Abstract
The major practical advantage of the direct oral anticoagulants (DOACs), comprising the thrombin inhibitor dabigatran and the factor Xa inhibitors apixaban, edoxaban, and rivaroxaban, over vitamin K antagonists is their fixed dosing without the need for laboratory monitoring. With the recent, rapid introduction of the DOACs for the treatment of acute venous thromboembolism (VTE), clinicians are now faced with various questions regarding the efficacy and safety of these compounds overall and in specific high-risk populations. The collective evidence from 6 large clinical trials involving 27,000 patients has demonstrated that DOACs are as effective as vitamin K antagonists (VKA) in preventing recurrent VTE while being associated with a significantly lower risk of major bleeding. These findings are consistent in subgroups of patients with pulmonary embolism, the elderly, and those patients with a high body weight or moderate renal insufficiency, making these agents suitable for a broad spectrum of patients with VTE. DOACs are also an attractive treatment option in patients with VTE and concomitant cancer, thrombotic antiphospholipid syndrome, or heparin-induced thrombocytopenia, but the currently available clinical data is insufficient to make evidence-based recommendations on the use of DOACs in these settings. Several studies evaluating the efficacy and safety of DOACs in these high-risk populations are underway.
Learning Objectives
Direct oral anticoagulants are an effective and safer treatment option for acute venous thromboembolism compared with standard treatment in the general population, as well as in patients with pulmonary embolism, the elderly, those with moderate renal impairment and patients with a body weight over 100 kg
The use of direct oral anticoagulants in patients with cancer, antiphospholipid syndrome, and heparin-induced thrombocytopenia is not yet recommended, but studies evaluating their efficacy and safety in these clinical conditions are ongoing
Background
Anticoagulant treatment of thrombotic conditions dates back to the 1930s and 1940s when unfractionated heparin (UFH) and vitamin K antagonists (VKA) were the first drugs to become available. They proved to be effective in preventing death in patients with venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), and remained the mainstay of anticoagulant therapy in the decades thereafter.1-3 The introduction of low-molecular-weight heparin (LMWH) in the 1980s marked the next breakthrough. These drugs could be injected subcutaneously in fixed doses, which simplified the initial management of acute VTE greatly and opened up the opportunity to treat these patients at home. From that time on, a treatment regimen of initial LMWH followed by long-term VKA for at least 3 months was considered standard of care for patients with VTE.
Recently, within a time span of nearly 4 years, the thrombin inhibitor dabigatran4,5 and the factor Xa inhibitors apixaban,6 edoxaban,7 and rivaroxaban,8,9 collectively termed direct oral anticoagulants (DOACs), were compared with conventional therapy for treatment of acute VTE in 6 large clinical trials. Now that these agents have been approved by the respective regulatory agencies around the world, physicians yet have another treatment option for their patients with VTE. This contemporary DOAC revolution has led to a quickly changing landscape of antithrombotic treatment. Clinicians are faced with various clinical questions regarding the efficacy and safety of these drugs overall and in subgroups of high-risk patients. Therefore, in the present review, we briefly discuss the collective evidence from the phase 3 clinical trials evaluating DOACs for VTE treatment. In addition, we will assess the potential role of DOACs in patients with cancer, the antiphospholipid syndrome (APS), or heparin-induced thrombocytopenia (HIT).
Methods of DOAC trials in the setting of acute VTE
More than 27,000 patients were enrolled in six randomized clinical trials that compared the efficacy and safety of either one of the DOACs with VKA therapy targeted at INR of 2-3 in the treatment of acute, symptomatic DVT or PE. The main features of the studies are shown in Table 1. Importantly, the definitions of recurrent VTE and bleeding were similar across all studies and followed the recommendations of the International Society on Thrombosis and Haemostasis (ISTH).10 Outcome events in all trials were adjudicated by the same independent committee who was blinded for treatment allocation, resulting in a similar objective judgement of recurrent VTE and bleeding. Noteworthy differences between the trials are the use of an initial LMWH lead-in period of 5 days in patients receiving dabigatran or edoxaban, whereas apixaban and rivaroxaban where given as oral monotherapy, and the open-label design of the studies evaluating rivaroxaban opposed to the double-blinding in the other studies.
Characteristics of phase 3 studies evaluating the efficacy and safety direct oral anticoagulants for treatment of acute venous thromboembolism
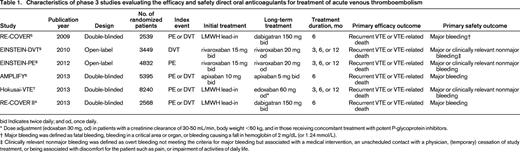
bid Indicates twice daily; and od, once daily.
* Dose adjustment (edoxaban 30 mg, od) in patients with a creatinine clearance of 30-50 mL/min, body weight <60 kg, and in those receiving concomitant treatment with potent P-glycoprotein inhibitors.
† Major bleeding was defined as fatal bleeding, bleeding in a critical area or organ, or bleeding causing a fall in hemoglobin of 2 mg/dL (or 1.24 mmol/L).
‡ Clinically relevant nonmajor bleeding was defined as overt bleeding not meeting the criteria for major bleeding but associated with a medical intervention, an unscheduled contact with a physician, (temporary) cessation of study treatment, or being associated with discomfort for the patient such as pain, or impairment of activities of daily life.
Recently, we reviewed and summarized the evidence from these phase 3 trials in a meta-analysis.11 Using these data, we will answer clinically relevant questions to aid the physician in choosing the optimal treatment for his or her patient with VTE. Specifically, we will discuss the overall efficacy and safety of DOACs compared with VKA, differences between dabigatran and the factor Xa inhibitors, and the efficacy and safety of DOACs in high-risk subgroups of patients with PE, elderly patients, obese patients, and patients with moderate renal impairment.
Clinical questions regarding the efficacy and safety of DOACs for VTE treatment overall and in high-risk subgroups
Clinical question 1: what is the overall efficacy and safety of DOACs in the treatment of acute symptomatic VTE compared to VKAs?
The main finding of the aforementioned meta-analysis was that DOACs are as effective as VKA in preventing recurrent VTE.11 Because all trials were designed to demonstrate noninferiority of DOACs, this should reassure the clinician of the overall efficacy of these agents. Most importantly, the comparable efficacy observed in DOAC recipients was paralleled by a 40% relative reduction in major bleeding compared with VKA. Consistent with this substantial reduction in major bleeding, DOACs were associated with a significant 60% relative reduction in the incidences of intracranial and fatal bleeding, the most feared components of major bleeding. In line with the reductions in major bleeding, patients on DOACs had a 25% lower relative risk of experiencing clinically relevant nonmajor (CRNM) bleeding. Of note, no significant difference in the incidence of major gastrointestinal bleeding between DOACs and VKA was observed, unlike earlier reports raising concerns on an increased risk of gastrointestinal bleeding in patients on DOACs.12 In support of this finding, a recent population-based study confirmed a similar risk of gastrointestinal bleeding associated with DOACs compared with VKAs in non-atrial fibrillation patients.13
Clinical question 2: are there any differences between the factor Xa inhibitors and thrombin inhibitors with respect to efficacy and safety?
Although the results of the studies evaluating oral direct thrombin and factor Xa inhibitors are frequently combined, in fact they are two distinct drug classes with important pharmacokinetic and pharmacodynamic differences. Compared to oral factor Xa inhibitors, dabigatran has a lower bioavailability requiring administration as a prodrug, has a longer half-life, and is more dependent on renal clearance. Dabigatran is not hepatically metabolized by the cytochrome P450 system and could therefore be a treatment option in patients with liver disease. In contrast, factor Xa inhibitors undergo significant CYP3A4 metabolism and thus are contraindicated in patients with significant liver impairment. Of note, also among the factor Xa inhibitors there are some differences in pharmacologic properties. Clinicians should take these drug characteristics into account when choosing the DOAC that best suits their individual patient, but they also want to know whether there are any important differential effects between these two classes of DOACs in terms of efficacy and safety. Table 2 shows the combined efficacy and safety results for the dabigatran studies and factor Xa inhibitor studies separately, based on data from the aforementioned meta-analysis. Overall, there are no significant differences in efficacy and safety between dabigatran and factor Xa inhibitors. Hence, the choice for a specific DOAC should primarily be based on drug characteristics (eg, renal clearance), treatment regimen (eg, once vs twice daily dosing), and preference for a LMWH lead-in rather than the efficacy or safety profile.
Clinical question 3: can I prescribe DOACs also to high-risk patients including those with PE, body weight >100 kg, the elderly (ie, age ≥75 years), or a moderate renal insufficiency?
The overall favorable efficacy and safety of DOACs in the setting of VTE compared with VKA is reassuring and justifies their use in the general population. However, not all clinicians are confident in prescribing these drugs in specific subgroups of high-risk patients. They may have questions regarding the efficacy of DOACs in patients presenting with PE, have concerns that patients with a high body weight will not reach sufficient drug blood levels putting them at risk of recurrent VTE, and fear that the risk of DOAC-related major bleeding complications may be higher in elderly patients, as well as in those with moderate renal insufficiency given the partial renal clearance of DOACs. Because the individual studies were powered only to detect differences in the primary outcomes in the total study population, the results of the meta-analysis provide valuable information as to the relative benefit of DOACs in these clinically important subgroups. Combined efficacy and safety data for the subgroups are presented in Table 3. The similar efficacy of DOACs compared to VKAs is consistent in patients presenting with PE, patients with a body weight >100 kg, patients aged 75 years or older, and patients with a creatinine clearance between 30 and 50 mL/min. Moreover, the overall significant reduction in major bleeding associated with DOACs is maintained in the subgroups of elderly patients and patients with moderate renal insufficiency. Based on these results, DOACs should be considered to be an appropriate treatment option for acute VTE in a broad spectrum of patients.
Recurrent venous thromboembolism and major bleeding associated with direct oral anticoagulants compared with vitamin K antagonists in clinically important subgroups
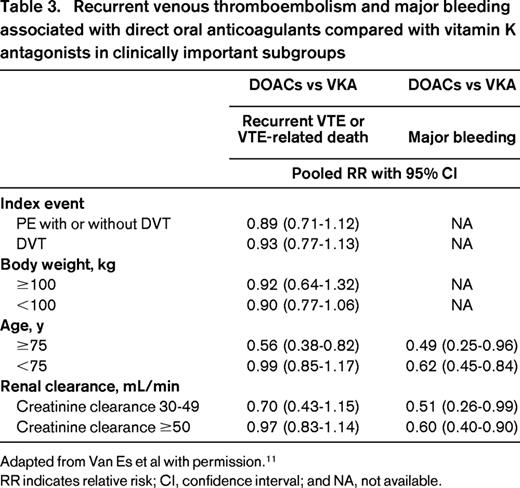
Adapted from Van Es et al with permission.11
RR indicates relative risk; CI, confidence interval; and NA, not available.
Other high-risk populations with VTE
Clinical question 4: are DOACs suited for my cancer patient with VTE?
It is estimated that 20% of all VTE are related to cancer and anticoagulant treatment in these vulnerable patients is often challenging.14 Cancer patients with VTE are at increased risk of developing recurrent VTE compared to noncancer patients, but also have a higher risk of major bleeding. LMWH is currently recommended over VKA for treatment of cancer-associated VTE due to its better efficacy in preventing recurrent VTE with a similar risk of bleeding compared to VKA.15,16 However, LMWH therapy may be burdensome, particularly in cancer patients that often require indefinite anticoagulant treatment. Because DOACs offer a simple oral treatment regimen without the need for anticoagulation control, they could be an attractive alternative. However, at present, clinicians should refrain from providing these drugs to cancer patients since they have not been compared directly to LMWH. Other concerns are possible drug-drug interactions with antineoplastic agents, in particular P-glycoprotein inhibitors, and the gastrointestinal absorption of DOACs in vomiting cancer patients or those with chemotherapy-induced intestinal mucosal defects. Nevertheless, the subgroup analysis of the 1500 cancer patients enrolled in the DOAC trials does provide guidance for those situations when LMWH is not an option, for instance when patients are unable or unwilling to take daily subcutaneous injections. Interestingly, in these patients, a possibly better efficacy of DOACs compared with VKAs without an increased risk of major bleeding was observed.11 It should however be realized that cancer patients in the trials probably had a favorable cancer prognosis compared to the real-world cancer population. Patients with a limited life expectancy were excluded from the trials, as were patients in whom long-term VTE treatment with LMWH was anticipated. This is reflected by the absolute recurrent VTE rate of 5.9% in VKA-treated cancer patients in the trials, which is substantially lower than the rate in the VKA groups of the CLOT trial17 (11%) and the recent CATCH trial18 (10%) after 6 months of treatment.
Recently, the pooled results of the subgroup of cancer patients enrolled in the trials comparing dabigatran with VKA was reported, providing information on the characteristics of these patients as well as on cancer-prognostic data.19 Active cancer was defined as cancer within 5 years prior to the VTE diagnosis. Compared with noncancer patients, the 221 cancer patients were older and had a lower renal clearance. The time spent within the therapeutic INR range of 2-3 was comparable between VKA-treated cancer and noncancer patients (54.5% and 58.3%, respectively) which is higher than in the CLOT17 (46%) and CATCH18 (47%) trials. The most prevalent tumor types were prostate, colorectal, and breast cancer, and 13% had metastasis. During 6 months follow-up, no significant differences were observed between dabigatran and VKA with respect to recurrent VTE or major bleeding. Detailed results of the cancer subgroups from the studies evaluating apixaban and edoxaban are expected shortly.
Before DOACs become an accepted treatment option for cancer-associated VTE, they have to be evaluated in a head-to-head comparison with LMWH. To this end, an important study comparing the efficacy and safety of edoxaban to LMWH in the setting of cancer-associated VTE has recently been started. One thousand patients will be randomized and followed for the occurrence of one of the components of the combined primary outcome of recurrent VTE or major bleeding. Following observations of a similar risk of recurrent VTE in patients with active cancer and those with a history of cancer,20 patients with VTE will be allowed to enter the study either with active cancer or cancer treated within 2 years prior to the VTE diagnosis. Moreover, incidentally detected lower extremity DVT or PE are allowed as an entry diagnosis because treatment and outcome is similar for this group of patients. A last innovative feature of the study is the follow-up time of 12 months, which will provide valuable data regarding the treatment of patients with VTE and cancer beyond 6 months.
In conclusion, DOACs may become an attractive treatment for cancer-associated VTE, but at present LMWH remains the preferred option. Although the results of the trial population with cancer are encouraging, further clinical studies are needed to evaluate the efficacy and safety of DOACs directly against LMWH in this population.
Clinical question 5: can I treat my patients with thrombosis and the antiphospholipid syndrome with a DOAC?
APS is an autoimmune disorder characterized by arterial or venous thrombosis or pregnancy complications in patients who repeatedly test positive for circulating antiphospholipid antibodies (aPLA), i.e. lupus anticoagulant, anti-β2-glycoprotein-1 antibodies, or anti-cardiolipin antibodies. It is estimated that 10% of all patients with VTE have circulating aPLA and these patients are at increased risk of recurrent VTE compared to VTE patients without aPLA.21 When patients test positive for all three types of aPLA, the risk of on-treatment recurrence is as high as 25% over 5 years.22 The treatment of thrombotic APS is similar to the treatment of VTE in the general population (VKA targeted at an INR of 2 to 3), but is often extended indefinitely in case of persisting aPLA.23
Now that DOACs are increasingly used for the treatment of VTE, the question arises whether these drugs are effective and safe for VTE treatment in the setting of APS too. From a patient perspective, DOACs may be a significant amelioration in this usually young population for whom lifelong anticoagulation with VKAs and frequent INR monitoring is not attractive.24 Given that aPLA are prevalent in patients with VTE, the aforementioned DOAC trials in the setting of VTE must have enrolled patients with APS because this was not an exclusion criterion. However, patients were not routinely tested for aPLA and outcomes for the patients with known aPLA have not been reported. Therefore, the current knowledge of the efficacy and safety of DOACs for thrombotic APS is exclusively derived from case series and retrospective cohort studies. These represent a select population of thrombotic APS patients that were switched to a DOAC often because of labile INRs, bleeding on VKA, or logistic problems with INR monitoring. In a retrospective multicenter cohort study, Noel et al25 enrolled 26 DOAC-treated patients who had thrombotic APS for a median duration of 3 years. Seven patients had tested positive for all 3 types of aPLA and another 12 patients had lupus anticoagulant. Six of 26 patients received rivaroxaban or dabigatran as first-line treatment, whereas the others had been treated previously with another anticoagulant for 1 to 23 years. During a median follow-up of 1.5 years, 1 patient (3.8%) developed recurrent VTE and in 2 patients (7.7%) a bleeding event occurred that led to discontinuation of DOAC therapy. Another report on the use of DOACs for thrombotic APS comes from Son et al.26 They describe a case series of 12 consecutive APS patients started on rivaroxaban as second-line treatment 12-46 months after their last thrombotic APS event. Five patients were triple-positive for aPLA, and another 3 tested positive for lupus anticoagulant. Follow-up ranged from 2-16 months during which 2 patients with the highest risk profile developed recurrent VTE. Although these 2 small patient series do not prove the effectiveness of DOACs in APS patients, they suggest that the VTE recurrence rate is likely not higher with DOACs than with VKA.
However, other authors have raised concerns about the effectiveness of DOACs to prevent recurrent APS-related VTE. Schaefer et al27 reported on a case series of 3 thrombotic APS patients that developed recurrent thrombosis 5-6 months after switching from VKA to a DOAC. Similarly, Win and Rodgers28 presented 3 thrombotic APS patients developing recurrent thrombosis 6-12 months following initiation of second-line DOAC treatment. The cause of these observations of DOAC-failures is unknown. The recurrence rate in APS patients is high and these failures may have otherwise developed under VKA therapy. Moreover, compliance may have been a contributing factor in these patients who were often switched to a DOAC due to difficulties with their INR control. Alternative explanations may be mechanisms intrinsically related to DOACs or their dosing regimens. For instance, inhibition of a single clotting factor might be insufficient to completely block the thrombotic potential in severe APS patients, or the short half-life of DOACs causes the procoagulant stimulus to overcome the anticoagulant effect at trough levels.
There are currently three studies ongoing or finished in thrombotic APS patients that will provide valuable data on the relative efficacy of DOACs compared with VKA. The Rivaroxaban in AntiPhospholipid Syndrome (RAPS) study (http://isrctn.org/ISRCTN68222801) is a randomized clinical trial of rivaroxaban versus warfarin in patients with thrombotic APS who have had a VTE and have been on warfarin for at least 6 months.29 The primary aim is to demonstrate that the intensity of anticoagulation achieved with rivaroxaban is not inferior to that of warfarin as assessed by the percentage change in endogenous thrombin potential from randomization to day 42. The study has been completed recently after enrollment of 156 patients and the preliminary results were reported in abstract form at the ISTH 2015 congress.
The second study is The Rivaroxaban in Thrombotic Antiphospholipid Syndrome (TRAPS) study (https://clinicaltrials.gov/ct2/show/NCT02157272) which is a randomized open-label trial that aims to demonstrate the noninferiority of rivaroxaban versus warfarin on the composite outcome of acute thrombosis, major bleeding or death during 4 years follow-up. The sample size is 536 and the estimated completion date is December 2018.
The third study is the Rivaroxaban in Antiphospholipid Syndrome Pilot Study. This feasibility study aims to enroll 150 APS with prior venous thrombosis patients by December 2016. Patients will be treated with rivaroxaban 20 mg once daily and followed for a minimum of 1 year for the occurrence of bleeding or (recurrent) venous or arterial thrombosis.
In summary, current anecdotal evidence on the use of DOAC in thrombotic APS patients is conflicting. As a consequence, physicians should be careful with prescribing DOACs in these patients until robust data become available.
Clinical question 6: are DOACs a treatment option for preventing or treating thrombosis in patients with heparin-induced thrombocytopenia?
HIT is a complication of treatment with UFH or, less commonly, LMWH and is characterized by formation of antibodies to heparin-platelet factor 4 (PF4) complexes.30 These antibodies may trigger platelet activation in turn resulting in venous or arterial thrombosis, so-called HIT associated with thrombosis (HITT). A decrease in platelet counts is usually observed 5-10 days after initiation of heparin exposure. In patients with confirmed HIT, cessation of heparin therapy alone is not sufficient to prevent HITT and treatment with an alternative anticoagulant is required. Traditionally, 2 classes of anticoagulants have been used for the initial treatment of HIT: parenteral direct thrombin inhibitors, including argatroban, bivalirudin, and desirudin, and nonheparin antithrombin-dependent anticoagulants, including fondaparinux and danaparoid.31 Although these drugs are widely used in the treatment of HIT, they have several disadvantages ranging from high costs, low availability, need for drug monitoring, dependence on renal clearance, and the absence of an antidote (Table 4). DOACs could potentially overcome all of these issues. The biochemical structure of DOACs has no similarities with heparin; hence, interactions with PF4 or heparin-PF4 antibodies are not to be expected. This has been confirmed in preclinical laboratory studies that demonstrated that rivaroxaban,32,33 dabigatran,33 and apixaban34 do not cross-react with HIT antibodies and do not induce platelet activation in the presence of HIT antibodies. Hence, on theoretical grounds, these drugs could be used to treat HIT.
Comparison of medications available for treatment of heparin-induced thrombocytopenia
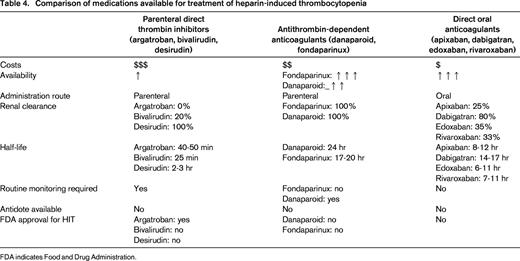
FDA indicates Food and Drug Administration.
Clinical data from case series is limited to 5 HIT patients treated with rivaroxaban and 1 patient with dabigatran as summarized in Table 5. During DOAC treatment, none of these patients experienced thrombotic events and platelet counts fully recovered in all cases. Similarly, in a retrospective cohort study of 22 HIT patients treated with dabigatran, apixaban, or rivaroxaban, no thrombotic events were observed during DOAC treatment. These observations suggest that DOAC are a safe alternative to traditional HIT treatment, but confirmatory studies are needed.
Case reports on the use of direct oral anticoagulants for treatment of heparin-inducted thrombocytopenia
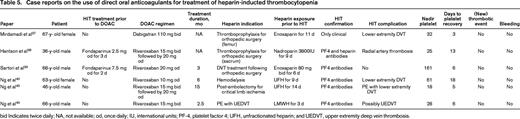
bid Indicates twice daily; NA, not available; od, once daily; IU, international units; PF-4, platelet factor 4; UFH, unfractionated heparin; and UEDVT, upper extremity deep vein thrombosis.
An ongoing study that will provide insight on the efficacy of DOACs for treatment of HIT is the “rivaroxaban for heparin-induced thrombocytopenia study” (https://clinicaltrials.gov/ct2/show/NCT01598168).35 This is a prospective cohort study that aims to recruit 209 patients with an intermediate or high clinical probability of HIT as assessed with the 4T's score.36 These patients will be started on rivaroxaban and subsequently tested for the presence of HIT antibodies. The primary objective is to determine the thromboembolic rate in all patients, irrespective of whether they ultimately are confirmed as having HIT or not. The goal is to show that the thromboembolic rate with rivaroxaban is similar to the rate in historical controls on argatroban. Results are expected in 2016.
In summary, the clinical experience with DOACs for HIT is currently limited, but they have the potential to become the mainstay of anticoagulant treatment in this setting. They do not interact with HIT antibodies and do not cause platelet activation in the presence of HIT antibodies. Together with their favorable pharmacokinetics, high availability, and lower costs, they could be particularly suited for patients with HIT. The results of the ongoing clinical study have to be awaited before the evidence-based use of DOACs in patients with HIT can be recommended.
Conclusion
With the recent introduction of the DOACs, clinicians now have multiple therapeutic options for oral treatment of patients with VTE. DOACs are as effective as VKA in preventing recurrent VTE, but are associated with a significant reduction in the risk of major bleeding and are easier to use with no need for dose adaptations. These findings in the general trial population are consistent with those in patients with PE, the elderly patients, heavy patients, and patients with moderate renal impairment, making DOACs a suitable treatment option for a broad spectrum of patients. Whether DOACs can also safely be used in patients with cancer-associated VTE, thrombotic APS, or HIT is currently not proven. Studies are underway to evaluate DOACs in these high-risk populations.
Correspondence
Harry Büller, Department of Vascular Medicine, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Phone: +31-20-56-65976; Fax: +31 20 696 8833; e-mail: h.r.buller@amc.nl.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.

