Abstract
RBCs can be targets of infection directly or indirectly. When the microorganism enters the RBC directly, RBC damage becomes a fundamental aspect of the disease process. Malaria is the best example of an organism that directly targets the RBC, but others are Babesia and Bartonella. RBCs can also be indirect targets of infectious agents. This can occur when molecules are bound to the surface of the RBC, leading to immunologic clearance; when microorganism-produced toxins damage the RBC membrane, leading to hemolysis; when previous crypt-antigens are exposed, leading to accelerated removal; when microorganism-produced toxins alter RBC antigens to a different phenotype, or when microorganism suppression of erythropoiesis occurs due to specific binding to RBC precursors.
Learning Objective
To understand the variety of infectious agents that affect RBCs directly or indirectly.
Introduction
This chapter reviews the obvious infections in which microorganisms specifically enter the RBC and also addresses infectious situations in which the RBC may be altered or damaged secondarily or its surface may be altered, leading to accelerated intravascular clearance. Litchman1 lists 39 infectious agents that cause hemolytic anemia. Some organisms invade the RBC and others damage the RBC, causing premature clearance; cause hemolytic anemia from the hemolysins that they produce or by stimulating an immune response due to alterations of the RBC surface; stimulate antibodies to RBC antigens; or deposit immune complexes, causing increased phagocytosis. Because this discussion focuses on the RBC, the other diseases are not discussed in detail.
RBCs as primary targets of infection (Table 1)
Malaria
Upon infection, malaria parasites multiply in hepatocytes and the engorged cells rupture, releasing merozoites into the circulation. These merozoites then invade the RBC. Invasion of the RBC involves receptors on the malaria parasite and ligands on the RBC surface. This is a multistep process involving several different pathways. Plasmodium falciparum, the most serious form of malaria, uses multiple ligands, one of which involves the glycophorins (GPs) A, B, and C.2 Plasmodium vivax uses only the receptor containing the Duffy antigen receptor for chemokines (DARC).3 The extent of parasitemia partly accounts for the destruction of infected RBCs. Low rates of parasitemia may have little effect on causing anemia, whereas high rates in which >10% of RBCs are parasitized may cause significant hemolysis.
Within the RBC, the parasites multiply, ultimately bursting the RBC and releasing more merozoites to invade other RBCs. The bursting and release of parasite products coincides with the occurrence of symptoms of malaria. However, the degree of anemia may also be greater than that suggested by the number of parasitized RBCs.
Merozoites are also found on the surface of the RBC and may be part of an immune complex also leading to accelerated RBC removal possibly related to complement-dependent phagocytosis. There is rossetting of parasitized cells, with nonparasitized cells leading to complement activation on the uninfected RBCs and accelerated clearance of those cells. Several uninfected RBCs are removed from the circulation for each parasite infected RBC, thus magnifying the extent of hemolysis. The osmotic fragility of nonparasitized cells is increased. Infected RBCs also develop some modified receptors binding to endothelial proteins, platelet glycoproteins, thrombospondin, intracellular adhesion molecule 1, and some vascular adhesion molecules.
Infected cells also have a highly irregular surface and there is activation of hepatosplenic macrophages that increases RBC clearance. There are changes on the surface of both parasitized and nonparasitized RBCs that increase RBC recognition and thus phagocytosis. The loss of RBC deformity in the parasitized cells leads to shortened RBC survival. There is sometimes deposition of IgG and complement on the RBC surface, which also increases clearance and may even result in a positive direct antiglobulin test.1
Role of the Fy (Duffy) blood group antigens in malaria.
Duffy antibodies are commonly encountered in routine blood banking and transfusion medicine. The Duffy system is composed of 6 different alleles and the molecule that contains the antigens is a glycoprotein called the DARC.4 DARC on RBCs binds excess chemokines to prevent inappropriate activation of neutrophils and disruption of chemokine gradients.2,5 DARC is the receptor for P. vivax and Plasmodium knowlesi. In the absence of the Duffy molecule, the parasites are not able to establish a junction site. Therefore, individuals with the Duffy null phenotype Fy(a−,b−) in which DARC is absent from RBCs, are resistant to infection with P. vivax and P. knowlesi. The Duffy antigen frequency varies in different populations and, not surprisingly the Fy(a−,b−) phenotype is very common, approaching 100% in some parts of sub-Saharan Africa.
Role of ABO blood group antigens in malaria.
One of the adhesion domains, called Duffy-binding ligand, binds primarily to the area on the RBC surface that contains the oligosaccharides of the A and B blood group system. Whereas the Duffy blood group is involved in malaria at the step of parasite binding to RBCs, the ABO blood group is involved in “adhesion of parasitized RBCs to other cells and this is central to the pathophysiology of severe malaria….”6 A and B antigens are also involved in the adherence of infected RBCs to endothelium; for example, GPA-deficient RBCs are resistant to invasion by merozoites.
P. falciparum–infected RBCs express membrane proteins, causing “sticky knobs” that bind A antigens on uninfected RBCs and rosettes of infected and uninfected RBCs. Rosetting is associated with severe disease by clogging the microvasculature of key organs, especially the brain, leading to cerebral malaria. Nonparasitized RBCs are removed from the circulation along with parasitized RBCs by adherence to the vascular endothelium or to other RBCs.
Rosette formation and adhesion to A and B blood group molecules is thus related to the severity of malaria.6 For example, individuals of blood group A have a 3-fold relative risk of severe malaria compared with those of blood group O.7,8 Not unexpectedly, the distribution of ABO blood groups is highly statistically significantly different between individuals with severe versus mild malaria.6 Therefore, the combination of the 2 blood groups plays a major role in malaria: Duffy involvement in parasite attachment to the RBC surface and ABO with the subsequent pathophysiology of RBC adhesion and thus the severity of the disease.
Knops blood group.
The Knops system antigens are located on the DR1 complement control protein. The Helgeson phenotype, a Knops-null phenotype, has very low levels of CR1. P. falciparum binds to CR1 on RBCs. However, infected RBCs do not form rosettes with RBCs of the Helgeson phenotype with low levels of CR1. Another association of P. falciparum with the Knopps blood group involves the CD 35 molecule. Individuals with low expression of CD 35 show reduced rosetting when parasitized with P. falciparum and thus are likely to have less severe disease.
Gerbich blood group.
The Gerbich antigens are on GPC and GPD. Because GPs are involved with malaria adhesion, RBCs of the Gerbich-negative phenotype are partially resistant to malaria parasitization.
Babesiosis
Babesiosis is a tick-borne disease that is transmitted to humans by the bite of an infected tick, the host of which is usually the deer mouse. The Babesia species are intraerythrocytic protozoans that primarily infect wild and domestic animals, but occasionally infect humans. The most common human infecting species, especially in the United States, is Babesia microti. Babesia divergens occurs mostly in asplenic patients and often involves severe hemolysis and a fulminant course. B. microti is usually subclinical or mild, but is also more severe in asplenic patients. What little is known about the interaction of Babesia with RBCs is from studies of Babesia bovis9 and B. divergens.10
Parasites directly invade RBCs without the need to first pass through other cell types. The specific receptor on the human RBC for the B. microti protozoan is not known. The important receptors for B. divergens are GPA and GPB.10 The Babesia parasites alter the structure and function of RBCs in which they reside. The merozygotes attach to the RBC and invaginate the membrane to form a vacuole, causing the RBC to be more rigid. This is due partly to the presence of the abnormal nondeformable parasite within the RBC, but also there is some alteration of the RBC skeleton and membrane due to parasite-produced proteins. Budding occurs, but the mode of exit of the parasite from the RBC is not known on a molecular level. However, because budding is asynchronous, massive hemolysis is unusual.
Infected RBCs develop ridges that appear to be alterations of the RBC membrane skeleton, but are poorly understood. The parasite delivers proteins that associate with the underside of the RBC membrane and proteins that are exposed on the RBC surface. These poorly understood factors contribute to an adhesive effect of the RBC and parasitized RBCs become abnormally adhesive, including to vascular endothelial cells. The exact role of surface adhesion molecules is not clear, but in the aggregate, these membrane abnormalities result in accelerated RBC splenic clearance. RBC destruction is due not only to the splenic sequestration due to changes in deformability, but also to alteration of immunologic properties that leads to splenic sequestration of the RBC.
The symptoms of babesiosis are caused by the reproduction of the protozoa in the RBC with subsequent hemolysis. The accumulation of parasitized RBCs in the microvasculature leads to severe clinical complications such as cerebral babesiosis, respiratory distress, and multiorgan failure. Symptoms may be mild to severe, necessitating RBC transfusion or even exchange transfusion. Usually, 1%–10% of RBCs are parasitized, although this may be up to 80% in asplenic patients. Babesiosis is fatal in 6%–9% of reported patients, although may reach 42% in asplenic patients (12 ). Transfusion-transmitted Babesia infections are one of the major current issues in transfusion medicine.11 In a review of 159 patients11 with transfusion-transmitted babesiosis, 18% died, but the role of Babesia in those deaths cannot be determined from the literature.
Bartonellosis (Oroya fever)
In 1865, a medical student named Carrion inoculated himself with blood obtained from the skin of a patient with a verrucas node of the skin. Unfortunately, Mr. Carrion developed a fatal hemolytic anemia that became known as bartonellosis or Oroya fever, caused by the agent Bartonella bacilliformis. The infection is transmitted to humans by the bite of the sandfly. After the bite, RBCs become infected with B. bacilliformis. The organism uses its flagella to penetrate the membrane, and this is followed by endocytosis of the organism and a resulting intracellular vacuole. It appears that some organisms do not penetrate the RBCs, but rather adhere to its external surface. For example, when infected RBCs are washed, free organisms are found and the RBCs are not hemolyzed. The osmotic fragility of the RBC may increase13 or remain normal, but RBCs are rapidly removed from the circulation by the reticuloendothelial system of the liver and spleen. Transfused normal RBCs also become coated with the organism and therefore are also removed from the circulation rapidly.14 The exact mechanism of removal is not well understood, but RBCs develop trenches or indentations and invaginations that apparently contribute to their premature removal from the circulation. The direct antiglobulin test is negative and cold agglutinins do not develop as a result of infection. Therefore, RBC removal does not appear to be antibody mediated.
The diagnosis of bartenellosis is made by demonstrating the presence of the organism on the surface of erythrocytes with a Giemsa-stained blood smear showing red rods varying in length from 1 to 3 μm. The second stage of Bartonella infection is a nonhematologic disorder characterized by an eruption over the face and extremities, which develops into bleeding, warty-appearing tumors. Other species of Bartonella cause human febrile infections such as cat scratch fever or trench fever, but these disorders are not associated with hemolytic anemia or RBC destruction.
Toxoplasmosis
Toxoplasmosis is one of the most common protozoal (Toxoplasma gondii) diseases, with ∼60 million people infected in the United States. It is usually caused by ingestion of undercooked meat. Many of those infected are asymptomatic or experience a mild, flu-like illness. Toxoplasmosis is mostly a disease of lymphadenopathy and cyst formation in multiple organs including brain, eye, heart, lung, skeletal muscle, GI tract, and pancreas. In infants or immunosuppressed individuals, the illness can be severe or even fatal. Although hematologic manifestations are not a major part of toxoplasmosis, a few cases have been reported.14 The parasite is able to penetrate early forms of erythrocytes.15 T. gondii may secrete an enzyme that acts on the host cell membrane to assist parasite penetration.
One patient, reported in detail, had anemia, erythroid hyperplasia, megablastic changes in the BM, and shortened RBC survival.14 In a summary of 10 cases, 6 had anemia. However, hematologic data from a small number of cases does not allow generalization as to the hematologic manifestations of Toxoplasma infection. It has been suggested that these might be a “reaction to parasitism rather than an independent process.”14
RBCs as secondary targets in infection
In many situations, the infecting microbe does not attack the RBC directly and cause intravascular hemolysis, but instead may cause hemolysis indirectly or accelerated RBC destruction usually via the liver and spleen. Several mechanism have been proposed including: absorption of immune complexes and complement onto the RBC surface, development of cross-reacting antibodies, and true autoimmunity with loss of tolerance secondary to the infectious agent.1 Alternately, RBC production can be influenced by the infecting agents.
Alterations of the RBC causing immunologic clearance
Mycoplasma pneumoniae.
M. pneumoniae infection usually leads to clinical pulmonary disease. As many as 50%–70% of patients infected with M. pneumoniae develop cold agglutinins. These are oligoclonal IgM immunoglobulins, usually with specificity for the I antigen that is present on all RBCs. The I antibodies may be formed by alteration of the I antigen from hydrogen peroxide secreted by the M. pneumonia, thus creating a newly antigenic form of I. An alternative hypothesis is that the anti-I is directed toward the M. pneumoniae and merely cross-reacts with the RBC-I antigen.
Low levels of cold agglutinins are common in healthy individuals and patients. These antibodies are of no clinical significance, so cross-matching for selection of RBCs for transfusion is done at 37°C to avoid detecting these nonclinically significant antibodies in vitro. In some situations, these antibodies change their character and react at higher temperatures (eg, 37°C), bind complement, and cause hemolysis. The I antibodies usually develop early (7-10 days) during infection, peak at about 2-3 weeks, and persist for 2-3 months. Severe hemolysis is unusual, but subclinical levels of RBC destruction are probably common. Safe transfusion can be accomplished by warming the blood as it is transfused.15
Haemophilis influenzae type B.
The capsular polysaccharide of H. influenzae B is released from the organism during infection and binds to RBCs.16 As infection progresses and antibodies develop against H. influenzae, they react with the polysaccharide-coated RBCs, causing complement activation. This results in a combination of RBC lysis and accelerated clearance via the liver and spleen. Although anemia is not common during infection with H. influenzae, severe hemolysis can occur rarely.
Salmonella.
Salmonella can cause in vitro agglutination, but it is not clear that this causes hemolysis in vivo.
Kala-azar.
RBCs in some kala-azar (ie, visceral leishmaniasis) patients are coated with complement, leading to accelerated clearance and anemia.1
Other infectious agents.
Other agents purportedly involved in immune hemolytic anemia are: measles, cytomegalovirus, varicella, herpes simplex, influenza A and B, EBV, HIV, and coxsackievirus.1
Enzymatic exposure of previously cryptic antigens: polyagglutination
Microbial enzymes can alter the RBC surface, revealing cryptantigens. This process is associated with septicemia, bowel or respiratory tract infections, wound infections, or processes that damage the bowel wall, allowing entry of the microbial enzymes into the circulation.17 The T-system is the most well described of these processes.
T-activation.
Anti-T develops after about 3 months of age and is present in all adults, probably as a result of exposure to various bacteria and environmental stimuli. The T antigen is present on the surface of RBCs, but normally is masked by N-acetyl neuraminic (sialic) acid. This acid can be removed from the RBC surface by neurominidase, which is produced by Clostridium perfringen species, Streptococcus pneumoniae, and influenza viruses. Removal of sialic acid exposes one or more different carbohydrate-related T antigens. The anti-T/T antigen reaction causes polyagglutination; that is, sera from all donors cause RBC agglutination in vitro. This makes it difficult to find compatible RBCs should the patients need RBC transfusions. Rarely, the anti-T may cause intravascular hemolysis of the T-activated RBCs.17
Toxin damage to RBCs causing accelerated destruction
C. perfringens.
C. perfringens is a gram-positive anaerobic organism usually associated with septic abortion, but also with cholecystitis, hepatic abscess, or amniocentesis. The alpha toxin of this organism is a lecithinase C, which reacts with lipoprotein complexes at the RBC surface, liberating the hemolytic substances called lysolecithins, which damage the RBC membrane, resulting in intravascular hemolysis.
Other toxins.
Some strains of enterohemorrhagic E. coli produce toxins that damage the RBC membranes as part of a hemolytic uremic syndrome. Some of these are known as Shiga toxins. GPs are receptors for some bacterial toxins, especially E. coli and Vibrio cholera, that can lyse RBCs.
Inhibition of RBC production
Parvovirus B-19.
The P antigen is a receptor for parvovirus B-19,18 although it alone is not sufficient for attachment of the virus to the RBC. Erythroid precursors, but not mature RBCs, are invaded by the virus and the virus only replicates in erythroid progenitors. Therefore, parvovirus B-19 infection causes direct inhibition of burst forming units E, so anemia may develop. This is especially severe and may result in aplastic crisis in patients with ongoing chronic hemolytic process such as sickle cell disease or hereditary spherocytosis. Individuals negative for the P antigen (pp) are resistant to infection with parvovirus B-19.17
EBV.
In patients with infectious mononucleosis, autoimmune hemolytic anemia is said to occur 0.5%–3% of the time.1 This is usually due to cold agglutinins with specificity for the RBC antigen i. Hemolysis occurs during the first 2–3 weeks of infection and spontaneously clears in 2-3 months.1 The underlying mechanism is not clear. The classic heterophil antibodies that occur in most cases of infectious mononucleosis and react with beef, horse, goat, and camel, but not human, RBCs are not responsible for anemia or hemolysis. EBV infection may cause RBC aplasia by T-cell suppression of erythroid colony forming units.
Acquired RBC antigens due to infectious agents
Acquired B antigen.
Bacterial enzymes, possibly deacetylases, may convert the blood group A determinant sugar into a form similar to the blood group B determinant sugar. This then gives the appearance that a type A individual has become type B. These individuals continue to produce anti-B, but this does not react with their own apparent type B RBCs. Bacteria shown to cause this phenomenon in vitro are C. perfringens species and E. coli.17
RBC blood group antigens in infection
The RBC may be involved in infection of other tissues indirectly. Many RBC antigens are located on molecules with important physiologic functions, some of which involve infectious agents. On other tissues, these may involve adhesion molecules, complement receptors, or microbial receptors.
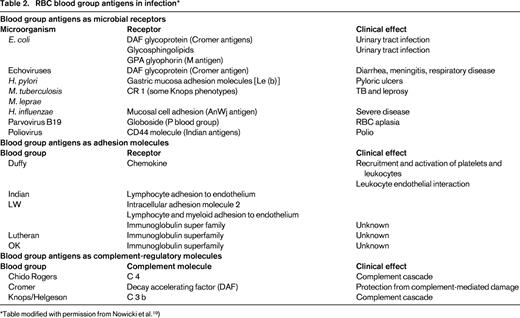
Blood group antigens as microbial receptors
Blood group antigens play a central role in host pathogen relations. “No other gene category shows similar levels of wide spread selection.”2 For example, many infectious agents attack the RBC surface determinants, although the pathogens targets are other tissues. As a first step in infection, an invading organism must bind to tissue.3,4 E. coli have several adhesion molecules. One group of these (the fimbriae) binds to the decay-excelerating factor (DAF) glycoprotein, which contains the Cromer blood group. Because the Cromer antigen is the receptor for E. coli binding, this facilitates infection with E. coli.19 The DAF molecule containing Cromer is located on cells other than RBCs and there is a suggestion that binding of E. coli to Cromer in the urinary tract facilitates urinary tract infection, cystitis, and diarrhea.20 DAF is also the ligand for echoviruses and coxsackieviruses, which can cause diarrhea, meningitis, and neonatal respiratory disease.
Such a mechanism is clearer with the P blood group system. Some E. coli adhesion molecules bind to the glycosphingolipids of the P system, thus increasing the likelihood of urinary tract infection in individuals who are P-antigen positive.22 Another receptor for E. coli is GPA containing the M antigen.
There are associations between the Lewis (b) determinant and binding to Helicobacter pylori. H. pylori produces adhesion molecules that bind to Le(b) on gastric mucosa cells. Therefore, whereas many people harbor H. pylori, Le(b+) individuals are more likely to become infected and develop pyloric ulcers.17
The CR 1 molecule containing the Knops antigen is a binding site for Mycobacterium tuberculosis and M. leprae. Some alternate Knops phenotypes provide protection to M. tuberculosis or M. leprae.17
The AnWj antigen binds H. influenzae. Strains of H. influenzae isolated from patients with more severe disease contain fimbriae that are probably involved in adhesion to mucosal cells, but that also agglutinate most RBCs except those of the AnWj-negative phenotype.
Some viruses also adhere to molecules containing blood group antigens. The parvovirus B19 adheres to globoside of the P blood group system.18 Enteroviruses such as coxsackievirus or echovirus use DAF containing the Cromer antigens as receptors. Poliovirus may use the CD44 molecule containing the Indian blood group antigens in its adhesive process.
Receptors and adhesion molecules
Some RBC surface proteins resemble receptor or adhesion molecules (Table 2). The role of the RBC chemokine receptors is to bind and thus inactivate chemokines in the blood, and several RBC antigen molecules fill this function. It is not known whether any of these RBC receptors and adhesion molecules are targets of or play a role in infection. However, because they are part of such a crucial and complex system, such a role could exist.
Complement regulatory molecules
Three blood group systems are part of molecules involved in the complement pathway. The Chido/Rogers antigens are part of the C4 molecule,21 which is absorbed onto RBCs from the circulation. The Cromer antigens are located on the DAF RBC membrane molecule,22 the role of which is to protect RBCs from complement damage. The Knops antigens are part of the C3b complement receptor.23 Despite these blood group antigen relationships with the complement system, there is no evidence that they are involved with complement in infectious processes.
Inflammatory mediators and cytokines damage RBCs, causing accelerated clearance
As part of most infections, there is generation of cytokines and inflammatory mediators. These may affect RBCs by causing membrane damage, altering the structure, leading to immunologic clearance, or other mechanisms. Because these are general pathologic processes, they are not discussed in detail here.
Conclusion
The RBC may be a target of infections in several different ways, ranging from direct attack by the microbe to damage, accelerated clearance, or lysis due to toxins produced by the infecting agent or alteration of the RBC surface. Some RBC blood group antigens are on molecules containing receptors for certain microbes, making infection with those agents more likely.
Disclosures
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for Fresenius/Kabe. Off-label drug use: None disclosed.
Correspondence
Jeffrey McCullough, MD, Professor, Department of Laboratory Medicine & Pathology, MMC 609, Mayo Building, 420 Delaware St. SE, Minneapolis, MN 55455; Phone: (612)626-3272; e-mail: mccul001@umn.edu.