Abstract
The detection of minimal residual disease (MRD) has become part of the state-of-the-art diagnostics to guide treatment both in pediatric and adult acute lymphoblastic leukemia (ALL). This applies to the treatment of de novo and recurrent ALL. In high-risk ALL, MRD detection is considered an important tool to adjust therapy before and after hematopoietic stem cell transplantation. Precise quantification and quality control is instrumental to avoid false treatment assignment. A new methodological approach to analyzing MRD has become available and is based on next-generation sequencing. In principle, this technique will be able to detect a large number of leukemic subclones at a much higher speed than before. Carefully designed prospective studies need to demonstrate concordance or even superiority compared with those techniques in use right now: detection of aberrant expression of leukemia-specific antigens by flow cytometry of blood or bone marrow, or detection of specific rearrangements of the T-cell receptor or immunoglobulin genes by real-time quantitative polymerase chain reaction using DNA of leukemic cells. In some cases with known fusion genes, such as BCR/ABL, reverse transcriptase-polymerase chain reaction has been used as additional method to identify leukemic cells by analyzing RNA in patient samples. MRD detection may be used to modulate treatment intensity once it has been demonstrated at well-defined informative checkpoints that certain levels of MRD can reliably predict the risk of relapse. In addition, MRD is used as end point to determine the activity of a given agent or treatment protocol. If activity translates into antileukemic efficacy, MRD may be considered a surrogate clinical end point.
Learning Objectives
To develop MRD detection as a clinical diagnostic tool
To determine the properties of different methods for MRD detection
To understand the issue of assay sensitivity in the context of treatment reduction
To determine clinically relevant time points in treatment for informative MRD detection
To understand that the prognostic impact of MRD positivity may vary between different ALL subsets
To recognize that MRD is probably an important tool to fine-tune allogeneic hematopoietic stem cell transplantation
To determine whether MRD detection may change the classical definition of relapse
To understand the role of MRD as an endpoint in clinical trials
Introduction
In vivo sensitivity of acute lymphoblastic leukemia (ALL), as measured by the early blast cell reduction in peripheral blood or BM after exposure to one or several antileukemic agents, is used to risk-stratify patients with ALL because response is of high prognostic relevance.1,2 Lack of adequate response, in particular at the end of remission induction, indicates poor prognosis, but this may vary significantly according to individual patient characteristics.3 Despite the clear separation of risk groups by BM cytology, 2/3 of the relapses occur in patients with M1 or M2 BM on day 15 of induction.4 Therefore, advanced and highly sensitive methods for response assessment were needed and developed to detect minimal residual disease (MRD). The choice of technique for MRD detection mainly depends on the aims of the clinical trial and on the availability of resources.5-9 When MRD analysis is used to identify high-risk (HR) patients, it may be sufficient to use a faster but less sensitive method.10 If the aim is to identify “super responders,” then it is necessary to use the method with the highest sensitivity because the lack of signal in the corresponding MRD investigation must be absolutely reliable; this is particularly true if such patients shall be spared additional therapy or even be assigned to reduced therapy. The second most important prerequisite is the prospective analysis with the MRD method of choice to determine the prognostic significance of certain MRD levels on the background of a predefined uniform chemotherapy regimen.8,9,11-18 It is now widely acknowledged that MRD detection is part of state-of-the-art diagnostics and is needed in the management of ALL. More importantly, MRD detection may even replace other prognostic factors.2,19-23 This short review first describes recent technological developments for MRD detection. The main focus then is on the clinical application of MRD detection in the treatment of ALL, with a special view on ALL subgroups, and on the relevance of MRD before and after hematopoetic stem cell transplantation (SCT). Finally, a few examples in which MRD was used for assessment of activity and efficacy of novel treatment modalities are briefly reviewed.
Techniques of MRD assessment
The choice of the appropriate MRD detection technique depends on the clinical purpose. It has been widely discussed that both flow cytometry (FCM)- and real-time quantitative polymerase chain reaction (RQ-PCR)-based techniques have specific requirements, advantages, and disadvantages.5,6,24 The concordance of the results generated by these 2 techniques is highly dependent on the respective time points of MRD assessment, as shown in a large series of pediatric ALL patients treated with identical induction and induction-consolidation regimens.25 Interestingly, the concordance rate appeared to be lower at the end of induction, but much better in the early phase of induction (day 15) and later at the end of induction-consolidation (week 12). Gaipa et al demonstrated that a FCM signal of the leukemic clone at a level of <0.01% at the end of induction (day 33) had a prognostic impact that depends on the PCR result of the same time point. If the PCR-based MRD detection also confers a (concordant) signal of <0.01%, then the prognosis is excellent [event-free survival (EFS) of 91.6%]. However, if the MRD PCR result of this same time point in this subgroup is ≥0.1%, then the EFS is only 77.1%. If the 0.01% threshold is used for end-of-induction MRD, the concordance is 70%. The concordance rate varies between reported series, but this may also be explained by the proportion of MRD-negative samples in the respective series: it is higher if the proportion of MRD (double-)-negative patients is higher.25
In ALL, if treatment aims at treatment reduction, it is important to choose a highly sensitive technique to avoid insufficient treatment of a patient who may have had a weak MRD signal that went undetected due to the lack of sensitivity of the assay. Treatment reduction has been the aim of 2 large pediatric trials using RQ-PCR-based MRD detection. Patients were considered low-risk if MRD was negative after induction and after consolidation.19,20,26 These study groups followed the strict guidelines and quality control panel meetings of the European MRD network.6,27 Considering this level of sensitivity, MRD positivity at any level, in particular after previous negativity, appears to be associated with increased risk of relapse, even in standard-risk adult ALL.16,17
Although reverse transcriptase (RT)-PCR may provide highly sensitive MRD detection in ALL with specific fusion genes (such as BCR/ABL or MLL rearrangements), the main limitation is the lack of such targets in the majority of ALL cases. It is highly relevant, however, for quantitative monitoring of BCR/ABL-positive ALL being treated with novel tyrosine kinase inhibitors (TKIs).5 Both PCR and FCM require minimum cell counts at diagnosis and during follow-up. This needs to be strictly observed because there is a risk of misinterpretation of quantitative readouts when cell counts are low.6,7 For reproducible quantification, clear guidelines are needed, in particular if several reference laboratories perform the initial and follow-up diagnostics in the same clinical trial.28
The recent advances in high-throughput technologies in molecular genetics may now provide a novel approach to detect MRD. The technique of choice appears to be next-generation sequencing (NGS).29,30 NGS may overcome some limitations observed when using allele-specific oligonucleotide RQ-PCR assays in ALL: the limits of sensitivity, the presence of oligoclonality at the time of diagnosis, and difficulties in identifying markers in some leukemia subtypes such as hyperdiploid leukemias. Ladetto et al compared NGS and RQ-PCR in 3 different types of B-cell malignancies focusing on clonal IgH rearrangements. In ALL, 20 of 26 follow-up samples (77%) from 15 patients showed concordance of both methods. Five qualitative and one quantitative discordance were found in the remaining follow-up samples (23%).30 The major discordance was found in a relapsed sample, which demonstrated clonal evolution by RQ-PCR; using NGS, this subclone could already be detected in the diagnostic sample. These results are very encouraging but, at this point, the question remains as to which technology confers the highest sensitivity and specificity in the follow-up of ALL. Obviously, the sensitivity of both methods may depend on the cell number used in the assay. The impact of this and other factors is being investigated by the European Study Group on MRD (ESG-MRD) at this time. In the United States, Faham et al looked at both IgH and TCR rearrangements and demonstrated an excellent sensitivity of the NGS-based assay compared with FCM- and RQ-PCR-based MRD assays, respectively. These investigators also pointed out that the turnaround time compared with development of patient-specific targets and RQ-PCR may be reduced significantly, potentially resulting in lower cost.29
MRD levels at predefined time points may predict success of therapy
In many pediatric ALL protocols, days 8 and 15 of induction therapy are considered the first checkpoints to test the in vivo sensitivity of the leukemia in the individual patient.1,4,31-33 The general message for the individual patient is simple: the fast reduction or even elimination of the leukemia (or its predominant clone) is highly predictive of superior relapse-free survival.8,34 Borowitz et al clearly demonstrated that distinct levels of MRD (by FCM) in the peripheral blood at day 8 of induction therapy in a large set of precursor B-cell (pcB) ALL patients (n = 2143) were associated with the probability of EFS (pEFS). Patients with 0.01% or less residual disease (called “MRD-negative”) had a 5-year pEFS of 90% ± 2%, whereas the worst group with MRD at >10% had a pEFS of only 54% ± 7%.8 Another large study performed by Basso et al compared BM day 15 MRD results generated by FCM with cytomorphological response assessment and PCR analysis of MRD at the end of induction (day 33) and at the end of consolidation (day 78). Levels of MRD in the BM at day 15 were well correlated with risk of relapse. Less than 0.1% of MRD conferred a cumulative incidence of relapse of 8% ± 1.7% in precursor B-cell ALL and only 3.3% ± 3.3% in T-cell ALL. These fast responders comprised 43% of pcB-ALL and 34% of T-ALL patients, respectively. In both major subgroups of ALL, a distinct poor-risk group was identified by high levels of MRD (≥10%) at day 15 comprising 10% of pcB-ALL and 21% of T-ALL, respectively: the 5-year cumulative incidence of relapse was 45.5% ± 6.8% in pcB-ALL and 55.6% ± 11.7% in T-ALL.9
In adult patients with ALL, early response as assessed by MRD delivered a slightly different picture even though the good prognosis associated with fast clearance of MRD remained to be the same. MRD negativity measured at day 24 of treatment was associated with only 68.6% disease-free survival.16 This finding appears to indicate that even earlier time points may be more advantageous to identify patients with the fastest clearance of disease. Investigators from the French Group for Research in Adult ALL (GRAALL) group demonstrated that MRD clearance in combination with early steroid response may define a large group of adult ALL patients with a low relapse risk.23
Ribera et al recently reported on the prognostic value of early response assessment by cytology and FCM-based MRD in Philadelphia-chromosome-negative (Ph−) adult ALL.35 They suggested that patients with this type of ALL who respond favorably early on (MRD <0.1% at the end of induction and MRD <0.05% at the end of consolidation) may be spared allogeneic SCT in first complete remission.
Therefore, results both from pediatric and adult ALL trials confirm the favorable prognostic impact of fast and early MRD clearance.
Prognostic information based on MRD from 2 consecutive time points
In the largest study for de novo ALL published so far, the BFM (Germany, Austria, Switzerland) and AIEOP (Italy) study groups used MRD (measured by RQ-PCR) for risk stratification in a total of 3184 pcB-ALL and 464 T-ALL patients. All patients were treated by identical chemotherapy in the first 9 weeks of therapy. Large differences in pEFS between MRD-defined subgroups were found.19,20 MRD was analyzed in the BM at the end of induction (day 33) and at the end of induction consolidation (day 78). Importantly, within pcB-ALL, the prognostic impact of MRD was maintained even in the 2 large subgroups of TEL/AML1+ and hyperdiploid ALL.19
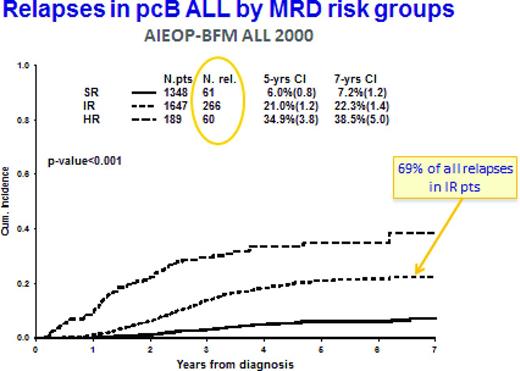
The AIEOP-BFM ALL 2000 trial confirmed the strong prognostic impact of MRD at the end of induction when combined with a subsequent time point. This trial also demonstrated that, in pcB-ALL, the MRD-intermediate risk group comprises the largest group of patients (51.7%), but also the majority of relapses (Figure 1): 69% of all relapses were found in this group, which showed an overall relapse rate of 21% at 5 years.19 The situation in T-cell ALL was similar: 62.9% of all patients were defined as intermediate risk, comprising 55% of all relapses (the cumulative incidence of relapse was 17.6% at 5 years).20 This observation illustrates the need for more refined approaches to identify the patients at high risk to relapse. Recent work of several study groups has identified intriguing properties in subsets of pcB-ALL, which appear to show prognostic impact independent of MRD. This may be clinically quite relevant because the distribution of relapses requires further refinement in risk assessment and treatment adaptation.36-43
Cumulative incidence (CI) of relapses in MRD-based risk groups in precursor-B-ALL treated in AIEOP-BFM ALL 200019 MRD-standard risk (SR) means no MRD detectable at days 33 and 78 from diagnosis (sensitivity for 2 targets must be at least 10-4); MRD-HR means that the MRD level at day 78 is ≥ 10-3; and MRD-intermediate risk (IR) is all other constellations of MRD.
Cumulative incidence (CI) of relapses in MRD-based risk groups in precursor-B-ALL treated in AIEOP-BFM ALL 200019 MRD-standard risk (SR) means no MRD detectable at days 33 and 78 from diagnosis (sensitivity for 2 targets must be at least 10-4); MRD-HR means that the MRD level at day 78 is ≥ 10-3; and MRD-intermediate risk (IR) is all other constellations of MRD.
MRD in special subgroups
Two major differences between pcB-ALL and T-ALL can be found: (1) MRD at the end of induction (day 33) is more informative in pcB-ALL, whereas MRD at the end of consolidation (day 78) is more informative in T-ALL, and (2) MRD levels in pcB-ALL correlate with risk of systemic relapse, whereas MRD in T-ALL is predictive of both systemic and extramedullary relapse. The results obtained here, in combination with the results obtained by FCM in the same trial, has changed the risk group definition for HR patients as used by AIEOP-BFM. Any patient who has >10% leukemic blasts by FCM on day 15 is enrolled into the HR group; any patient with pcB-ALL who has MRD ≥10-3 (0.1%) at day 33 and is still MRD+ at day 78 is stratified into the HR group; any pcB-ALL patient with MRD <0.1% at day 15 is considered standard risk if PCR results (with at least one highly sensitive molecular target, quantitative range up to 10-4) at day 33 and day 78 are also negative.9,25
Postremission MRD surveillance: should MRD before and after hematopoietic SCT be monitored?
Additional postremission MRD assessment was performed in several clinical trials. In AIEOP-BFM ALL 2000, all patients with MRD at a level of ≥10-3 at day 78 were stratified into the HR group and then monitored after each reconsolidation element. This strategy is currently used to adjust further chemotherapy and to prepare for allogeneic SCT. Although postinduction MRD was also found to have a significant prognostic impact in relapsed ALL,14 MRD monitoring of pre-SCT response demonstrated the necessity of optimizing the quality of remission before transplant to prevent post-SCT relapse.44-46 In addition, the use of allogeneic hematopoietic SCT may also abrogate in part the negative impact of pretransplantation MRD.47 Another recent investigation of MRD in adult patients with HR-ALL demonstrated that high MRD at day 71 after induction was associated with only 32% ± 6% disease-free survival compared with 66% ± 8% in HR patients with molecular complete remission. Allogeneic transplantation appeared to be beneficial in patients with molecular failure, because those patients who were not transplanted had a disease-free survival of only 6% ± 5%. In standard-risk patients, similar but less pronounced observations were made. If MRD at week 16 was analyzed, this difference between transplanted and nontransplanted patients could be shown as well.22 Therefore, allogeneic transplantation should be offered rather soon because relapse will occur early in such patients. The prognostic impact of MRD positivity before and after SCT was also confirmed in another recent retrospective study of adult ALL patients treated in the United States. It showed the adverse prognostic of MRD positivity before SCT, but a particularly bad prognosis was associated with MRD reappearance after SCT.48
Ph+ ALL
The long-term follow-up of Ph+ patients treated in the Children's Oncology Group (COG) with imatinib in addition to intensive chemotherapy provides interesting insights into the efficacy of a tyrosine-kinase inhibitor when combined with chemotherapy.49,50 In those patients treated most intensively with imatinib, the prognostic value of MRD was nearly abrogated. At the same time, the investigators were able to demonstrate that additional genetic abnormalities had a significant impact on EFS.49 In adult Ph+ ALL, the picture was similar, demonstrating a limited prognostic impact of persisting MRD when patients were treated with a TKI in addition to chemotherapy.51 Very intriguingly, Foa et al demonstrated that a TKI (dasatinib) combined only with steroids and intrathecal chemotherapy can induce complete remission in patients with Ph+ ALL, even though a significant number relapsed later on.52 This indicates a potentially significant progress, because such approaches may contribute to future treatment regimens that carry less toxicity due to the replacement of (some) chemotherapy by more targeted agents.
MRD in relapse and clonal evolution
MRD monitoring in relapsed patients carries some potential pitfalls that are mainly due to clonal evolution.53 Detailed analysis of all molecular markers at first diagnosis and at time of relapse may reveal a different origin of the predominant clone.54 This implies that regular monitoring of MRD by those markers defined at first diagnosis may fail if clonal evolution occurs, also when occurring after the first relapse.55 Ongoing research will determine whether NGS is the appropriate tool to prevent such diagnostic “failures.”29,30
In adult patients, a striking difference was found between de novo and relapsed Ph+ ALL with regard to the levels of bcr-abl kinase domain mutations. This may not only explain the lack of treatment efficacy when treating with TKI, but also may also affect the utility of RT-PCR monitoring of relapsed Ph+ ALL patients.56
Definitions of remission and relapse revisited?
Although there is large consensus about the definition of complete cytological remission in ALL,3 it has become more difficult to achieve common definitions for certain MRD terms. This is urgently required for comparison of results in clinical trials but, more importantly, to provide safe guidelines for patient management. Therefore, the consensus proposal summarized by Brüggemann et al seems to be a useful basis to provide the terms for remission assessment and postremission monitoring (Table 1).
Proposals for definition of MRD terms in ALL5

*Treatment modifications depending on these measurements are strongly recommended to be based on at least 2 analyzed samples.
†Time points should be specified within the respective protocol. At least one relevant treatment element should be administered in between.
‡MRD reappearance can be equated to “MRD relapse” (which would then be considered “event”) if a particular ALL protocol has provided unequivocal evidence that MRD reappearance is closely associated with hematological relapse.
MRD as an end point in clinical trials: activity versus efficacy
After MRD analysis became a common diagnostic tool in most multi-agent clinical trials for ALL, the question arose of whether MRD response can also be used as a primary end point in clinical trials. It needs to be taken into account, however, that MRD response is only a more sensitive tool to assess response compared with the cytological response. It may completely depend on the scenario if activity translates into improved efficacy of a given intervention, thus improving the relevant clinical end points such as EFS and overall survival. This said, it is noteworthy that recent novel immunotherapeutic strategies, for example, with an anti-CD3, anti-CD19 bispecific monoclonal antibody showing strong antileukemic activity (as measured by MRD response) may contribute to long-term remission because such agents may improve the quality of remission needed to perform subsequent allogeneic SCT successfully.22,57,58 In contrast, a British study comparing reinduction therapy with mitoxantrone versus idarubicin in relapsed pediatric ALL demonstrated that MRD response (activity) was not predictive of treatment efficacy.59 This observation indicates that MRD response may be misleading in drug evaluation as long as activity is considered to be equal to efficacy.
Summary
In summary, MRD has evolved as one of the most powerful diagnostic tools in the clinical management of ALL. At this time, it cannot yet be replaced, but may be supplemented by upfront diagnostic markers. This should allow us to predict response and relapse with even higher specificity. New techniques such as next-generation sequencing may overcome some of the shortcomings of current MRD technologies if validation is successful. Clinical intervention based on MRD results must rely on robust prospective data indicating the precise prognostic impact of a given MRD level at predefined time points. Under well-defined preconditions, MRD detection can be a very useful guide for modulating therapy for ALL.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Martin Schrappe, Department of Pediatrics at the Christian-Albrechts-University of Kiel, University Medical Center Schleswig-Holstein, Campus Kiel, Arnold-Heller-Str. 3, Building 9, 24105 Kiel, Germany; Phone: 49-431-597-1621; Fax: 49-431-597-3966; e-mail: schrappe-office@pediatrics.uni-kiel.de.