Abstract
Obstetric hemorrhage remains a leading cause of maternal morbidity and mortality worldwide. Many postpartum hemorrhages (PPHs) do not have identifiable risk factors; maternity units should therefore have obstetric hemorrhageprotocols in place for all parturients as every pregnancy has the potential to be complicated by hemorrhage. This review will examine the epidemiology of PPH as well as current recommendations for key elements in obstetric hemorrhage protocols. Recent advances in hematologic management of PPH will be also be reviewed, including: (1) recognition of hypofibrinogenemia as a risk factor for severe PPH, (2) use of antifibrinolytic therapy, and (3) strategies for fibrinogen replacement therapy.
Learning Objectives
List recommended elements of obstetric hemorrhage protocols, including estimation of blood loss, provision of emergency blood products, and laboratory testing
Recognize fibrinogen levels consistent with hypofibrinogenemia in postpartum hemorrhage and its association with progression to severe postpartum hemorrhage
Discuss the current data on antifibrinolytic therapy and fibrinogen replacement in postpartum hemorrhage
Obstetric hemorrhage is the leading cause of maternal mortality worldwide. In 2013, the World Health Organization estimated worldwide deaths from obstetric hemorrhage at >78,000 women, comprising 27% of all maternal deaths.1 Furthermore, women in developing countries suffer disproportionately compared to those in developed countries. An analysis of >34 worldwide databases found that 13.4% of maternal deaths in developed countries (including the US, Canada, and Western Europe) resulted from obstetric hemorrhage, as opposed to 33.9% of African women, 30.8% of Asian women, and 20.8% of women from Latin America and the Caribbean.2 Reducing maternal deaths is a worldwide priority, as reflected in the UN Millennium Goals for reducing the maternal mortality ratio by three-quarters from the 1990 baseline by 2015. Substantial progress toward that goal has been achieved, with a 45% reduction between 1990 and 2013.3 In the US, 11.4% of all pregnancy-related mortality was due to hemorrhage from 2006–2010,4 a small decline from the 12.5% reported in 1998–2005.5 However, these optimistic statistics belie that maternal hemorrhagic deaths are among those causes of peripartum mortality which are most avoidable. A statewide review of maternal deaths in North Carolina from 1995–1999 found that 93% of obstetric hemorrhage deaths were preventable6 ; in California from 2002–2005, 70% of such deaths were deemed preventable.7
Mortality from obstetric hemorrhage is an important metric, but incompletely captures its impact on women's health. The World Health Organization (WHO) Multicountry Survey on Maternal and Newborn Health found that 1.2% of women experienced postpartum hemorrhage, with 17.6% of those events resulting in severe maternal outcomes as measured by death or a “near miss” of resultant organ-system dysfunction.8 The US Centers for Disease Control have put forth an alternate definition for identifying severe maternal morbidity, with criterion of either: (1) ICU admission, or (2) transfusion of 4 or more whole blood or packed RBC units.9 Identifying not only maternal deaths, but also severe maternal outcomes, will allow for development and refinement of obstetric hemorrhage interventions.
Definition and etiology of postpartum hemorrhage
Obstetric hemorrhage can broadly be categorized as antepartum, primary postpartum (within 24 hours following birth) and secondary postpartum (from 1 day to 6 weeks following delivery), with the vast majority of cases being primary postpartum. Postpartum hemorrhage (PPH) has traditionally been defined as >500 mL blood loss during vaginal delivery and 1000 mL blood loss during cesarean delivery. A recent PPH definition proposed by the American College of Obstetricians and Gynecologists (ACOG) states it is the “cumulative blood loss of ≥1000 mL or blood loss accompanied by signs/symptoms of hypovolemia within 24 hours following the birth process”.10 This definition also includes the recommendation of “cumulative blood loss of 500–999 mL alone should trigger increased supervision and potential interventions as clinically indicated”. Estimates of PPH prevalence vary widely between regions, with an overall worldwide prevalence of 11% and ranging from 8.5% in Asia to 25.7% in Africa.11 In the US, 2.7% of maternal discharges from 1994–2006 received an ICD-9-CM code for PPH.12
Postpartum hemorrhage is an event resulting from multiple potential causes, including medical, obstetric, and surgical etiologies. An oft-used mnemonic for PPH etiologies is the “4 Ts”: (1) Tone (uterine atony), (2) Tissue (retained placenta or placental abnormalities), (3) Trauma (injury to the uterus, birth canal, and supporting structures), and (4) Thrombin (coagulopathies). Many PPHs will not have an identifiable risk factor prior to hemorrhage, but identification of such risk factors allows for planning to ensure necessary resources and personnel are available at delivery (Table 1). The most common cause of PPH is uterine atony: failure of the uterine muscles to contract after delivery of the placenta. The gravid uterus at term has >500 mL/min flowing through its circulation, with swift compression of the dilated uterine spiral arterioles by uterine muscles necessary to prevent excessive blood loss. Some risk factors for uterine atony include prolonged labor induction, chorioamnionitis, multiple gestation, and fetal macrosomia. Cesarean delivery is a risk factor in subsequent pregnancies for placentation abnormalities, such as placenta previa (placenta partially or completely covering the cervical os) and placenta accreta (abnormal invasion of the placenta into the myometrium). Both placenta previa and accreta have been found to be risk factors for PPH requiring massive transfusion.13 Alarmingly, PPH incidence is increasing in developed countries, driven primarily by increased incidence of uterine atony.12,14
Risk factors associated with postpartum hemorrhage

Adapted from Abdul-Kadir, et al26 with permission.
Obstetric hemorrhage protocols
Management of PPH must coordinate multiple elements rapidly and simultaneously: diagnosing the underlying cause, providing targeted therapies, and delivering blood products swiftly. There is widespread agreement from professional societies and global healthcare agencies, including ACOG, WHO, and the Royal College of Obstetrics and Gynaecology (RCOG), on the need for PPH protocols.15-17
Published protocols are available with recommendations for obstetric, surgical and medical PPH interventions. The National Partnership for Maternal Safety in the US has presented an obstetric patient safety bundle (Table 2) which is recommended for every US maternity unit to have available for every delivery.18 An additional useful resource is the California Maternal Quality Care Collaborative (CMQCC), who updated their comprehensive, web-based obstetric hemorrhage toolkit in 2015.19 A central strategy in PPH protocols is early recognition through regular assessment of uterine tone and vital signs, prophylactic and therapeutic use of uterotonics and active management of the third stage of labor.16 Postpartum hemorrhage is most successfully prevented and treated with uterotonics, but protocols should also have provisions for PPH not controlled by initial strategies. Further obstetric interventions include uterine packing or balloon tamponade of the uterus, with surgical interventions including compressive uterine sutures, arterial ligation, or hysterectomy. Lastly, a critical element for these protocols is clear, explicit communication directed to and acknowledged by team members regarding orders and patient status.19
National Partnership for Maternal Safety: Obstetric hemorrhage patient safety bundle18

Rapid delivery of emergency released, universally-compatible blood products, including O-negative RBC units, is another key elements of PPH protocols. High quality data on transfusion in PPH is lacking, compelling the obstetric community to make recommendations for transfusion management based on the trauma literature.20 Both trauma and obstetric patients may face massive hemorrhage, but obstetric patients have a different coagulation profile at baseline as compared to nonpregnant patients (see below), raising questions if trauma data can be generalized to obstetric patients. The CMQCC recommends delivery of 4-6 RBC units, 4 plasma units, and 1 platelet dose as an initial emergency release blood package for PPH protocols, with use of laboratory data to direct transfusion (and other) therapies to the greatest extent possible.19 Providing a foundation of blood products is a prudent recommendation for resuscitation and coagulopathy treatment, but clinical trials are needed to determine optimal transfusion strategies in PPH.
Obstetric hemorrhage protocols should have clear criteria for protocol activation and early recognition of excessive blood loss is critical for timely activation. Vital sign changes may provide indications of occult hemorrhage, with changes of heart rate, respiration, or blood pressure serving as a trigger for protocol activation. However, it is important to recognize that many parturients will experience hypotension as a late vital sign change only after significant blood loss (1500 mL or more). Blood loss exceeding critical thresholds can also serve as a protocol activation trigger. The CMQCC defined stages of blood loss in PPH progression, with recommendations for interventions based on vital sign changes or cumulative blood loss.19 Visual estimation of blood loss has historically been employed in obstetrics, but it is inaccurate and underestimates actual blood loss volume.21 Quantitative assessments of blood loss is preferable to visual estimation. Two quantitative methods recommended by the CMQCC include: (1) weight of blood-soaked materials (ie, pads, sponges), and (2) use of under-buttock graduated cylinder drapes.19 Providers can avoid capture of nonblood fluids, such as urine or amniotic fluid, by awaiting quantitation of shed blood until after delivery of the infant. As many cases of PPH do not have an identifiable risk, it is recommended that all deliveries have quantitation of blood loss as an additional “vital sign” during the postdelivery period. During PPH protocols, every effort should be made to measure hemorrhage volumes at regular intervals and ensure that this data is clearly communicated with the team leader.
Hypofibrinogenemia in postpartum hemorrhage
Pregnancy is a well-recognized hypercoagulable state, speculatively thought to have evolved as an adaptation to the hemostatic challenge of human childbirth. Progressive elevations in procoagulant factors during pregnancy include von Willebrand factor (vWF), FVII, FVIII, FIX, as well as decreased anticoagulant factors, such as Protein S.22 Notably, fibrinogen levels at term pregnancy are nearly double that of nonpregnant individuals (350-650 mg/dL compared to 200-400 mg/dL).22 Successful diagnosis of coagulopathy in the setting of PPH needs to account for the physiologic elevations of these factors during pregnancy and recognize abnormal values despite being reported within reference range of nonpregnant individuals.
Hypofibrinogenemia in the setting of PPH has in recent literature emerged as a predictor for progression to severe PPH. A study by Charbit et al performed a prospective analysis of 128 women with PPH unresponsive to uterotonics, correlating coagulation laboratory values (including procoagulant, anticoagulant, and fibrinolytic markers) to severity of hemorrhage.23 Severe PPH was defined as transfusion of 4 or more RBC units, Hgb decrease of <4 g/dL, surgical interventions such as hysterectomy, or death. The only marker identified in multivariate analysis as predictive of severe PPH was fibrinogen levels, with those levels <200 mg/dL having a 100% positive predictive value for severe PPH. Similar results were found in a secondary analysis of the PITHAGORE cohort study in France, which examined a subset of 738 women (of the >9000 patient cohort) in whom fibrinogen levels were collected.24 In this study, fibrinogen levels below 2 g/L in women experiencing PPH after vaginal delivery was an independent predictor of severe PPH (odds ratio 11.99; 95% CI: 2.56–56.06). Although these results support that hypofibrinogenemia identified after onset of PPH is predictive of severe PPH, a recent prospective observational of >1900 parturients found no correlation between prelabor fibrinogen levels and either PPH (rs = 0.003, P = 0.90) or PPH in excess of 1000 mL (odds ratio 1.01; C.I 95% 0.85–1.19, P = 0.93).25 These findings emphasize the importance of intrahemorrhage laboratory testing in identifying those women at risk of progression to severe PPH.
Coagulation testing in postpartum hemorrhage
Obstetric hemorrhage can be complicated by consumptive or dilutional coagulopathy. Laboratory assessment of coagulopathy should be performed at time of PPH recognition and repeated frequently (every 30-45 minutes) until the hemorrhage is controlled.19,26 One recommended laboratory panel for PPH includes a platelet count, prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen levels.26 Use of standard coagulation testing during massive hemorrhage has been criticized for slow turnaround times. Fibrinogen levels in particular can have turnaround time of an hour or greater, which either delays goal-directed therapy or necessitates empiric treatment for suspected hypofibrinogenemia. To address this problem, Chandler and colleagues implemented an “emergency hemostasis panel” (EHP) at their institution, comprised of a prothrombin time, fibrinogen level, hematocrit, and platelet count.27 Prior to the EHP, turnaround times for coagulation panels were 35 ± 37 minutes. By ensuring sample prioritization in work queues, streamlining pre-analytical sample processing and adjustment of calibration curves, the turnaround time was reduced to 14 ± 3 minutes. These findings are encouraging for maternity units, as these described process improvements are relatively low cost and do not require purchase of new equipment.
If available, viscoelastic coagulation testing such as thromboelastography (TEG) or rotational thromboelastometry (ROTEM) may be performed in conjunction with standard coagulation testing. Both the TEG and ROTEM platforms measure the viscoelastic properties of clot initiation, formation, strength, and lysis in whole blood, representing these properties in graphical tracings and numeric values, which reflect the contribution of clotting factors, platelet, and fibrinogen to clot strength. Similar to Clauss fibrinogen levels, detection of hypofibrinogenemia by ROTEM in the setting of PPH has recently been shown to predict severe PPH. A prospective, observational study of 365 women experiencing PPH >1000 mL measured standard fibrinogen levels and early assessment of fibrinogen by ROTEM using the Fibtem A5 value, which reflects fibrinogen contribution to clot strength 5 minutes after onset of clot formation.28 The Fibtem A5 value was found to be predictive of PPH progression to >2500 mL blood loss (OR 0.85; 0.77-0.95). An additional advantage of TEG and ROTEM compared with standard coagulation testing is rapid identification of excessive fibrinolysis.20 Empiric antifibrinolytic therapy in PPH has an uncertain safety and efficacy profile (see below), but detection of hyperfibrinolysis by viscoelastic whole-blood testing may support antifibrinolytic therapy in uncontrolled PPH.
Antifibrinolytic therapy and postpartum hemorrhage
Tranexamic acid (TXA), a lysine analogue antifibrinolytic agent, has been shown in perioperative settings (including trauma) to reduce hemorrhage and transfusion requirements.29,30 Use of TXA in prevention and treatment of PPH has gained attention in recent studies. Regarding TXA for prevention of PPH, a Cochrane review by Novikova and Hofmeyr31 of 2 trials, as well as a review of 12 trials by Senthilhes et al32 found the available quality of evidence to be poor. The EXADELI open-label, randomized trial compared high-dose TXA (4 g IV bolus followed by 1 g/hr infusion over 6 hours) versus no treatment in women with PPH in excess of 800 mL following vaginal delivery (n = 72 in each arm).33 There was a statistically significant difference in the primary endpoint of blood loss 6 hours after enrollment (173 vs 221 mL, P = 0.041). Although these results are promising, this trial is limited in that it was not blinded, was not placebo-controlled and that the differences in blood loss, although statistically significant, were of questionable clinical benefit.
Safety concerns have been voiced related to use of TXA in obstetrics. Maternal thromboembolic risk needs to be determined, as well as risk to the infant as TXA can cross the placenta and is excreted in low quantities in breast milk. A retrospective case-controlled analysis of women with bleeding disorders given TXA during pregnancy found that 2 of 256 women given TXA experienced thromboembolic complications, whereas 4 of the 1846 controls experienced thromboembolic complications.34 The authors concluded that there was not a significant thromboembolic risk; however, generalizability to women who do not have bleeding disorders is difficult. An additional concern recently raised is whether TXA use in PPH may be associated with renal injury. A retrospective analysis performed on 198 women with PPH admitted to the ICU compared those who developed acute renal failure (ARF) to those who did not.35 Univariate analysis found that patients with ARF received TXA more frequently (54% vs 38%, P = 0.03), but this association did not persist in multivariate analysis. Lastly, a French Periodic Safety Update Report was issued after observation of severe renal impairment in PPH patients managed with high-dose (4 g) TXA.36 Ongoing studies of TXA safety in PPH should therefore include evaluation of renal function as well as thromboembolic complications.
Recent PPH management guidelines widely, but not universally, recommend use of TXA, with most recommendations urging TXA use only in uncontrolled PPH unresponsive to first-line therapies (summarized in Table 3). An ongoing international, double-blinded, randomized trial enrolling 20 000 women with PPH (the WOMAN trial) is currently underway, investigating a primary endpoint of maternal mortality after subjects receive 1 g TXA versus placebo.37 The results of this trial are highly anticipated for providing data on both efficacy and safety in PPH, with secondary endpoints including thromboembolic events in both mother and infants. Additionally, a nested cohort study in the WOMAN trial will investigate impact of TXA on laboratory markers of fibrinolysis, including D-dimer, fibrinogen levels, and ROTEM markers associated with increased clot lysis.
Fibrinogen replacement in postpartum hemorrhage
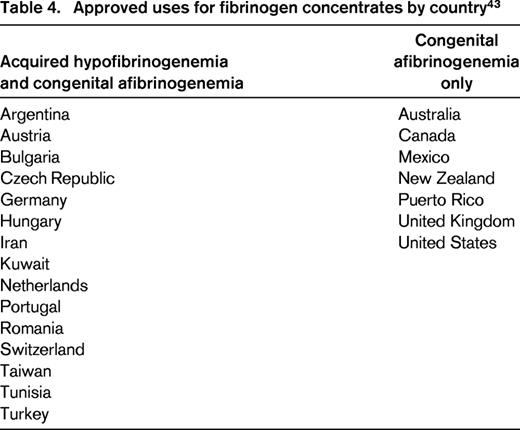
With the recognized relationship of severe PPH and hypofibrinogenemia, fibrinogen repletion has gained attention as a promising therapeutic intervention. In North America, cryoprecipitate has traditionally been used to treat acquired hypofibrinogenemia arising in the setting of consumptive coagulopathies such as PPH. Cryoprecipitate is manufactured from fresh-frozen plasma thawed at 1°C–6°C, precipitating large molecular weight proteins including fibrinogen, von Willebrand factor, and factor VIII, and is preferable to plasma for fibrinogen repletion due to its higher fibrinogen concentration (∼15 g/L compared to 1-3 g/L).38 In several countries throughout the world, purified fibrinogen concentrates are approved for the treatment of both congenital afibrinogenemia and acquired hypofibrinogenemia (Table 4).43 Currently in the US, fibrinogen concentrates (RiaSTAP, CSL Behring, Marburg, Germany) are approved only for treatment of congenital afibrinogenemia, but interest has been growing in their potential use for management of severe hemorrhage complicated by low fibrinogen, such as PPH.38 Dosage of fibrinogen concentrates (FCs) in congenital afibrinogenemia when fibrinogen levels are unknown are recommended at 70 mg/kg, with a half-life of 82.3 ± 20.0 hours.44 Fibrinogen concentrates have some advantages as a fibrinogen source compared with cryoprecipitate: (1) they are pathogen-reduced; (2) dosing of FC is more precise than dosing of cryoprecipitate, which can have widely varying fibrinogen concentration; (3) FCs may be available more rapidly in some centers, where thawing and pooling of cryoprecipitate can take in excess of 30 minutes; and 4) cryoprecipitate bears the risk of noninfectious adverse transfusion events, such as TRALI. However, the level of evidence for use of FC until recently has been limited to case series and retrospective studies, and risk of thromboembolic complications from use of FC in obstetric patients remains uncertain.
The recently published multicenter FIB-PPH trial from Denmark randomized 249 women with PPH to receive either 2 g empiric FC versus saline placebo, examining a primary outcome of RBC transfusion up to 6 weeks postpartum.39 No significant difference was found, with 20% of the women treated with FC receiving postpartum RBC transfusion as compared with 22% of the placebo group (P = 0.88). However, only 2.2% of the subjects had fibrinogen levels <2 g/L at baseline; accordingly, the results suggest only that empiric FC in PPH with normal or near-normal fibrinogen levels does not significantly alter RBC transfusion incidence. While the study was not powered to evaluate adverse outcomes, the authors noted no thromboembolic complications in either arm. Another randomized, placebo controlled trial which recently completed enrollment compares women with ongoing PPH and evidence of hypofibrinogenemia on Fibtem analysis to receive either FC or saline placebo (n = 30 for each arm).40 Primary outcome will be the total number of allogeneic blood products administered from time of study drug administration to discharge. Results from this trial will help further inform the role of FC in women with PPH and identified hypofibrinogenemia. Table 5 summarizes current guideline recommendations for fibrinogen replacement in PPH.
Role of the hematologist
Hematologists and those who have expertise in hemostasis play a valuable role in the multidisciplinary teams caring for women with PPH. By providing guidance on laboratory interpretation and therapeutic interventions, hematologists can address coagulopathy-complicating PPH as other obstetric and surgical interventions are being used. Every person working on these teams—obstetricians, anesthesiologists, nurses, hematologists, transfusion medicine, and laboratory medicine physicians—has the opportunity to save a mother's life, and by working together those odds are improved.
Correspondence
Evelyn Lockhart, University of New Mexico Health Science Center, MSC08 46401, Albuquerque, NM 87131-0001; Phone: 505-925-7765; Fax: 505-272-6726; e-mail: ELockhart@salud.unm.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has consulted for CSL Behring, Octapharma, TEM Systems and Bayer; has received honoraria from CSL Behring, Octapharma, TEM Systems; and has received travel support from TEM Systems, Bayer, and Octapharma.
Author notes
Off-label drug use: For my lecture at the ASH 2015 annual meeting, I will be discussing use of fibrinogen concentrates in obstetric hemorrhage.