Abstract
Women with acquired and inherited thrombophilia are thought to be at increased risk for pregnancy complications, including recurrent pregnancy loss and, depending on the type of thrombophilia, severe preeclampsia. This review discusses the associations between the types of thrombophilia and types of complications, as well as the currently available clinical trial evidence regarding the use of aspirin and heparin to prevent these pregnancy complications. In women with antiphospholipid syndrome, guidelines recommend prescribing aspirin and heparin to women with recurrent miscarriage. The same regimen is suggested for late pregnancy complications by some, but not all, experts. Aspirin or low-molecular-weight heparin to improve pregnancy outcome in women with unexplained recurrent miscarriage has no benefit and should not be prescribed. Whether anticoagulant therapy prevents recurrent miscarriage in women with inherited thrombophilia or in women with severe pregnancy complications remains controversial because of inconsistent results from trials. Aspirin modestly decreases the risk of severe preeclampsia in women at high risk.
Learning Objectives
To describe the association between pregnancy complications and thrombophilia both quantitatively and qualitatively
To describe the state of the evidence with respect to prevention of pregnancy complications with aspirin and/or heparin in various subgroups of women, particularly women with antiphospholipid syndrome and those with inherited thrombophilia
Introduction
Whether women with placenta-mediated pregnancy complications, including recurrent miscarriage, late pregnancy loss, preeclampsia, intrauterine growth restriction, and placental abruption, benefit from anticoagulant or antithrombotic agents such as aspirin or heparin is a frequently occurring clinical question.1-3 The role of the coagulation specialist is particularly evident for women with some form of thrombophilia. Pregnancy failure is extremely distressing for couples who desire to have children and preeclampsia and HELLP syndrome (hemolysis, elevated liver enzymes, low platelet counts) are leading causes of maternal and perinatal mortality and morbidity.4 A presumed benefit of antithrombotic therapy, in the perceived absence of harm, has led many clinicians to prescribe low-molecular-weight heparin (LMWH), aspirin, or both to women with placenta-mediated pregnancy complications, sometimes but not exclusively based on the presence of thrombophilia. This review discusses the associations of thrombophilia and pregnancy complications and focuses on the current evidence on antithrombotic therapy to improve pregnancy outcome.
Clinical case
A 38-year-old woman who has moved to the Netherlands many years ago is referred to my outpatient clinic because of a history of 3 early miscarriages and the presence of the prothrombin 20210A mutation. She and her husband are convinced that aspirin and LMWH should be prescribed to improve the prognosis in a next pregnancy because this was suggested by an obstetrician whom she consulted in her country of birth. The patient has been pregnant 4 times in the past 3 years. The first pregnancy ended in spontaneous miscarriage at approximately 6 weeks after the first day of her last menstrual period (gestational age); the second pregnancy went well until she developed preeclampsia at a gestational age of 34 weeks. At 35 weeks, she prematurely delivered, after induction, a healthy son with a birth weight of 2800 g. Her third and fourth pregnancies ended in spontaneous miscarriages at weeks 7 and 6; in the third pregnancy, a fetal heart beat had been detected before the pregnancy loss. She is otherwise healthy and has no personal or family history of venous thromboembolism. She has a normal body weight (BMI 22) and normal blood pressure. Thrombophilia screening reveals no laboratory criteria for antiphospholipid syndrome (APS); hereditary thrombophilia tests showed normal results for antithrombin, protein C, and protein S levels and activated protein C resistance (indicative of absence of factor V Leiden) and heterozygosity for prothrombin 20210A.
Definition of thrombophilia
Changes in blood composition are part of Virchow's triad, which he proposed in the 19th century as one of the mechanisms that predispose to thrombosis. These changes, known as thrombophilia, indicate the presence of a hypercoagulable state leading to a thrombotic tendency. The currently most commonly tested inherited thrombophilias include deficiencies of antithrombin, protein C, or protein S and the gain-of-function mutations factor V Leiden and prothrombin G20210A, which affect either the anticoagulant or procoagulant pathways, respectively.5 Lupus anticoagulant, anticardiolipin antibodies, and anti-beta 2 glycoprotein I antibodies are laboratory features of acquired thrombophilic APS (Table 1).6 Although some laboratories include other tests, both established and less well established, such as levels of coagulation factor VIII or polymorphisms such as MTHFR 677TT and PAI-1 4G/5G in their thrombophilia panels, these are not discussed here because their associations with pregnancy complications are most uncertain.
Biological plausibility for a role of thrombophilia in pregnancy complications
The mechanisms of how thrombophilia leads to pregnancy complications are difficult to study in humans and remain largely unknown. It is attractive to hypothesize that hypercoagulability with thrombosis of placental vasculature is the pathophysiological substrate for an association with both acquired (APS) and inherited thrombophilia. However, coagulation and inflammation are closely related pathways and several observations have implicated a role for both procoagulant and inflammatory pathways in pregnancy failure. In vitro experiments have shown that antiphospholipid antibodies inhibit extravillous trophoblast differentiation and subsequent placentation.7 Although tissue factor seems to play a central role, this appears independent of its role in coagulation.8 Similar mechanisms may be true for inherited thrombophilia. Thrombomodulin-deficient mice, which are lacking the important natural anticoagulant protein C pathway, are unable to carry their fetuses beyond 8.5 weeks gestational age and dead fetuses are usually resorbed within 24 hours. Fetal demise is caused by tissue-factor-dependent activation of blood coagulation at the feto–maternal interface. Activated coagulation factors were found to induce cell death and inhibit growth of trophoblast cells.9 Furthermore, both heparin and aspirin attenuate trophoblast apoptosis in vitro.10 Therefore, mere hypercoagulability is unlikely to be the sole mechanism by which thrombophilia, either acquired or inherited, increases the risk for pregnancy failure or defective placentation.
Association between thrombophilia and pregnancy complications
Pregnancy failure and pregnancy complications are amongst the clinical criteria for APS (Table 1)6 and many studies have observed a relationship between inherited thrombophilia and pregnancy failure and complications. However, most associations are modest in strength and vary with type of thrombophilia and type of pregnancy complications because most studies have been underpowered11-14 (Table 2). Even for APS, the associations between various types of antiphospholipid antibodies and placenta-mediated pregnancy complications were found to be inconsistent in a recent large systematic review.12 Particularly for anti-beta 2 glycoprotein I antibodies, the relationship between these antibodies and pregnancy complications was not evident. The investigators concluded that it is questionable whether a diagnosis of APS in the setting of pregnancy complications should be established if only these antibodies are present at elevated levels as part of the laboratory criteria. Furthermore, the most recent and larger prospective cohort studies found lower odds ratios for inherited thrombophilia than older and smaller case control studies, which may point to a bias in the observed associations.12,13,15,16 Therefore, it can be concluded that even the association between thrombophilia and placenta-mediated pregnancy complications is controversial.
Associations between pregnancy complications and several forms of thrombophilia
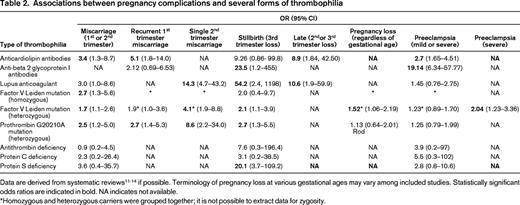
Data are derived from systematic reviews11-14 if possible. Terminology of pregnancy loss at various gestational ages may vary among included studies. Statistically significant odds ratios are indicated in bold. NA indicates not available.
*Homozygous and heterozygous carriers were grouped together; it is not possible to extract data for zygosity.
Clinical trial evidence of effect of aspirin or heparin on pregnancy complications
Comprehensive systematic reviews summarizing the clinical trial evidence in the field of antithrombotic therapy and pregnancy complications have been published recently.3,17 For extensive details, I refer to the original trial reports and meta-analyses. Interpretation of the current clinical trial evidence is provided here.
General considerations
First, depending on the type of pregnancy complications, the natural history of subsequent pregnancies without pharmacological intervention is often uncertain. For example, recurrence rates of preeclampsia in women with a history of severe preeclampsia range from 25% to 65% and solid estimates of whether this is higher in women with thrombophilia are largely unknown. Furthermore, these women also have a 3% risk of placental abruption and 10% risk of having a small-for-gestational-age baby (<10th percentile).18,19 For pregnancy loss, the prognosis of a subsequent live birth ranges from 0% to 99%, indicating the difficulty in drawing conclusions.3 Study populations varied and the onset of follow-up differed per study. Live birth rates in women recruited in very early pregnancy generally were substantially lower than in women who were recruited from 12 weeks gestation, which was likely explained by the fact that women with early miscarriages are not included in the latter study population.
Second, beneficial effects of antithrombotic agents have been suggested by results from observational studies that have intrinsic methodological issues undermining their validity to assess efficacy of an intervention.1
Third, although clinical trials have been performed in recent years, these are generally limited by small sample sizes and often lacked a control arm without active intervention. Furthermore, study populations vary widely. Some trials used very stringent inclusion criteria that limit the generalizability of the findings to women with other or coexisting complications. Other trials used very broad inclusion criteria that make it difficult to draw conclusions for subgroups with specific pregnancy complications or thrombophilia. Some meta-analyses purposely pooled effects of heterogeneous interventions in heterogeneous patient populations. It should be realized that the summary effects from such analyses do not have the same scientific strength as a randomized controlled trial of sufficient size.
Summary of randomized controlled trials and meta-analyses
Aspirin to prevent pregnancy loss
In women with APS, almost no data are available to support the use of aspirin only to prevent recurrent miscarriage. The pooled results of 3 very small trials (total number of 71 participants) showed no effect of aspirin only compared with no treatment [risk ratio (RR) of pregnancy loss = 1.05, 95% confidence interval (CI) = 0.66–1.68], but from the 95% CI, it can be concluded that neither benefit nor harm can be ruled out.20 A small observational study suggested that starting aspirin before conception may be associated with a better pregnancy outcome than starting it once pregnancy is established.21 To my knowledge, no randomized controlled trials evaluating the efficacy of aspirin in women with inherited thrombophilia and recurrent pregnancy loss have been performed.
Aspirin to prevent preeclampsia
A meta-analysis of individual patient data from 31 randomized primary prevention trials with 32 217 women showed that aspirin was associated with a 10% relative risk reduction in preeclampsia, premature birth (<34 weeks gestation), and a pregnancy with a serious adverse outcome.22 There was no significant effect on the risk of death of the fetus or baby, having a small-for-gestational-age infant, or bleeding events for either the women or their babies. No particular subgroup of women who were more or less likely to benefit from antiplatelet agents could be identified. A very recent systemic review observed clinically important and statistically significant reductions in several important outcomes, but the investigators stated that their confidence was tempered by potential small-study effects and observed modest effects on outcomes in the 2 largest trials.23 Depending on baseline risk, aspirin use was associated with absolute risk reductions of 2% to 5% for preeclampsia (RR = 0.76; 95% CI = 0.62–0.95), 1%–5% for intrauterine growth restriction (RR = 0.80; 95% CI = 0.65–0.99), and 2%–4% for preterm birth (RR = 0.86; 95% CI = 0.76–0.98). The investigators did not observe different effects in trials in which aspirin was started before 16 weeks gestational age. The American College of Chest Physicians (ACCP) 2012 guidelines give a grade 1B recommendation to treat women considered at risk for preeclampsia with aspirin throughout pregnancy, starting from the second trimester, over no treatment.24 However, what constitutes high risk for preeclampsia is not strictly defined, but does include a history of severe preeclampsia, prior early preterm birth, renal disease, and autoimmune disease. Therefore, aspirin should be offered on an individualized basis and decisions made on the basis of the woman's risk profile from her obstetric and medical history. For women with a history of severe preeclampsia in the context of APS, I offer aspirin in subsequent pregnancies to improve their outcome (Table 3). Whether women who have a diagnosis of APS based on venous thromboembolism only should be considered at high risk for preeclampsia is basically unknown. Contrary to other views,25 in my opinion, there is no evidence that aspirin on top of regular antepartum thrombosis prophylaxis with LMWH improves pregnancy outcome in women with APS without a history of pregnancy complications. Women with a history of preeclampsia, with or without inherited thrombophilia, are also being counseled regarding the small reduction in risk of recurrence in a next pregnancy and prescribed aspirin on an individualized basis.
Heparin, with or without aspirin, to prevent pregnancy loss
The efficacy of antithrombotic agents in women with unexplained (eg, in the absence of abnormal parental karyotype, uterine anomalies, or APS) recurrent pregnancy loss was compared with no treatment or placebo in 2 relatively large randomized trials.26,27 In the SPIN study, 294 women with 2 or more unexplained pregnancy losses were randomized to enoxaparin 40 mg combined with aspirin 75 mg plus standard surveillance or standard surveillance only.26 No effect of the medical intervention was observed (odds ratio for successful pregnancy = 0.91, 95% CI = 0.52–1.59). In the ALIFE study, we randomized 364 women with 2 or more unexplained pregnancy losses to nadroparin 2850 IU combined with aspirin 80 mg daily, aspirin 80 mg only, or placebo (for aspirin) before conception or at a maximum gestational age of 6 weeks.27 Of these women, 299 became pregnant. The chance of live birth did not differ between the treatment groups [RR of live birth for women who became pregnant was 1.03 (95% CI = 0.85–1.25) for nadroparin combined with aspirin and 0.92 (95% = CI 0.75–1.13) for aspirin only compared with placebo]. Based on the available evidence that also included trials comparing 2 active treatments,28 various guidelines recommend against the use of antithrombotic agents in women with unexplained recurrent pregnancy loss.24,29
In women with recurrent pregnancy loss and APS, a landmark randomized trial showed a large beneficial effect on live birth in women treated with unfractionated heparin combined with aspirin compared with aspirin only.30 A meta-analysis summarized the data of 2 studies in women with APS and 2 or more pregnancy losses31 ; treatment with unfractionated heparin combined with aspirin (n = 103) reduced the chance of first trimester miscarriage compared with aspirin only (n = 109; RR = 0.26; 95% CI = 0.14–0.48); treatment with LMWH combined with aspirin (n = 96) compared with aspirin only (n = 90) showed a pooled relative risk for pregnancy loss of 0.70 without reaching statistical significance (95% CI = 0.34–1.45). Comparing any heparin (unfractionated heparin or LMWH) combined with aspirin (n = 199) with aspirin only (n = 199), the beneficial effect of heparin of reducing the risk of first trimester miscarriage was maintained (RR = 0.39; 95% CI = 0.24–0.65), with little statistical heterogeneity.31 An important question is whether there is a differential effect of unfractionated heparin from LMWH. In a few small studies, the use of LMWH and unfractionated heparin (both combined with aspirin) were compared directly and the results did not suggest a difference in efficacy.3 Furthermore, it is noteworthy that the chances of a live birth in the aspirin-only arms were only 44% and 42% in the studies that observed a profound effect of unfractionated heparin added to aspirin; this is markedly lower than in the comparator arms of studies comparing LMWH and aspirin with aspirin only or aspirin with placebo, in which the chances of a live birth varied between 68% and 80%. This indicates clinical heterogeneity between the trials.3 In a recently published trial in women with APS and at least 2 consecutive miscarriages before 20 weeks gestation, the LMWH bemiparin (2500 U once daily, n = 80) was compared with aspirin 100 mg (n = 61) and a modest benefit of bemiparin over aspirin was observed (RR for live birth for women treated with bemiparin = 1.20; 95% CI = 1.00–1.43).32 The trial used a quasirandomized approach in which allocation to a treatment group was done in an alternating manner, which leads to inadequate concealment of allocation. The ACCP guidelines recommend unfractionated heparin or LMWH combined with aspirin for women with APS and 3 or more pregnancy losses, but refrain from recommendations for women with APS based on clinical criteria of a single late pregnancy loss or placental insufficiency.24 The Royal College of Obstetricians and Gynaecologists guidelines state that pregnant women with APS should be considered for treatment with aspirin combined with heparin to prevent further miscarriage, without specifying clinical criteria of APS in the recommendation.29
Therefore, although evidence for a beneficial effect of heparin combined with aspirin in women with APS and 3 or more miscarriages is compelling, it is based on very small studies in heterogeneous populations. The effect of antithrombotic agents in different subgroups of women with APS based on laboratory or clinical criteria (eg, women with one late pregnancy loss, severe preeclampsia, or those with beta 2 glycoprotein I antibodies) is unknown. Nevertheless, based on the currently available evidence of studies with small numbers of participants, clinicians worldwide have adopted the practice of prescribing aspirin with or without heparin to all women with APS.
For women with recurrent miscarriage and inherited thrombophilia, no sufficiently sized trials have been performed that show an effect of heparin on the prognosis of a subsequent pregnancy. The Habenox trial randomized women with at least 3 consecutive first trimester miscarriages to enoxaparin 40 mg and placebo once daily (n = 68), enoxaparin 40 mg and aspirin 100 mg (n = 63), or aspirin 100 mg (n = 76); there was no control group without intervention.33 A live birth rate of 71% (RR = 1.17; 95% CI = 0.92-1.48) was found for enoxaparin and placebo and 65% (RR = 1.08; 95% CI = 0.83-1.39) for enoxaparin and aspirin compared with aspirin alone (61%, reference group). Almost a quarter of the included women had either hereditary thrombophilia or anticardiolipin antibodies <40 GPL or beta 2 glycoprotein I antibodies, and no differential effects of the interventions were observed. One clinical trial found promising results in women with a single previous pregnancy loss after 10 weeks gestation and heterozygous factor V Leiden mutation, prothrombin G20210A mutation, or protein S deficiency; they were allocated to enoxaparin 40 mg once daily (n = 80) or to aspirin 100 mg (n = 80).34 Women treated with enoxaparin had a much higher chance of a live birth than those allocated to aspirin (86% and 29%, respectively; 57% absolute risk reduction; odds ratio = 15.5; 95% CI = 7–34). However, several methodological issues were raised, and the results of this single study have not been confirmed by other trials; therefore, this regimen was not endorsed by the ACCP guidelines for women with a single previous pregnancy loss after 10 weeks gestation and inherited thrombophilia.24,35
In the SPIN and ALIFE studies, small proportions of the study populations consisted of women with inherited thrombophilia26,27 ; the subgroup analyses of these women were insufficiently powered to address the effect of antithrombotic treatment. In the ALIFE study, a nonsignificant increase in live birth was observed in the 2 active treatment arms for women with inherited thrombophilia (RR for live birth = 1.22; 95% CI = 0.69–2.16 for aspirin and RR = 1.31; 95% CI = 0.74–2.33 for aspirin combined with nadroparin compared with placebo), highlighting the urgent need for new randomized controlled trials. We are currently performing the ALIFE2 study (NTR 3361; www.trialregister.nl) that started recruiting in 2013; in this trial, women with inherited thrombophilia and recurrent pregnancy loss are being randomized to either treatment with LMWH plus standard pregnancy surveillance or standard pregnancy surveillance only.
Heparin to prevent preeclampsia
Finally, a few trials have investigated the use of LMWH with or without aspirin compared with no treatment in women with a history of various pregnancy complications, including preeclampsia, small-for-gestational age babies, and placental abruption, to reduce the risk of recurrence in subsequent pregnancies. These 6 studies were recently summarized in a meta-analysis.17 The primary outcome was a composite of preeclampsia, birth of a small-for-gestational-age newborn (<10th percentile), placental abruption, or pregnancy loss later than 20 weeks. Overall, 67 of 358 (18.7%) women randomized to prophylactic LMWH had recurrent severe placenta-mediated pregnancy complications, compared with 127 of 296 (42.9%) women with no LMWH (RR = 0.52; 95% CI = 0.32-0.86). The included studies are relatively small, heterogeneous with regard to type of complications, and the inclusion or exclusion of thrombophilia. Although the pooled risk reduction is statistically significant, the results are strikingly positive in some studies, with relative risk reductions up to 85%,36-39 whereas in the 2 most recently published studies, one in thrombophilic women, no effect on the risk of recurrence of severe pregnancy complications was observed.40,41 This is reflected by the statistical heterogeneity that was also observed in the meta-analysis (I2 = 69%).17 In all studies combined, 25% of women had thrombophilia and only the FRUIT study was dedicated to thrombophilic women only.40 In this trial, women with inherited thrombophilia and a history of preeclampsia or intrauterine growth restriction, <10th percentile requiring delivery before 34 weeks of gestation, were randomized between prophylactic dose LMWH (dalteparin, 5000 IU) with aspirin and aspirin alone. The primary outcomes were recurrence of a hypertensive disorder (preeclampsia, HELLP, or eclampsia) before 34 weeks gestation or recurrence at any gestational age. The overall primary outcome did not differ between the 2 groups; hypertensive disorders occurred in 18.6% of the women randomized to LMWH + aspirin (n = 13/70) and 21.7% (n = 15/69) of women on aspirin alone (risk difference = 3.1%; 95% CI = 10.5-16.7%). None of the women in the LMWH + aspirin group developed recurrent hypertensive disorders before 34 weeks gestational age, whereas 6 (8.7%) women in the aspirin-only group delivered before 34 weeks due to the development of recurrent hypertensive disorders, with 3 women delivering at 19-22 weeks (risk difference = 8.7%; 95% CI = 1.9-15.5%). The recently finished TIPPS study, which included thrombophilic women either at high risk for pregnancy-mediated complications or at high risk of pregnancy-related venous thromboembolism, randomized women between LMWH and no LMWH and found no effect of the intervention.42
Why should we prescribe prudently?
In addition to LMWH being very costly, the potential adverse effects of antithrombotic therapy include bleeding, local skin reactions such as itching and swelling, and the rarer complications of heparin-induced thrombocytopenia and heparin-induced osteopenia.24 Although the incidence of major bleeding is low, avoiding any bleeding by withholding ineffective therapy is preferred. Delayed-type allergic skin reactions due to administration of LMWH occur in ∼ 20% of women and were reported in 40% of women treated with nadroparin in the ALIFE study.27,43 Finally, the use of antithrombotic therapy raises specific issues with regard to planning and induction of delivery and neuraxial anesthesia.
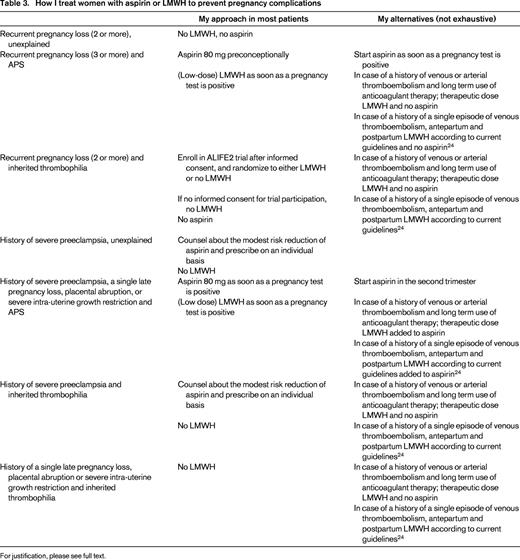
Conclusions, management of the clinical case, and future perspectives
In my view, the body of evidence shown in the above-mentioned intervention studies have not clearly and unequivocally shown the benefit of LMWH with or without the addition of aspirin. This is even true for women with APS, but foremost for women with inherited thrombophilia and recurrent pregnancy loss. Women with a history of severe preeclampsia likely benefit modestly from aspirin, whereas the evidence that LMWH reduces the risk for recurrent severe placenta-mediated pregnancy complications in women with or without inherited thrombophilia is extremely inconsistent.
Nevertheless, we have to deal with clinical cases every day. I have summarized my approach in clinical practice in Table 3. For the presented clinical case, I have counseled her to participate in the ALIFE2 trial. If she will not give consent for participation, I will only prescribe her aspirin based on her history of preeclampsia and refrain from prescribing LMWH.
The huge evidence gaps should be filled in in the next few years by multinational collaborative studies. Acquiring funding and ethical approval for such studies and finding patients who are willing to participate may be a hurdle that can only be overcome by mutual scientific enthusiasm and persistence. I strongly believe that it is our responsibility to further advance the field, rather than prescribing potential harmful and costly medication for which the benefits are still unclear.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from GSK, BMS/Pfizer, Meda Pharma, and Sanquin and has received honoraria from GSK, Bayer, Boehringer Ingelheim, and Daiichi Sankyo. Off-label drug use: LMWH and aspirin for pregnancy complications.
Correspondence
Saskia Middeldorp, MD, Professor of Medicine, Department of Vascular Medicine, Academic Medical Center, F4-276, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Phone: 31-20-5665976; Fax: 31-20-6968833; e-mail: s.middeldorp@amc.uva.nl.