Abstract
This chapter focuses on the recent advances in adoptive T-cell immunotherapies, not only for patients after hematopoietic stem cell transplantation, but also in the autologous setting using T cells early in the disease process for the treatment of the highest-risk patients with leukemias and lymphomas. The particular emphasis is to highlight the role of T-cell therapies for hematologic malignancies using a non-gene-transfer approach to direct specificity, including the clinical use of T-cell therapies for EBV-associated lymphomas and strategies for targeting nonviral lymphoma- and leukemia-associated antigens.
Learning Objectives
To understand the different T-cell strategies targeting leukemia- and lymphoma-associated antigens without gene modification
To evaluate the clinical use of these leukemia- and lymphoma-directed T-cell approaches
Introduction
T cells play a major role in the GVL effect that confers cure in patients who are incurable by chemotherapy and “small-molecule” drugs. However, the GVL effect is limited, especially in patients with advanced disease and those with aggressive malignancies. Adoptive T-cell therapy strategies have arisen out of the need to improve GVL. The first attempts to boost GVL used donor lymphocyte infusions (DLI). Since then, the field has diversified and the underlying biology of the interactions between T cells and tumor cells has become better understood. Here, we address the current developments in allogeneic T-cell therapy using a non-gene-transfer approach to direct specificity and describe ways in which similar strategies can be applied in the autologous setting.
Anatomy of the T-cell–tumor cell interaction
With advances in immunology over the last 20 years, we now have a molecular-level picture of the interplay between the T lymphocyte and the malignant cell that results in cytotoxic suppression of the malignancy. Tumor antigen presentation through MHC class I and II molecules engages CD4+ and CD8+ cytotoxic T lymphocytes (CTLs) through their TCR. If the avidity of the TCR to the antigen is sufficient, costimulatory molecules engage with ligands on the tumor cell and an immune synapse forms between the T cell and its target, binding the 2 cells together and mediating the delivery of cytotoxic molecules into the tumor cell, leading to cell lysis by perforin and apoptosis by granzymes. FAS/FAS ligand interaction is an additional cytotoxic mechanism exhibited by some T cells. Although CD4+ T cells are classically viewed as helper cells facilitating CD8+ T-cell function, it is now clear that both cell subsets can exert cytotoxicity against tumor targets.
Factors controlling the T-cell–target cell interaction
This idealized picture of T-cell mediated toxicity is modified by factors involving the CTL, the tumor target, and the milieu in which the interaction occurs. The following considerations are important in the successful generation and administration of functional tumor-specific T cells.
CTL function.
CTL function is dependent on the maturational state of the effector cell. To expand into clones of effector cells, T cells must engage with their cognate antigen presented by an APC expressing the appropriate costimulatory molecules. Dendritic cells (DCs) represent the most efficient type of APC for this purpose. In the laboratory manufacture of CTL, standard approaches use DCs generated from CD34+ cells or CD14+ monocytes. As T cells expand, they generally acquire CD45RO and lose CD45RA surface molecules. Continued expansion results in the production of a CD57-expressing end-effector “exhausted” T cell. Only the early effector cells are capable of persistence in vivo and successful CTL manufacture plays close attention to growth conditions that avoid the generation of exhausted T cells. Other factors affecting CTLs are cells that suppress T-cell proliferation and function, chiefly regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs).
The tumor cell.
It is now apparent that malignant cells use a variety of mechanisms to evade T-cell attack. These include the down-regulation of HLA surface molecules, thus preventing tumor-associated antigen (TAA) presentation, the down-regulation of TAA synthesis, or the clonal selection of cells negative for the TAA. Critically, tumor stem cells (especially dormant stem cells) may not express the TAAs present on more mature cells, resulting in disease persistence despite the elimination of more mature progeny.
The milieu.
T-cell homing is controlled by an array of homing molecules that determine whether the CTL reaches the tumor target. At the site of the tumor (the BM in the case of leukemia), local conditions can be protective for the malignancy. Protective sites include the stem cell niches in the BM, which help maintain the leukemia cell in a dormant state, the presence of MDSCs in the tumor, and anti-inflammatory molecule production by the malignant cell (eg, TGF-β), which negatively affect CTL function. The presence of appropriate cytokines and growth factors is needed for transfused CTL to expand and persist. T-cell infusions have a greater propensity to expand when administered to a recipient rendered lymphopenic by chemotherapy. The consequent surge of IL-15 and IL-7 in particular in the absence of competing lymphocyte populations ensures that the transfused CTLs have the maximum opportunity to expand and persist in the recipient.
Approaches to T-cell therapies from basic to sophisticated
T-cell therapy owes its origins to 2 lines of research in the 1980s. First, the clinical grade production of IL-2 paved the way for the in vitro expansion of CTLs (so-called lymphokine-activated killer cells) and pioneering attempts by Rosenberg at tumor immunotherapy using the patient's lymphocytes.1 Second, there was a growing realization that allogeneic hematopoietic stem cell transplantation (HSCT) can confer a powerful antileukemic effect. This was dramatically demonstrated when some patients with myeloid malignancies relapsing after HSCT achieved durable remissions after DLI.2 In the ensuing years, DLIs have been widely applied with varying success to prevent or treat relapse after HSCT in patients with leukemia or lymphoma.3 In the field of tumor immunology, the field has moved to the generation of more tumor-specific T cells through the selective expansion of tumor-infiltrating lymphocytes and the generation of TAA-specific T cells both in the autologous and allogeneic setting.
T-cell therapies for lymphoma
Targeting EBV antigens
Because B cells are a reservoir for EBV, lymphomas can often present viral antigens that can be exploited for immunotherapies using EBV-directed T cells. When EBV infects an immunocompetent host, it sets up lifelong latency in B cells and oral epithelial cells. EBV-infected B cells are maintained at a level of ∼1% of the B-cell pool, predominantly because of the potent EBV-specific T-cell response. However, in immunosuppressed individuals, the impairment in cell-mediated immunity provokes an EBV-driven lymphoproliferative process. In addition, a proportion of B-cell-derived non-Hodgkin's lymphomas (NHLs), NK-T lymphomas, and some Hodgkin lymphomas (HLs) retain latent EBV in the immunocompetent setting.4
In either situation, the expression of EBV antigens by the lymphoma represents a potential target for T-cell immunotherapy. Most experience comes from the use of donor-derived T cells to prevent and treat EBV-associated lymphomas after HSCT.5 Because these tumors arise in the immunocompromised host in the absence of T-cell immunity, such lymphomas are highly immunogenic and can be targeted by DLIs, as first demonstrated by O'Reilly. Such unselected DLIs, although effective at restoring immunity to EBV and eradicating lymphoproliferative disorder (LPD), carry the risk of GVHD.6 This has driven investigators to develop strategies to generate EBV-antigen-specific T cells free of GVHD risk.7 Donor-derived EBV-specific T cells given as prophylaxis for EBV-induced lymphoma in >100 patients after HSCT have been highly effective, as highlighted in one study showing that none of the patients treated with this approach developed posttransplantation LPD, compared with an incidence of 11.5% in a historical cohort.8 Moreover, this tumor has proved to be exquisitely sensitive to the adoptive transfer of EBV-CTLs and even multivirus CTLs when used as treatment for patients with posttransplantation LPD in the HSCT setting; even large tumors can shrink and disappear rapidly.8,9
However, in other less immunogenic EBV-associated lymphomas developing in the immunocompetent host (eg, type II latency tumors), the expression of EBV antigens is limited to subdominant EBV antigens such as LMP1, LMP2, BARFO, and EBNA1. These weak target antigens for CTL activity allow the lymphoma to evade an intact immune system. Type II latency is seen in EBV-associated HL and NHL. Polyclonal EBV CTL generated using lymphoblastoid cell lines elicited a small percentage of CTLs recognizing these subdominant EBV antigens.10 Nevertheless, using this approach, some CTLs can be generated against the subdominant EBV antigens LMP1 and LMP2, We evaluated autologous EBV-specific CTL in patients with EBV-positive HL in a phase 1 dose escalation study. We found a 20% tumor response rate and documented persistence of the adoptively transferred T cells for up to a year.11 Presumably due to the limited numbers of EBV-specific T cells targeting the EBV antigens expressed by these type II latency lymphomas, these responses were clearly inferior to those seen in studies targeting type III latency EBV LPD and only occurred in patients with low tumor burden.
To improve the treatment for patients with type II latent lymphomas, we then created CTL lines using APCs uniquely presenting LMP2 with or without LMP1. We treated patients with EBV+ HL, B-cell NHL, and T/NK cell NHL in a phase 1 study in which patients received 4 × 107 to 1.2 × 108 CTLs/m2.12,13 There was no immediate toxicity from infusion and, of 29 patients without radiological evidence of disease receiving CTLs after HSCT or chemotherapy, 28 remain in remission. In 21 patients with relapsed disease 13 showed a tumor response, complete in 11. Therefore, this study demonstrated both safety and efficacy of autologous LMP-CTLs.13
Immunotherapy for HIV
Patients with HIV may also develop EBV-associated lymphomas, and the risk appears to correlate with increasing viral load in the absence of an EBV-specific immune response.14 This observation implies that these complications may be prevented if the EBV-specific T-cell response could be augmented. Immunotherapy approaches targeting HIV and EBV are now being explored.15
Targeting nonviral lymphoma antigens
In contrast to highly immunogenic viral antigens, TAAs are often self-proteins that are mostly weakly immunogenic. The majority of T cells lack receptors capable of avidly binding to self-antigens. Nevertheless, in the lymphoma setting, tumor-specific T cells targeting survivin, PRAME, NY-ESO1, SSX2, and MAGE A4 can be expanded from patients with NHL and HL, and this approach is currently being evaluated clinically (www.ClinicalTrials.gov identifier #NCT01333046).16
T-cell therapies for leukemia
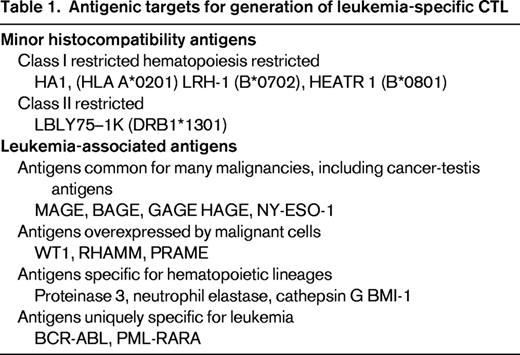
After the initial success in the use of DLI to control chronic myeloid leukemia, DLIs were widely used to treat all types of leukemia relapsing after HSCT. It rapidly became clear that, with a few exceptions (chronic myeloid leukemia, some lymphomas, and occasional patients with myelodysplastic syndrome and acute leukemia), most patients did not show any leukemic regression. Strategies to enhance DLI with IFN, GM-CSF, and IL-2 have sometimes shown greater efficacy, but it is generally agreed that although preemptive DLIs can reduce relapse rates, they represent an unsatisfactory approach to treating leukemic relapse. Further improvement in the efficacy and safety of leukemia-specific T-cell therapies has come from a clearer understanding of the antigens on leukemia cells that can be targeted by CTLs. These antigens, listed in Table 1, have led to distinct strategies to generate leukemia-specific T cells that have application both in the context of allogeneic HSCT and in the autologous setting.17,18
T-cell therapy using minor histocompatibility antigens
The first attempts at using minor histocompatibility antigen (mHag)–specific CTLs followed the discovery of the first hematopoietic-restricted mHag HA-1 by Goulmy et al.19-21 HA1-specific CTLs have been successfully used to treat leukemia relapsing after HSCT, but HA-1-specific T cells also have unwanted side effects due to targeting of inflammatory cells of hematopoietic origin causing bystander damage to non-HA-1-expressing tissues. Because class II molecules are predominantly restricted in their expression to hematopoietic cells, these class II mHags are of particular interest because of the potential leukemic specificity of CD4+ T-cell alloresponses.22,23 The Leiden group has subsequently focused on such HLA-DR-restricted mHags. Bleakely, et al24 demonstrated a feasible approach to generate mHag CTLs in vitro from unsensitized donors by stimulating naive CD8+ T cells with recipient DCs. Leukemia-reactive T-cell clones are selected and tested to exclude recognition of patient fibroblasts and for the ability to eliminate leukemia in a mouse model. Leukemia-recognizing T-cell clones are further analyzed for their recognition element and the HLA restriction of these CTLs. This approach successfully identified mHag-reactive T cells in every donor-recipient pair tested, leading to the description of new mHags defined at the genetic and protein sequence level.25 However, these sophisticated, time-consuming, and expensive approaches are not widely available at present. Furthermore, the narrow repertoire of mHag-specific CTLs recognizing only hematologic cells that can effectively eliminate leukemia without off-target side effects on nonhematopoietic tissue limits clinical applicability.26
T-cell therapy using LAA specific CTL
To avoid some of the inherent logistical difficulties associated with the nonuniversality of mHag-based approaches, we focused on targeting antigens widely expressed by a variety of hematological malignancies. With the aim of creating a single CTL product targeting myeloid and lymphoid malignancies in general, our strategy has been to create DCs expressing entire sequences of multiple TAAs. In this way, the constraint of HLA restriction is removed because the responding T cell can choose a cognate peptide from the entire array of TAA peptides. Using multiple TAAs, multispecific CTLs can be generated, eliminating the need to identify the specific TAA in the individual leukemia and decreasing the risk of tumor escape from down-regulation of a single TAA. We used overlapping peptide libraries of WT1, PR3, PRAME, NE, and MAGE-A3 to target myeloid malignancies and WT1, PRAME, NY-ESO, survivin, and MAGE A4 to target acute lymphoblastic leukemia.17,18 The approach generated CD8 and CD4 T cells of an effector memory phenotype. Cocultured with primary leukemia blasts or lymphoma cells matched at one or more class I or class II HLA antigens, these CTL lines showed specific recognition of leukemia targets and eliminated even single HLA-class I or II allele-matched target cells. It was possible to generate multileukemia-antigen-specific CTLs from healthy donors and from patients with hematological malignancies, opening the way for both allogeneic and autologous CTL treatments.
Limitations to successful T-cell therapy and ways to overcome them
Tumor escape and immune editing
Under the selection pressure of an immune response, hematological malignancies are prone to evolve immune resistant subclones with a competitive survival advantage. Two types of immune evasion have been identified: (1) tumor escape, the loss of antigen presentation through down-regulation of MHC and costimulatory molecules or epigenetic down-regulation of TAA through demethylation, and (2) immune editing, T-cell suppression through the production of cytokines such as TGF-β, the recruitment of suppressor cells, and other less defined mechanisms that favor an exhaustion T-cell phenotype.
Because time favors clonal evolution, one simple approach is the administration of T cells early in the course of the disease. Targeting multiple TAAs can reduce the risk from loss of TAA presentation, as we have shown for our leukemia and lymphoma strategies.16-18 In addition, demethylating agents such as 5 azacytidine can be used to make the leukemia blasts or lymphoma cells reexpress antigen. We have shown that patient-derived multi-TAA CTLs can efficiently kill autologous tumors and that this effect was increased in the presence of demethylating agents.27,28
To counteract TGF-β blockade, we have generated a retrovirus vector expressing the dominant-negative TGF-β type II receptor (DNR) that prevents the formation of the functional tetrameric TGF-β receptor.29 Transduction of cytotoxic T cells with the DNR can render the T cells resistant to this inhibitory cytokine. In vitro studies showed that DNR-transduced CTLs were resistant to the antiproliferative effects of recombinant TGF-β. In addition, although transduced CTLs were protected from the negative effects of TGF-β, long-term expression of this construct had no deleterious effects on the function, phenotype, or growth characteristics of the transduced CTL lines.29 Additional support for this approach comes from murine models in which transgenic mice genetically engineered so that all of their T cells are insensitive to TGF-β signaling were able to eradicate tumors,30 and adoptive transfer of TGF-β-DNR transduced T cells resulted in eradication of tumor cells.31,32 CTLs expressing the DNR may therefore have a selective advantage in vivo in patients with TGF-β-secreting tumors such as HL. We have now begun a study using DNR-transduced LMP-CTLs for patients with relapsed EBV+ HL and have shown persistence of the transduced CTLs for up to 3 years, with clinical responses seen in the first 8 patients ranging from stable disease to complete remissions (www.ClinicalTrials.gov identifier #NCT00368082).
Identifying the so-called chemoresistant lymphoma stem cell or leukemia initiating cell and targeting it will be necessary if immunotherapy is to eradicate residual disease, and it is increasingly clear that many hematological malignancies represent complex hierarchies of cells with varying abilities to initiate leukemia/lymphoma.27,33 Knowing the antigenic nature of the lymphoma or leukemia stem cell is critical to designing effective T-cell immunotherapy. Unfortunately, it is clear that true tumor-initiating cells are protected by a low metabolic state and the BM niche that favors dormancy, although preliminary studies suggest that it may be possible to target such cells with immune therapies.27
Experience in the immunology of solid tumors has shed light on the powerful suppressive effect exerted by MDSCs. It appears that some myeloid malignancies have strong MDSC capacity, which may limit the ability of T cells to eradicate bulk disease. T-cell therapy may be more effective in remission when the leukemic bulk of suppressor cells is at its lowest. Tregs are increased in many lymphomas (eg, HL) and leukemia (eg, acute myeloid leukemia) and their presence may limit the full potential of T-cell therapy. Several approaches to limiting Tregs, such as antibody strategies and the use of pulsed chemotherapy (“metronomic” cyclophosphamide), are under evaluation. Further, because T cells have been found to persist much longer and to show much greater efficacy if they are infused into a lymphopenic milieu, conditioning of patients with lymphodepleting agents can also provide a lymphoregenerative environment (driven by largely by IL-15 production and Treg depletion) that can greatly expand infused T cells into the empty lymphoid “space.”34
Quality of the T cells
Several factors are critical in the generation of potent CTLs. First, it is abundantly clear that short culture periods with limited exposure to IL-2 favor the induction of effector-memory T cells that are associated with prolonged persistence and self-renewal in the recipient. Our current culture systems use IL-7 and IL-15 to induce and expand CTLs from their naive or memory precursors. IL-2 is used only after the second round of antigen pulsing. This generates within 3 weeks a T-cell product that has the capacity to persist and further expand in the recipient. Culture conditions that generate both CD4+ and CD8+ T cells favor the long-term persistence of CTLs in vivo. T cells, whether CD4 or CD8, with potent cytotoxicity have specific characteristics: they express CD27 but not CD57 (effector-memory phenotype) and produce multiple cytokines (polyfunctionality). A potential problem with such high-avidity T cells is their propensity to cause “off-target” side effects: tissue and organ damage from cytokine release. This has been a particular problem with genetically transformed T cells, leading to serious complications, especially from IL-6 toxicity. Such reactions respond to steroid treatment and anti-IL-6 monoclonal antibody.35
The milieu
The milieu into which CTLs are delivered can have profound influence on the ability of the infused cells to expand and survive. CTLs infused into a lymphocyte-rich environment have to compete for growth factors and have little room for expansion. Studies of lymphocyte recovery after allogeneic HSCT have identified the first few weeks after transplanatation as a period of massive lymphocyte growth factor production in response to the profound lymphopenia induced by the conditioning regimen. Infusion of T cells during this period favors their rapid expansion. This has led to the use of lymphodepleting chemotherapy using steroids, pentostatin, or fludarabine before DLI. Such approaches have been shown to significantly augment the potential of DLI to expand and persist after allogeneic HSCT, and have also been adopted in the autologous setting to favor the survival of tumor-specific T cells.34 Finally, developments aimed at modifying T-cell function by stimulating T-cell proliferation and increasing cytotoxic function, such as lenalidomide36 and blocking antibodies to enhance antitumor immunity through blockade of the negative costimulation through anti CTLA4 and anti PD1 to prevent T-cell exhaustion,37 are under investigation, but are beyond the scope of this review.
Conclusions
This century has seen the coming of age of adoptive T-cell therapy. This has been helped by new technologies that support our ability to generate potent antigen-specific CTLs expanded to clinical grade products. The focus of research now moves toward clinical trials with adoptive T cells of defined function to establish efficacy in diverse hematological malignancies. In the course of these trials, we will inevitably discover optimal approaches to T-cell therapy and also its limitations, which may require genetic adaptations to the T cells infused and treatments to optimize the target susceptibility of the malignancy and to block inhibitory mechanisms to CTL function.
Disclosures
Conflict-of-interest disclosures: C.M.B. has received honoraria from Cellmedica. A.J.B declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Catherine M. Bollard, Children's National Health System, 111 Michigan Ave NW, Washington, DC 20010; Phone: (832)824-4781; Fax: (202)476-6498; e-mail: cbollard@cnmc.org.