Abstract
Platelets are primary effector cells in hemostasis. Emerging evidence over the last decade, however, demonstrates that platelets also have critical roles in immunity and inflammation. These nontraditional functions of platelets influence the development, progression, and evolution of numerous diseases, including arthritis, cancer, cardiovascular disease, and infectious syndromes. This chapters reviews recently discovered attributes of platelets that contribute to human disease, paying particular attention to the inflammatory activities of this anucleate cytoplast.
Learning Objective
To provide a brief understanding of recently discovered inflammatory functions of platelets that contribute to human disease
Background
Since their discovery, platelets have been known for their roles in hemostasis, in which they rapidly bind to damaged blood vessels and prevent excessive bleeding. The hemostatic functions of platelets have been and continue to be the focus of intense investigation, especially in disease situations in which platelets inadvertently occlude vessels that should remain patent. Indeed, drugs that dampen the adhesive functions of platelets are commonplace in the clinical setting because they have proven benefits in the treatment of cardiovascular disease. Because of their primary role in hemostasis, many view platelets as “sacks of glue.” However, emerging evidence demonstrates that platelets are far more complex than previously considered and their intracellular machinery is not used solely for hemostatic activities. The newly recognized inflammatory functions of platelets are the focus of this review.
Phylogeny of platelets
The phylogeny of platelets suggests that they are destined to have prominent inflammatory functions. In-depth discussion of how platelets evolved from primitive, multifunctional cells can be found in previous reviews.1,2 In brief, platelets likely evolved from invertebrate cells that have ancient origins in host defense.2 By the vertebrate stage, blood cells adapted to perform more distinct functions. For example, fish, reptiles, and birds have circulating thrombocytes that are phenotypically and functionally similar to platelets.2 Unlike platelets, however, thrombocytes are nucleated cells that regulate hemostatic and inflammatory processes.3 Some of the activities of thrombocytes are driven and controlled by their nucleus. Therefore, one might expect that platelets do not express nuclear components or perform “nuclear-like functions” because they circulate as anucleate cytoplasts. New evidence, however, suggests otherwise. Platelets possess numerous transcription factors that have nongenomic functions,4-6 an active spliceosome,7,8 and an extensive repertoire of miRNA and mRNAs.9-12 Platelets also have diverse inflammatory functions, including a recently described inflammasome function.13 Therefore, platelets are complex cells with highly evolved, nonhemostatic functions.
Platelets induce inflammatory responses in leukocytes
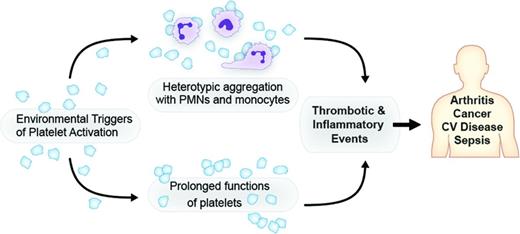
Platelets have a diverse array of surface receptors that allow them to interact with leukocytes, pathogens, pathogen-released products, and inflamed endothelium.1,14 These interactions contribute to microvascular occlusion, thrombosis, and propagation of inflammatory damage elicited at the vascular wall.1 The reader is referred to recent reviews that detail the types and functions of these receptors in platelet-mediated responses, especially in relation to interactions with bacteria, bacterial-derived toxins, and endothelial cells.1,14-16 Under homeostatic conditions, platelets generally do not bind to leukocytes. However, upon activation, platelets adhere to neutrophils and monocytes and interactions with lymphocytes have also been reported.1,17,18 These heterotypic aggregates have several inflammatory consequences, which may be good or bad depending on the pathologic situation (Figure 1). Although less studied, reciprocal activation of platelets also occurs.19 Interactions of platelets with neutrophils and monocytes have received the most attention in regard to inflammatory responses.
Platelets are effectors of thrombotic and inflammatory injury. Platelet signaling of leukocytes and/or prolonged newly recognized functions contribute to the pathophysiology of arthritis, cancer, cardiovascular (CV) disease, sepsis, and other clinical syndromes.
Platelets are effectors of thrombotic and inflammatory injury. Platelet signaling of leukocytes and/or prolonged newly recognized functions contribute to the pathophysiology of arthritis, cancer, cardiovascular (CV) disease, sepsis, and other clinical syndromes.
As described in previous reviews, binding between the cell types is primarily mediated by P-selectin, which translocates to the surface of activated platelets, and P-selectin glycoprotein-1 (PSGL-1) on target leukocytes, but P-selectin/PSGL-1 interactions tend to be more transient in neutrophils than in monocytes.20 Platelet-neutrophil and platelet-monocyte aggregates (PMAs) have been detected in the blood of humans with a variety of diseases1 and are considered one of the most sensitive markers of platelet activation in human whole blood.21
When platelets bind neutrophils, they trigger the release of chemokines and the formation of neutrophil extracellular traps (NETs),17,22 which are lattices of chromatin, histones, and granule enzymes that are intended to capture and kill microbes.23 NETs have known roles in bacterial sepsis, but they are also generated in transfusion-related acute lung injury (TRALI), venous thrombosis, and a variety of other infectious syndromes.24-26 Untimely generation of NETs can also induce vascular injury and trap platelets, which may lead to adverse responses in many disease settings. Lipopolysaccharide-stimulated platelets trigger NET formation by mechanisms that remain unresolved and, in TRALI, NETs accumulate in lung microvessels in a platelet-dependent fashion.22,24 Human platelets also trigger NET formation via the release of β-defensin 1,27 a potent antimicrobial and signaling molecule.
Platelets also induce an array of inflammatory responses in monocytes, and targeted disruption of PMAs may have therapeutic value in mitigating thromboinflammation at the vascular wall. Circulating PMAs are elevated in a variety of diseases, and cigarette smoke acutely increases the number of PMAs in the bloodstream.1,28 Several studies have shown that as platelets bind to monocytes, they induce monocyte-derived synthesis of critical cytokines including IL-8, IL-1β, cyclooxygenase-2 (COX-2), and monocyte chemotactic protein-1 (MCP-1).18,29 Recent studies also demonstrate that PMAs synthesize soluble Flt-1,30 which has critical functions in preeclampsia, and dengue infection induces the formation of PMAs and synthesis of both proinflammatory and antiinflammatory products.31
Platelets also release a variety of factors that signal leukocytes, including angiogenic factors, chemokines, and CD40L, which is a pivotal immune signaling molecule in the adaptive immune response.32 Taken together, these findings indicate that platelets regulate and amplify responses in leukocytes through a variety of mechanisms that involve direct binding and release of soluble products. These activities, and others, are now widely recognized in the field and have led to the growing appreciation that platelets are critically involved in thrombosis and inflammation.
Platelet RNAs: effectors of cellular function and predictors of disease
Although it is not hard to imagine that platelets can induce inflammatory gene expression in other cells, one might not predict that platelets themselves are capable of synthesizing proteins. This was our premise in 1998 when we examined the expression of B cell lymphoma 3 (Bcl-3) in PMAs and unexpectedly found that platelets, not monocytes, synthesize Bcl-3 protein upon activation.4 We subsequently demonstrated that Bcl-3, a presumed transcription factor, regulates cytoskeletal processes including platelet-dependent clot retraction.4,33 Perhaps more importantly, we realized that platelets function for extended periods of time and at least one of their prolonged functions is de novo synthesis of proteins.
We have since shown that platelets also synthesize IL-1β, a proinflammatory cytokine.34 Synthesis of IL-1β is controlled at many steps, starting first with splicing of IL-1β pre-mRNA into mature message.7 Demonstration that platelets splice pre-mRNAs provided the first example that an active spliceosome, one of the most complex eukaryotic signaling systems, resided outside of the nuclear milieu. It also demonstrated that platelets are far more sophisticated than previously thought despite their anucleate stature. In addition to splicing pre-mRNA, platelets process newly synthesized pro-IL-1β protein into its mature, active form.7,34 Processing of IL-1β protein occurs via the inflammasome, another complex intracellular system recently described in platelets.13 Ultimately, platelets package IL-1β into microparticles that induce endothelial cell permeability.13,34 Recent evidence has also shown that IL-1β is produced at higher levels when platelets are exposed to endotoxin compared with classic platelet agonists, and dengue induces significant amounts of IL-1β synthesis by platelets.8,13 These findings demonstrate that platelets sense infectious stimuli and respond in a discrete fashion to produce active, inflammatory products.
In addition to expressing transcripts for Bcl-3 and IL-1β, it is now known that platelets possess thousands of mRNAs.12 The advent of next-generation RNA sequencing has shown that mRNAs in platelets have classic features, including capped and polyadenylated tails that control stability and translation.35 The repertoire of mRNAs in platelets is similar among healthy subjects, but levels of expression often differ between individuals. Perhaps the best example of this is a recent publication by Edelstein et al showing that several platelet mRNAs are differently expressed between white and black subjects, including phosphatidylcholine transfer protein (PCTP).36 This group demonstrated that PCTP contributes to racial differences in PAR4-mediated platelet activation, which indicates that the effects of race need to be considered when developing future antiplatelet drugs. It also opens the door for personalized therapeutic regimens in cohorts of subjects with defined risk factors. Platelet mRNA expression analysis has also been used to predict whether genes are conserved between mouse and humans,12 which has proven particularly useful as one considers whether the expense, time, and effort to manipulate a gene in mice will yield interpretable results. Platelet mRNA expression profiling also provides key insights into the types of genes that are being regulated in megakaryocytes,37 especially genes that are actively transcribed in response to environmental cues such as toxins, cytokines, and lipid mediators.
In addition to mRNAs, platelets have a very diverse and abundant repertoire of miRNAs.9 Platelet miRNA expression has been particularly useful in predicting how mRNAs are regulated in megakaryocytes.38 It is likely, however, that miRNAs also function in platelets. In support of this, human platelets contain functional pre-miRNA processing machinery, including Dicer and Ago2.38 Platelet miRNAs are also transferred to other cells, as are platelet mRNAs, and both types of RNA continue to function in target cells.39,40 Paracrine delivery of miRNAs and mRNAs to target cells represents yet another fascinating function of platelets that was not previously considered until the last few years.
Summary
This brief review highlights several nontraditional functions of platelets, but it is not intended to be a comprehensive review of the literature. Instead, its sole intent is to introduce readers to previously unrecognized functions of platelets that contribute to human disease. These unexpected functions of platelets are reviewed in more detail elsewhere1,14,15 and will continue to evolve and be refined over the coming years. With each discovery, we should expect the unexpected. One thing we can say for sure, however, is that platelets are far more complex than a “sack of glue.”
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Andrew S. Weyrich, PhD, Professor of Internal Medicine, Eccles Institute of Human Genetics, Bldg 533, Rm 4220, University of Utah, Salt Lake City, UT 84112; Phone: (801)585-0727; Fax: (801)585-0701; e-mail: andy.weyrich@u2m2.utah.edu.