 has been reported previously in these patients.32 Interestingly, recent RNA sequencing studies in the TF-1 cell line expressing SF3B1 (K700E) showed that missplicing of ABCB7 introduces a frame shift, generating a premature stop codon that leads to nonsense mediated decay of the RNA; this would explain the reduced expression of ABCB7.33 It remains to be seen how this and the abovementioned abnormal splicing events in genes of heme biosynthesis play a role in the pathogenesis of mitochondrial iron overload.
SF3B1-mutant MDS as a distinct nosologic entity
To identify mutation patterns that affect disease phenotype and clinical outcome, we recently performed a comprehensive mutation analysis in patients with a myeloid neoplasm and 1% or more ring sideroblasts.34 SF3B1 mutations were detected in 129 of 159 cases (81%) of RARS or RCMD-RS. Among the other patients with ring sideroblasts, a lower prevalence of SF3B1 mutations and a higher prevalence of mutations in other splicing factor genes were observed. In multivariate analyses, patients with SF3B1 mutations showed a significantly better overall survival and a lower cumulative incidence of disease progression compared with SF3B1-unmutated cases. The independent prognostic value of an SF3B1 mutation was retained when the analyses were restricted to MDS subgroups without excess blasts, as well as to sideroblastic categories (RARS and RCMD-RS). Among SF3B1-mutated patients, coexisting mutations in DNA methylation genes were associated with multilineage dysplasia but had no effect on clinical outcome. TP53 mutations were frequently detected in patients without an SF3B1 mutation and were associated with poor clinical outcome. We concluded that the presence of an SF3B1 mutation identifies a distinct MDS subtype that is unlikely to develop detrimental subclonal mutations and is characterized by an indolent clinical course and a favorable outcome.34
Clinical features and treatment of clonally acquired sideroblastic anemias
RARS is characterized by isolated anemia, erythroid dysplasia only, <5% blasts, and ≥15% ring sideroblasts in the bone marrow. Anemia is most often macrocytic (MCV >100 fL), whereas white blood cell and platelet counts are generally normal at presentation.22 Most patients have some evidence of iron overload, as indicated by increased serum iron, transferrin saturation, and serum ferritin, and inappropriately low hepcidin levels have been described previously, indicating that RARS behaves as an iron loading anemia.35 However, parenchymal iron overload becomes clinically relevant only in patients who become transfusion dependent. The anemia of RARS is usually mild and stable for many years but tends to worsen with time, eventually leading to regular transfusion dependence.36 A small fraction of patients may progress to AML.
RCMD-RS is characterized by dysplasia in ≥10% of cells in ≥2 cell lineages in the bone marrow. In contrast to RARS, patients with RCMD-RS have a significantly higher risk of death from bone marrow failure or evolution into AML.36
Patients with RARS or RCMD-RS without adverse cytogenetic features and asymptomatic cytopenias do not need any treatment and should be followed regularly. The safety of the watchful-waiting approach is dependent on regular monitoring aimed at early recognition of worsening cytopenias, increasing number of bone marrow blasts, and cytogenetic evolution.
When moderate-to-severe anemia occurs, patients may benefit from treatment with recombinant human erythropoietin alone or in combination with granulocyte-colony stimulating factor.37 Patients without a transfusion requirement before erythropoietin treatment and with low pretreatment serum erythropoietin levels (<100 U/L) have a higher probability of response.38 However, most patients who improve with treatment lose responsiveness over time.
The onset of a regular red blood cell transfusion requirement in patients with RARS or RCMD-RS is associated with a worsening of survival.39 Severe anemia results in a significantly higher incidence of cardiac complications in these elderly patients.40 In addition, taking into account life expectancy, transfusion-dependent patients with RARS are more likely to develop deleterious consequences of parenchymal iron overload41 : therefore, iron chelation therapy may be considered in these individuals.42
A novel therapeutic perspective: targeting ineffective erythropoiesis in RARS
Two recent studies have shown that GDF11 is a regulator of erythropoiesis and that its inhibition in mouse models of anemia caused by ineffective erythropoiesis restores normal erythroid maturation and ameliorates anemia.45,46 More specifically, compounds such as ACE-53 or its mouse version RAP-536, targeting molecules of the transforming growth factor-β (TGF-β) superfamily and specifically inhibiting GDF11, were found to correct anemia by promoting late-stage erythropoiesis in a mouse model of MDS.46 A recent clinical trial in patients with MDS showed that ACE-536 (also known as luspatercept) ameliorates anemia in patients with RARS carrying an SF3B1 mutation.47 Similar results have been obtained with ACE-011 (also known as sotatercept).48 Thus, administration of inhibitors of TGF-β superfamily members may represent a novel way of targeting ineffective erythropoiesis in patients with RARS, but phase 3 clinical trials are needed to confirm the efficacy of these compounds.
Correspondence
Mario Cazzola, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, I-27100 Pavia, Italy. E-mail: mario.cazzola@unipv.it.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: Luspatercept and sotatercept.