Abstract
In blood, oxygen is transported principally by hemoglobin tetrameric molecules in erythocytes, which allow for the delivery to tissue cells. When anemia occurs, such as perisurgically or after trauma, blood transfusion is administered to replace the deficit in oxygen-carrying capacity. During embryogenesis and later in adult life, tissue oxygen levels control multiple key cellular functions. Low tissue oxygen levels in particular are physiologically relevant to stem cells by controlling their metabolism and cell fate. In adult life, hematopoietic stem cells reside in specified BM microenvironments/niches, where their quiescence and differentiation are presumably also influenced by cell-intrinsic and cell-extrinsic (niche) factors. Novel imaging technologies have allowed determination of the spatial localization of hematopoietic stem/progenitor cells (HSPCs), as well as the topography of oxygen distribution in BM cavities. Together, these recent advances have contributed to the emergence of a novel model that challenges the previous concept of a hypoxic hematopoietic stem cell niche characterized by poorly perfused endosteal zones with the deepest hypoxia. HSPCs display a hypoxic phenotype despite residing in close association with arterial or sinusoidal vascular networks. The entire BM cavity is hypoxic and unexpectedly exhibits an opposite oxygen gradient to the one initially proposed because arteriole-rich endosteal zones are relatively less hypoxic than deeper regions of the BM perfused by dense sinusoidal networks. Therefore, further studies are warranted to elucidate to what extent differences in oxygen tensions in these diverse microenvironments influence HSPC homeostasis.
Learning Objective
To understand the fundamental basic research carried out to investigate the relevance of hypoxic conditions in bone marrow microenvironments that support hematopoietic stem cell maintenance
Oxygen in tissues and stem cell biology
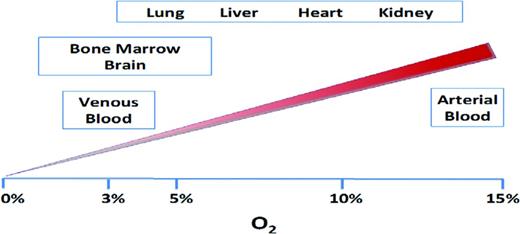
During evolution, fluctuations in atmospheric oxygen concentration have defined eukaryotic life forms on earth, exemplified by the adoption of efficient oxidative phosphorylation (ie, aerobic metabolism) to support more complex and multicellular organisms.1 Oxygen is the primary intracellular acceptor of electrons that are generated during mitochondrial respiration. Therefore, proper tissue function strictly relies on the constant delivery of adequate oxygen levels to all cellular environments in the organism. Physiologic oxygen concentrations in multiple organs have been measured by various methods and oscillate within a wide range of values (1%–14%). Whereas some tissues, such as the lung, liver, and heart, have relatively higher partial pressures of oxygen (pO2), others, such as the BM, brain, and eye, are relatively more hypoxic (Figure 1).2 Substantial regional variations in microenvironmental oxygen tensions also exist within individual tissues and depend on a variety of factors, such as topography of the vascular networks, oxygen permeability of the endothelium, and blood flow rate.
Therefore, even in homeostatic conditions, oxygen levels may be as low as 1% in certain tissue microenvironments or niches in which physiologic hypoxia is critically linked to specific cellular functions. Indeed, in the past decade, a growing body of evidence has supported the idea that local maintenance of relatively low pO2 is required during embryonic development and constitutes a key regulatory feature of adult stem cell niches.3,4 At a molecular level, hypoxic conditions lead to the induction of many well-characterized intracellular signaling pathways, including the ones elicited by hypoxia-inducible transcription factors (HIFs), which influence numerous cell functions related to the preservation of stem cell identity, quiescence, and the metabolic shift toward anaerobic glycolysis.
Hematopoiesis: a paradigm to study tissue stem cell–based maintenance
Hematopoiesis is a dynamic hierarchical process in which hematopoietic stem and progenitor cells (HSPCs) continuously generate exceptionally large numbers of relatively short-lived mature blood cells, which can rapidly increase in response to enhanced demand during stress conditions such as bleeding and infections.5 Postnatally, hematopoiesis occurs inside soft tissues of BM cavities. During the past decades, a tremendous body of knowledge has been gained regarding the multistep stem cell differentiation process and its regulation by transcription factor pathways. However, only recently are the tools being developed to investigate the specific localization of HSPC residency within the complex environment of BM tissue (ie, HSPC niches) and the specific interactions that these cells establish with hematopoietic and nonhematopoietic BM components. The concept of a hematopoietic stem cell (HSC) niche was proposed almost 40 years ago as an anatomically confined microenvironment in the BM that uniquely controls HSPC quiescence, self-renewal, and differentiation.6 Residency in these specific tissue microenvironments grants HSPCs access to vital maintenance signals from adjacent cells, extracellular matrix, and soluble growth factors.7 HSPC niche-related studies using microscopy have mostly concentrated on determining the localization of HSPCs within the context of entire BM cavities and subsequent analysis of their spatial relationship with respect to putative niche components. The physiological implications of such histological observations have typically been confirmed by assessing the effects that perturbations of either the functionality or the numbers of candidate niche cells exert directly on the number and function of HSPCs. These strategies have yielded significant advances in our understanding of HSPC cellular associations, which have been extensively reviewed in recent publications to which we refer the reader.7,8
In brief, initial studies, which used mostly ex vivo purified, fluorescently labeled adoptively transferred cells or label-retaining methods to detect quiescent HSPCs, highlighted the importance of mature osteoblasts in regulating primitive hematopoietic cells and tentatively placed the HSPC niche along the inner bone surfaces of BM cavities. Nonetheless, using simplified phenotypic combinations to track endogenous HSPCs, multiple groups have now shown that HSPCs are scattered in perivascular locations and in contact with a variety of stromal cell subsets of mesenchymal and neural origin,9-15 which, together with endothelial cells, provide specialized niches for HSPC maintenance.
Hypoxic HSPC niche model
A key feature distinguishing HSCs from their progenitors is their quiescent state, presumably regulated by the niches in which they reside. A prevailing model applied to stem cells and tumor stem cells hypothesized that induction into quiescence is, at least partially enforced by the shortage/lack of oxygen in the local tissue microenvironment.16,17 According to this model, low oxygenation exerts a protective role by promoting a metabolic shift toward anaerobic glycolysis that would safeguard stem cells from oxidative stress, thus minimizing DNA damage that could be potentially transmitted to the mature progeny.
A functional link between stemness and reduced oxygen availability has been in fact reported for multiple stem cell types and extensively studied in the case of HSPCs.4,16 In vitro studies have shown that hypoxic culture conditions preserved quiescence and to some extent enhanced the repopulating ability of HSPCs, suggesting that hypoxia could be a hallmark of the native tissue context in which HSPCs are preserved.18 Further proof for this hypothesis came from studies that demonstrated selective labeling of HSPCs with a probe for intracellular hypoxia (pimonidazole),19 as well as the stable expression and functional role of HIF in primitive hematopoietic populations20 (discussed in detail in the following section). Based on these data, it was speculated that quiescent and self-renewing HSCs reside in defined zones with the deepest hypoxia, presumably endosteal regions, either a distance away from vascular structures and/or near vascular structures with limited perfusion. However, evidence in support of this model has been indirect and inconclusive. Recent studies have sought to further define the metabolic and/or hypoxic phenotype of HSPCs in the context of their spatial distribution and cellular associations within distinct zones of the BM cavity. In the following sections, we discuss recent findings that have led to an emerging view regarding the role of microenvironmental oxygen levels on HSPC quiescence and metabolism.
HIF-1α protein expression
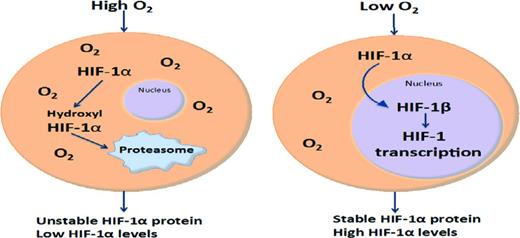
In tumors and in some organs, oxygen gradients exist that allow tissue cells to adapt to hypoxic environments/niches as low as 1%. One of the principal stress responses to hypoxia involves the up-regulation and stable expression of HIF-1α, which is tightly regulated by intracellular oxygen levels. When oxygen pressures increase above certain levels (∼5%), HIF-1α becomes hydroxylated and is targeted for ubiquitinylation and degradation in the proteasome.17 However, when cells are exposed to low oxygen environments, or possibly when intracellular oxygen levels are inadequate, oxygen-dependent mechanisms involved in HIF-1α degradation are deactivated, thereby permitting stable expression of HIF-1α, which translocates to the nucleus and heterodimerizes with HIF-1β. This heterodimer binds to hypoxia response element sequence domains on multiple chromosomes, leading to the activation of a broad transcriptional program that includes >100 genes involved in survival, proliferation, neovascularization, and cell cycle.17 Stable expression of HIF-1α in HSCs has been shown to regulate quiescence and to stimulate anaerobic glycolysis mediated by pyruvate dehydrogenase kinase 1 (PDK1)21,22 PDK1 also represses mitochondrial function by limiting the influx of glycolytic metabolites. The resultant metabolic state is necessary for HSC homeostasis and self-renewal potential (Figure 2).22
Schematic model of HIF-1α expression. At low oxygen levels, HIF-1α protein is stable, heterodimerizes, and translocates to the nucleus. At higher oxygen levels, HIF-1α is hydroxylated and targeted to the proteasome.
Schematic model of HIF-1α expression. At low oxygen levels, HIF-1α protein is stable, heterodimerizes, and translocates to the nucleus. At higher oxygen levels, HIF-1α is hydroxylated and targeted to the proteasome.
Hypoxyprobe pimonidazole
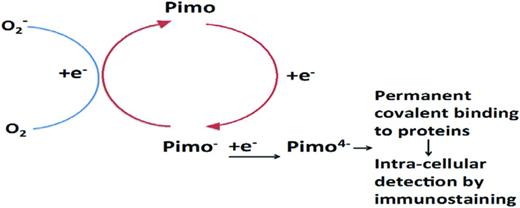
Pimonidazole (Pimo) is the most widely used and extensively validated nitroimidazole hypoxic marker.23 Pimo is an oxygen mimetic that competes with molecular oxygen as an acceptor of electrons generated during mitochondrial respiration. In the absence/deficit of intracellular oxygen, Pimo becomes increasingly reduced, thereby converting into an alkylating agent that irreversibly forms adducts with thiol groups in proteins. Protein adducts of reduced Pimo are in turn potent immunogens that can be detected using enzymatically or fluorescently labeled polyclonal and monoclonal antibodies and therefore quantified in flow cytometry or microscopy techniques. Therefore, Pimo incorporation is a consequence of an intracellular state in which oxygen influx from the extracellular environment is insufficient to serve as effective electron acceptor during mitochondrial respiration (oxidative phosphorylation). For these properties, Pimo had been broadly used as a surrogate marker of low intracellular oxygen availability in solid tumors. Subsequent studies aimed at understanding oxygenation dynamics in the BM demonstrated that HSPCs were among the cellular fraction of the BM that contained the highest levels of Pimo adducts 90 minutes after intraperitoneal administration of Pimo, as measured by flow cytometry.19
Altogether, the increased avidity for Pimo incorporation of HSPCs, the stable expression and functional role of HIF-1α in HSPC homeostasis, and the finding that HSPCs preferentially activated glycolytic metabolic pathways were seen as “hypoxic features” strongly supporting the notion that HSPCs niches would localize in areas of the BM with minimal oxygenation (Figure 3).
Hypoxia marker detection by immunostaining. Intracellular nitroimidazole molecules compete with oxygen for electrons that are generated by oxidative phosphorylation. In the absence of oxygen or in the presence of an insufficient amount of oxygen, nitroimidazoles such as Pimo become increasingly reduced, which enables irreversible binding to protein macromolecules.
Hypoxia marker detection by immunostaining. Intracellular nitroimidazole molecules compete with oxygen for electrons that are generated by oxidative phosphorylation. In the absence of oxygen or in the presence of an insufficient amount of oxygen, nitroimidazoles such as Pimo become increasingly reduced, which enables irreversible binding to protein macromolecules.
HSPCs are perivascular and accumulate in endosteal regions
Since the original postulation of the existence of localized HSPC regulatory niches in the BM by Schofield in 1978, multiple studies have attempted to define the specific positioning and cellular makeup of these regulatory entities. Until recently, a comprehensive analysis of HSPC localization in BM had not been feasible, leaving open the question of whether HSPC frequencies would still be higher in defined districts within the BM cavity. Two recent reports have addressed this issue and reached comparable results. Using quantitative imaging cytometry to analyze entire whole longitudinal 2D femoral BM sections, our group revealed that HSPCs distribute in BM according to a steep gradient in which maximal frequencies are found in perivascular locations of bone-proximal endosteal zones (not restricted to bone-lining areas and defined by 100 μm), gradually decreasing toward bone distal areas.9 Using confocal 3D imaging of sternal bones, Kunisaki et al observed similar patterns of HSPC distribution with regard to bone surfaces,15 a spatial profile comparable to the one described for progenitor cells in human BM and humanized mouse models.24,25 .
3D imaging of the complex BM vascular network
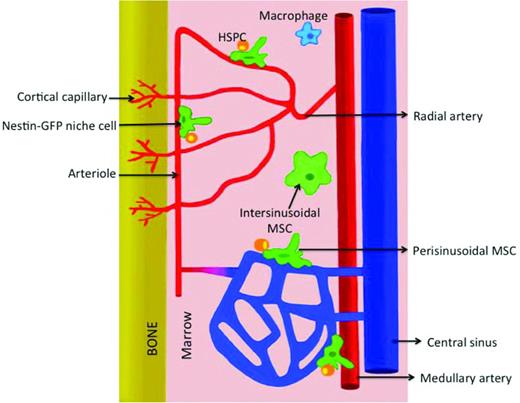
A detailed overview of the micro-architectural conformation and cellular heterogeneity of the microvascular system of the BM had been difficult to obtain due to the technical difficulties faced when applying advanced imaging techniques to study bone-enclosed BM cavities. Given the strong experimental evidence suggesting a close spatial and functional relationship of HSPCs with the BM microvascular network, our group developed novel immunohistology protocols to deliver full 3D reconstructions of confocal microscopy image stacks of large volumes of BM with unprecedented resolution.9 Using this novel technological approach to visualize whole longitudinal slices of murine femoral bones, our studies revealed a highly organized microvascular system. Consistent with an earlier study,26 within the diaphysis of the BM, large nutrient arteries run longitudinally throughout the length of the cavity, splitting into smaller arteries that migrate outwardly toward the inner margins of the bone. As they approach the endosteum, arterial branches give rise to arterioles. Along the surface of the endosteum, arterioles transition into sinusoidal microvessels, which constitute the venous equivalent of BM circulation and exhibit unique morphological and functional features (Figure 4). Sinusoids spatially arrange as a honeycomb-like network of large, wide vessels that form frequent anastomoses and migrate radially to merge into a central sinus into which BM diaphyseal circulation drains.9 The precise organization of the microvasculature in areas of trabecular bone (metaphysis) of the femur was to a certain extent less clear, with multiple thin arterial braches penetrating through the growth plate and running, together with a dense sinusoidal network, throughout intratrabecular BM spaces. As for the diaphysis within trabecular bone regions, arterial to sinusoidal transitions were mostly observed along endosteal areas. Similar immunohistological approaches have been used by different groups to study the 3D organization of microvascular compartments in tibial, sternal and calvarial BM cavities.15,27,28 Altogether, these studies demonstrate that endosteal surfaces are perfused by a dense and heterogeneous vascular network.
Vascular organization in the BM cavity. Within a longitudinal view of the diaphysis, ascending and descending medullary arteries branch out to give rise to radial arteries. As they approach the endosteum, radial arterial branches give rise to progressively smaller arterioles. Along the surface of the endosteum, arteriolar circulation transitions into the honeycomb-like network of sinusoidal microvessels, which then collect into the venous central sinus. Periarteriolar environments harbor specific stromal populations such as nestin-GFPhi mesenchymal stem cells and are niches for quiescent HSPCs.
Vascular organization in the BM cavity. Within a longitudinal view of the diaphysis, ascending and descending medullary arteries branch out to give rise to radial arteries. As they approach the endosteum, radial arterial branches give rise to progressively smaller arterioles. Along the surface of the endosteum, arteriolar circulation transitions into the honeycomb-like network of sinusoidal microvessels, which then collect into the venous central sinus. Periarteriolar environments harbor specific stromal populations such as nestin-GFPhi mesenchymal stem cells and are niches for quiescent HSPCs.
The definition of specific molecular markers of sinusoidal and arterial blood vessels in the BM enabled us to identify these vascular types in 2D tissue sections and to determine that HSPCs do not just interact with sinusoidal microvessels, as was proposed previously. In fact, a significant fraction of HSPCs and progenitor cell subsets were found in the vicinity of central arteries and periendosteal arterioles.9 Notably, periarterial niches harbor specific stromal cell subsets, which include nestin-expressing mesenchymal stem cell populations and nonmyelinating Schwann cells that collectively contribute to the maintenance of HSPCs in a quiescent state.14,15,29
In situ analysis of BM oxygenation dynamics and the hypoxic status of HSPCs
The so-called hypoxic profile of HSPCs lent experimental support to a conceptually attractive model in which intracellular hypoxia was the direct consequence of poor oxygenation in the local extracellular microenvironment of HSPCs, and thus theoretically assigned HSPC niches to areas of minimal oxygen availability within BM parenchyma. Nonetheless, the hypoxic nature of HSPC niches was hard to reconcile with their proposed perivascular localization. Therefore, according to this model, endosteal or osteoblastic niches were proposed as specific low-oxygenated sanctuaries for quiescent HSPCs, whereas perivascular niches would harbor activated proliferating HSPCs. However, whether “superhypoxic” tissue regions harboring Pimohi and HIF-1α+ HSPCs exist in BM and in fact localize in the proximity of the endosteal surfaces remained to be determined, either through in situ quantification of the hypoxic profile of cells within histologic samples of BM or by direct in vivo measurement of partial oxygen pressures in distinct BM areas. Recent studies have systematically addressed these questions revealing a rather complex scenario.
Hypoxic phenotype of HSPCs is independent of their localization in specific microenvironments of the BM
Our group recently performed a global, in-depth analysis on the effect of anatomical localization in distinct BM areas on the referred hypoxic profile of HSPCs. These studies clearly demonstrated that the high Pimo incorporation levels and constitutive HIF-1α expression found in HSPCs as well as early multipotent progenitor cell subsets do not depend on their residency at specific sites of the BM. Indeed, Pimohi and HIF-1α+ HSPCs are found scattered throughout perivascular sites in BM parenchyma and adjacent to B cells, which do not display hypoxic features despite sharing the same extracellular space.9 Furthermore, we observed that cycling HSPCs conserve hypoxic features and, when forced to massively enter into cycle through 5-fluorouracil or poly-IC treatment, both Pimo incorporation and HIF-1α expression are increased in the HSPC pool.9 These results strongly argue against the suggested causal relationship between hypoxic status and quiescence and demonstrate that, during extensive proliferation, HSPCs maintain intracellular hypoxic features.
Direct in vivo measurement of oxygen concentrations in diverse BM locations
Expression of HIF-1α and Pimo incorporation only provide an indirect assessment of the intracellular oxygenation of cells and may be regulated by oxygen-independent factors, as has been shown in the case of HIF-1α.30,31 Therefore, the finding that the hypoxic features of HSPCs were not related to specific positioning in BM microdomains did not rule out that local microgradients of pO2 exist locally in BM regions and may influence HSPC fate. To test this possibility, Spencer et al pioneered the use of 2-photon phosphorescence-based microscopy to detect and measure local extracellular oxygen concentrations in the BM of the skull in live animals, thereby providing for the first time an accurate picture of the topography of oxygen distribution in the BM.29 These studies revealed that oxygenation is low in all areas throughout the BM (ranging from 1% to 4%), which can therefore be considered as a hypoxic organ as a whole. Contrary to the initial prediction, they found highest oxygen tensions in endosteal zones and a gradual decrease toward the deepest, more central regions of the BM. This unexpected gradient is explained by the direction of blood flow because endosteal regions contain the highest density of arterioles carrying oxygenated blood, which gets rapidly depleted of oxygen as it enters sinusoidal circulation and penetrates the densely populated environment of the BM.29 Therefore, cells found in perisinusoidal areas further away from the bone surfaces encounter the lowest oxygen concentrations (pO2 of ∼5-20 mmHg or 2%) compared with those in the proximity of arterioles (pO2 of ∼35 mmHg or 4%–5%). These studies further demonstrated that, during irradiation and/or chemotherapy, severe disruption of vascular integrity, as well as reduced oxygen consumption due to the dramatic decrease in cellular density, lead to a general increase in oxygen partial pressures in the BM and the disruption of the described gradient.29
Revised model for hypoxia in HSPC niches: new paradigms and open questions
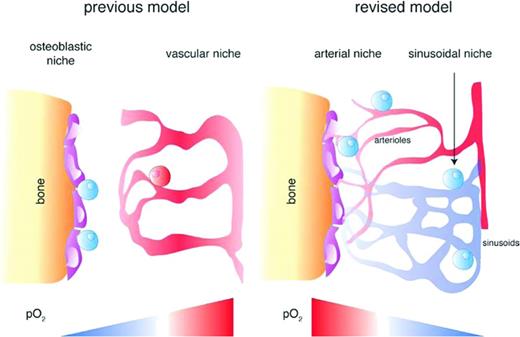
Collectively, the knowledge gained through the studies described herein supports the emergence of a revised and complex model of HSPC niches that integrates novel anatomic and functional data (Figure 5). The majority of HSPCs and early primitive progenitors are found in heterogeneous perivascular compartments (sinusoidal or periarterial). Periarteriolar niches, mostly found in endosteal zones, contain a specific set of mesenchymal and neural stromal cells and are suggested to preferentially harbor quiescent stem cells. Oxygen tensions in all BM parenchyma, including perivascular regions, are low, but significant regional differences exist, being slightly higher in arteriole-rich endosteal zones and gradually decreasing toward central areas of the BM containing higher densities of sinusoidal vessels. Therefore, HSPCs do not necessarily reside in the lower end of the oxygen gradient of the BM, and periarteriolar quiescent HSPCs are subject to higher oxygen levels than actively cycling HSPCs. Local oxygen availability in the BM increases after irradiation or treatment with chemotherapeutic agents. Finally, HSPCs and early multipotent progenitors exhibit a hypoxic profile irrespective of their precise localization in the BM and their cycling status.
Hypoxia niche models for HSPCs. The previous hypoxia niche model proposed that “hypoxic” HSCs reside in poorly perfused endosteal zones with deepest hypoxia at a certain distance from vascular structures and get oxygenated and activated as they approach the proximity of oxygen-rich blood vessels. In the revised model, the BM cavity exhibits an opposite oxygen gradient with the highest pO2 in arteriole-rich endosteal zones and the lowest in deeper areas of the BM. HSPCs and early primitive progenitor cells reside in heterogeneous perivascular niches (perisinusoidal and periarteriolar) and exhibit similar hypoxic profiles irrespective of their positioning in different BM regions.9 Blue cells depict a “hypoxic” state, whereas red cells depict a “normoxic” state. (Adapted and used with permission from Spencer et al.29 )
Hypoxia niche models for HSPCs. The previous hypoxia niche model proposed that “hypoxic” HSCs reside in poorly perfused endosteal zones with deepest hypoxia at a certain distance from vascular structures and get oxygenated and activated as they approach the proximity of oxygen-rich blood vessels. In the revised model, the BM cavity exhibits an opposite oxygen gradient with the highest pO2 in arteriole-rich endosteal zones and the lowest in deeper areas of the BM. HSPCs and early primitive progenitor cells reside in heterogeneous perivascular niches (perisinusoidal and periarteriolar) and exhibit similar hypoxic profiles irrespective of their positioning in different BM regions.9 Blue cells depict a “hypoxic” state, whereas red cells depict a “normoxic” state. (Adapted and used with permission from Spencer et al.29 )
Many open questions remain regarding the functional relevance of microenvironmental oxygen levels in HSPC physiology. Specifically, a major unresolved issue is whether differences in pO2 in periarterial versus perisinusoidal HSPC niches are functionally relevant and ultimately dictate HSPC cell fate decisions such as self-renewal and differentiation. In addition, it is yet unclear whether and to what extent microenvironmental conditions specifically enforce glycolytic pathways in HSPCs and how this characteristic metabolic wiring shared by other stem cells may be related to their hypoxic phenotype. The fact that both HIF-1α expression and Pimo incorporation in HSPCs increase during chemotherapy, whereas oxygen levels also rise in the BM, and that HSPCs mobilized into the blood and spleen conserve these features, supports the notion that such hypoxic profile is at least partially independent of environmental oxygen levels and could be influenced by the metabolic pathways and rates at which oxygen is used in these cells. Finally, future investigations will be needed to determine how pathologic conditions such as inflammatory or malignant processes in the BM affect the physiological features, topography, and ultimately the function of HSPC niches.
These questions are highly relevant to the ongoing challenges in bioengineering of blood cells and BM transplantation and call for an in-depth analysis. The studies described herein illustrate the value of using advanced quantitative imaging technologies to gradually advance our understanding of the microarchitecture and physiology of HSPC niches.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Leslie E. Silberstein, MD, Boston Children's Hospital, 300 Longwood Ave., 807 Enders Building, Boston, MA 02115; Phone: (617)919-2588; Fax: (617)730-0765; e-mail: leslie.silberstein@childrens.harvard.edu.
César Nombela-Arrieta, Department of Hematology, University Hospital Zurich, Schmelzbergstrasse 12, 8091 Zurich, Switzerland; Phone: 41-442-555-396; e-mail: cesar.nombelaarrieta@usz.ch.