Abstract
The availability of new oral anticoagulants (NOACs) targeting either thrombin (dabigatran etexilate) or factor Xa (rivaroxaban and apixaban) for the prevention and treatment of thrombosis has been highly anticipated. NOACs have major pharmacologic advantages over vitamin K antagonists (eg, warfarin), including rapid onset/offset of action, few drug interactions, and predictable pharmacokinetics, eliminating the requirement for regular coagulation monitoring. Regulatory agencies have approved several NOACs for specific indications based on the results of clinical trials demonstrating efficacy and safety that are at least as good, if not better, than warfarin (for stroke prevention in atrial fibrillation and treatment and secondary prevention of venous thromboembolism) or low-molecular-weight heparin, which is injectable (for initial treatment of venous thromboembolism and thromboprophylaxis in patients undergoing hip or knee arthroplasty). However, the adoption of this new therapeutic class into clinical practice has been slower than expected due to several factors including concerns regarding medication adherence without laboratory monitoring, uncertainty about dosing in some patient populations (eg, renal dysfunction, marked extremes of body weight), and higher drug costs compared with warfarin. Other issues are the current absence of specific antidotes for NOACs and assays to measure drug levels at most centers. The indications for NOACs on the market will expand and at least one additional agent (edoxaban) will likely gain approval within the next 2 years. As practitioners gain familiarity with the drugs and healthcare systems adapt to their use, NOAC use will increase substantially over time. Warfarin, however, will continue to be an appropriate anticoagulant choice for many patients.
Introduction
For more than 50 years, vitamin K antagonists (eg, warfarin) were the only available oral anticoagulants. It has now been 3 years since the first of the new oral anticoagulants (NOACs), dabigatran etexilate, gained approval for stroke prevention in atrial fibrillation in the United States. This was followed by the approval of rivaroxaban and apixaban for this indication. Rivaroxaban has been approved for thromboprophylaxis after total hip or knee replacement and the initial treatment and secondary prevention of venous thromboembolism (VTE). In other countries, dabigatran etexilate and apixaban were approved for the prevention of VTE after hip and knee replacement. Edoxaban and betrixaban are other oral factor Xa inhibitors in development. Phase 3 trials comparing edoxaban to warfarin for the prevention of stroke and systemic embolism in patients with atrial fibrillation and the treatment of VTE have been completed. Betrixaban is being evaluated in a phase 3 trial versus enoxaparin for the prevention of VTE in medically ill patients undergoing hospitalization.
Unlike warfarin, which has a narrow therapeutic window and requires individualized dosing based on the international normalized ratio (INR), the NOACs have a wide therapeutic window, thereby facilitating fixed dosing in adults without the need for laboratory monitoring or dose adjustments for body weight. NOACs are renally cleared and lower doses of rivaroxaban and apixaban were used in patients with atrial fibrillation and impaired kidney function (creatinine clearances between 25 and 49 mL/min). In general, the results for the approved indications have been robust, with NOACs being either noninferior or superior to standard treatment regimens. Despite the advantages and potential simplification of anticoagulation afforded by NOACs, their uptake for the approved indications after launch has been less than anticipated in some areas of the United States and other parts of the world.1
The results of the phase 3 trials of NOACs have been published in peer-reviewed journals and have been the topic of numerous review articles,2,3 including recent ASH education sessions.4,5 The issue of whether NOACs or warfarin should be used as frontline agents for stroke prevention in atrial fibrillation has been, and continues to be, vigorously debated6,7 ; therefore, the clinical trials will not be reviewed here in detail. This chapter primarily focuses on the pros and cons of the NOACs for the various indications that have gained approval in the United States by the middle of 2013, particularly focusing on real-world concerns related to their effectiveness, safety, and use.
Pharmacologic properties of warfarin and NOACs
The pharmacologic properties of warfarin, dabigatran etexilate, rivaroxaban, and apixaban are summarized in Table 1.2,4
Properties of warfarin and oral inhibitors of thrombin and factor Xa inhibitors approved for use in the United States
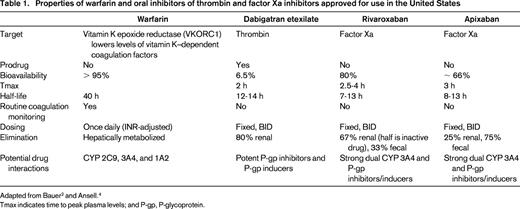
Tmax indicates time to peak plasma levels; and P-gp, P-glycoprotein.
Warfarin is associated with > 10-fold interindividual variations in dose to achieve therapeutic anticoagulation. Its pharmacokinetics and pharmacodynamics are influenced by genetic polymorphisms (CYP 2C9 and VKORC1), dietary vitamin K intake, concomitant medications, alcohol use, patient age, body weight, and various disease states, necessitating regular coagulation monitoring to ensure that the patient's INR remains within the target range.
Dabigatran etexilate is an oral prodrug that is converted to dabigatran, a competitive direct thrombin inhibitor (Ki 4.5 nmol/L), by hydrolytic cleavage mediated by plasma esterases in vivo.8 It is not metabolized by cytochrome P450 (CYP) enzymes or oxidoreductases. Dabigatran etexilate has relatively low oral bioavailability and is encapsulated with tartaric acid to facilitate absorption in the gastrointestinal tract; however, consistent absorption of dabigatran etexilate is not dependent on overall gastrointestinal acidity and dose modifications are not required with the use of proton pump inhibitors. The capsules, however, must be ingested intact (ie, they cannot be crushed, broken before administration, or chewed) and the medication cannot be administered to patients receiving nutrition and oral medications via nasogastric, gastrostomy, or jejunostomy tubes. The capsules are hygroscopic and expire 4 months after the seal of the bottle is broken upon opening. Dabigatran etexilate has relatively few drug interactions, but p-glycoprotein transporter inhibitors such as amiodarone, verapamil, or quinidine increase drug exposure. The use of the drug with rifampin, a p-glycoprotein inducer, should be avoided because it reduces the drug's anticoagulant effect. In patients with moderate renal impairment (creatinine clearance 30-50 mL/min) taking potent p-glycoprotein inhibitors (eg, ketoconazole or dronedarone), it is recommended that consideration be given to reducing the dose of dabigatran etexilate to 75 mg twice daily (BID).
Rivaroxaban (Ki 0.4 nmol/L) and apixaban (Ki 0.08 nmol/L) are not prodrugs and bind competitively to the active site of factor Xa. Although both agents have high oral bioavailability, it is necessary to take higher doses of rivaroxaban (15 or 20 mg tablets) with food to ensure optimal drug absorption. Rivaroxaban is absorbed best in the stomach, whereas apixaban is absorbed throughout the gastrointestinal tract. Rivaroxaban may be crushed and mixed and administered with food through a gastrostomy tube. Rivaroxaban has a dual mode of elimination; one-third is excreted unchanged by the kidney and 67% is converted by the liver (CYP 3A4) to inactive metabolites.9 Rivaroxaban should not be prescribed to patients taking strong inhibitors (ketoconazole/itraconazole, HIV protease inhibitors)/inducers (carbamazepine, phenytoin, rifampin, St John's wort) of both CYP 3A4 and p-glycoprotein or those with moderate or severe hepatic disease (Child-Pugh B and C). Apixaban is also metabolized by the liver (partially by CYP 3A4); 75% is eliminated in the feces and 25% by the kidneys.10 Strong inhibitors of both CYP 3A4 and p-glycoprotein can raise plasma levels of apixaban and it is recommended that the dose be reduced to 2.5 mg BID; it is also recommended that the drug not be prescribed to patients on strong dual inducers of CYP 3A4 and p-glycoprotein. There is no dose adjustment of apixaban for mild hepatic impairment; it should not be prescribed to patients with moderate or severe liver disease.
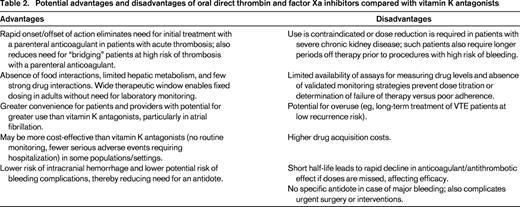
Although relatively few drugs have strong interactions with NOACs, care must be taken to ensure that patients are not taking one of these medications; this is best done by computerized pharmacy programs, but requires that all of their other medications be inputted. If there is a strong drug interaction, it is best not to prescribe the NOAC rather than altering doses as suggested by the label. As yet, there are neither assays available to measure drug levels on a routine basis nor evidence-based algorithms for adjusting doses. Although warfarin has innumerable drug interactions, the dose can be adjusted by monitoring the INR more frequently to account for any concomitant interactions. Data are also not available regarding NOAC absorption in patients who have undergone gastric-bypass or lap band surgery for obesity or resection of large portions of the small bowel; NOACs should therefore not be prescribed to such patients until data or algorithms for adjusting dose become available. Some of the potential advantages and disadvantages of oral direct thrombin or factor Xa inhibitors compared with warfarin are listed in Table 2.
Efficacy and safety of the NOACs in atrial fibrillation
The key findings of the randomized trials comparing dabigatran (RE-LY),11 rivaroxaban (ROCKET-AF),12 and apixaban (ARISTOTLE)13 with warfarin for stroke prevention in atrial fibrillation were as follows.
NOACs are noninferior to warfarin for the prevention of stroke and systemic embolism (see Table 3 for approved doses and schedules). Dabigatran at the Food and Drug Administration (FDA)–approved dose of 150 mg BID significantly reduced the risk of ischemic stroke. All reduced mortality by ∼ 10% per year compared with warfarin.
Recommended doses of the dabigatran etexilate, rivaroxaban, and apixaban for approved indications in the United States (2013)

QD indicates daily.
*Pradaxa (dabigatran etexilate mesylate) [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc.
†Xarelto (rivaroxaban) [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals Inc.
‡Eliquis (apixaban) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Co.
The rates of major bleeding for dabigatran at the 150 mg BID dose and rivaroxaban were similar to warfarin. Apixaban demonstrated a significantly lower rate of major bleeding (hazard ratio = 0.69; 95% confidence interval, 0.60-0.80; P < .001) than warfarin. All 3 agents had rates of intracranial bleeding that were about half that of warfarin; this difference likely arises from the target-specific mechanism of action of the NOACs. However, both dabigatran and rivaroxaban caused more extracranial bleeding, particularly gastrointestinal bleeding. For dabigatran, this appears to be an age-related phenomenon and is most relevant to those older than 85 years.14
All of the NOACs approved for atrial fibrillation now carry labels warning that discontinuing the medication without providing an alternative immediately acting anticoagulant can increase the risk of stroke. Stroke can result from the rapid dissipation of the antithrombotic effect of NOACs if patients do not take their medication as prescribed (either 12 or 24 hours after the previous dose in accordance with the label). In the phase 3 trials of rivaroxaban and apixaban, there was a clustering of thrombotic events at the end of the double-blind, double-dummy ROCKET-AF and ARISTOTLE trials, which was likely due to transitioning of NOAC patients back to warfarin without “bridging” with low-molecular-weight heparin (LMWH) or unfractionated heparin.15 In transitioning patients from a NOAC to warfarin, this problem can be overcome by giving the NOAC concomitantly with warfarin until the INR at “trough” NOAC level (just before the next scheduled dose) is 2 or higher.
Although warfarin prevents more than 60% of strokes in compliant patients with atrial fibrillation and has been recommended for those with this rhythm abnormality and one additional risk factor,16 only ∼ 50% of patients with this rhythm disorder take the drug. There are many reasons for this, including perceptions of both physicians and patients regarding the relative benefits and risks of taking a vitamin K antagonist on a long-term basis. Furthermore, only ∼ 50% of patients prescribed warfarin are well managed with respect to their time in therapeutic range (ie, TTR > 60%-65%). Poor control of warfarin places patients at increased risk for adverse events (ie, bleeding) and reduces its benefit in preventing thrombotic events.17,18
It is conceivable that patients deemed unsuitable for warfarin would do better on a NOAC providing they are adherent in taking their medications. The AVERROES trial compared apixaban with aspirin for stroke prevention in atrial fibrillation in such a study population.19 This trial was stopped early due to the clear efficacy of apixaban in stroke prevention and its associated risk of major bleeding was surprisingly no different from aspirin. The results of the AVERROES study suggest that aspirin should not have a role in the prevention of stroke in atrial fibrillation. Nevertheless, the efficacy and safety of NOACs in patients with atrial fibrillation for whom warfarin is not prescribed remains uncertain. In the United States, atrial fibrillation treatment rates did not increase in the first year that dabigatran was available.20 Observational data on the safety of NOACs in this population of atrial fibrillation patients is required for them to fulfill their promise of expanding the proportion that receive effective prophylaxis for stroke prevention.
Patients in clinical trials of NOACs are generally highly motivated to comply with the requirement for regular visits and follow-up calls and are provided education regarding their disease state and the importance of medication adherence (monitored with pill counts). This will not be the case in practice for some patients who are prescribed NOACs given the absence of a requirement for regular coagulation monitoring.21 This is especially critical in atrial fibrillation patients, most of whom have not experienced a thrombotic event. Warfarin's requirement for INR monitoring effectively allows adherence to be monitored.
Rivaroxaban in the prevention and treatment of VTE
Rivaroxaban starting 6 to 8 hours postoperatively at a dose of 10 mg daily is approved in the United States for VTE prevention after major orthopedic surgery based on the RECORD trials; 2 were in patients undergoing total hip replacement, with 1 trial having different durations of therapy,22,23 and 2 were in total knee replacement using 2 different enoxaparin dosing regimens, 40 mg 12 hours preoperatively and then daily postoperatively or 30 mg BID starting postoperatively.24,25 Rivaroxaban was superior to enoxaparin in preventing VTE in all 4 trials, with similar risks of major bleeding. A pooled analysis of the 4 trials, however, indicated a very small increase in bleeding,26 an issue of particular concern to some orthopedic surgeons who will also be reticent in initiating immediate-acting anticoagulants until 12 to 24 hours postoperatively.27 Rivaroxaban nonetheless is a convenient and highly effective agent for the prevention of venous thrombosis after total hip or knee replacement (see Table 3 for dose/schedule) and is a good alternative to anticoagulants that require subcutaneous injections (eg, LMWHs, fondaparinux) or regular laboratory monitoring (eg, warfarin).
In the EINSTEIN studies of patients presenting with acute deep vein thrombosis (DVT) or pulmonary embolism, rivaroxaban alone was compared with enoxaparin followed by a vitamin K antagonist for 3, 6, or 12 months; the rivaroxaban dose was 15 mg BID for 3 weeks followed by 20 mg daily.28,29 Rivaroxaban was noninferior with respect to the primary efficacy outcome versus enoxaparin/vitamin K antagonist therapy. The principal safety outcomes of major and clinically relevant nonmajor bleeding were not significantly different between the groups. In the continued-treatment study, rivaroxaban reduced the recurrence rate by 82% compared with patients on placebo, albeit with a small increased risk of major (0.7%) and clinically relevant nonmajor bleeding bleeding (5.4%) over a mean treatment duration of 190 days. In the AMPLIFY trial, a fixed-dose regimen of apixaban alone for 6 months was also shown to be noninferior to standard therapy for the treatment of symptomatic VTE, but was associated with significantly less bleeding.30
A single oral drug approach greatly simplifies the management of symptomatic VTE by eliminating the need for a “bridging” approach (unfractionated heparin/LMWH/fondaparinux followed by warfarin); it may reduce the costs of treating VTE by reducing the need for, or duration of, hospitalization and the need for coagulation monitoring, which is particularly burdensome during initiation of a vitamin K antagonist.
Although the Einstein DVT and pulmonary embolism trials included patients who were representative of those seen in routine practice, questions have arisen as to which patients should be treated with rivaroxaban alone. A post hoc analysis of pooled data from the 2 trials did not find any difference in outcomes among “fragile” patients (age > 75 years or creatinine clearance < 50 mL/min or body weight ≤ 50 kg), those with active cancer, or those with large clot burdens.31 The percentages of patients with body weight ≤ 50 kg and creatinine clearance < 50 mL/min were, however, low at 2% and 8%, respectively. The percentage of cancer patients enrolled in the trials was also relatively small (< 5%) because oncologists generally treat such patients with chronic LMWH, which has superior efficacy compared with a standard 2 anticoagulant approach with LMWH followed by a vitamin K antagonist.32 There is also a potential for interactions between various chemotherapeutic and immunosuppressive drugs and the NOACs based upon their known metabolic pathways.33 Therefore, the use of NOACs is not currently recommended for patients with cancer-associated VTE.34 In addition, women with pregnancy-associated VTE or those who are breastfeeding should not be prescribed NOACs. Standard therapy is also preferred among patients in intensive care units with pulmonary emboli and hemodynamic instability or a very extensive DVT (eg, phlegmasia cerulea dolens) in whom thrombolytic therapy is being considered. Caution should also be exercised in prescribing rivaroxaban at approved doses to patients at increased risk of bleeding complications or those with low body weight (< 50 kg), morbid obesity, or renal dysfunction. Although there is a dose reduction of rivaroxaban for creatinine clearance < 50 mL/min in atrial fibrillation, such a dose modification is not recommended for VTE (Table 3). Caution is advised in switching “difficult” to manage patients without cancer (ie, recurrent VTE while on therapeutic doses of an anticoagulant, either warfarin or LMWH). There are not currently data regarding the efficacy of rivaroxaban or other NOACs in such patients who develop recurrences while on warfarin with a therapeutic INR; these patients may be best managed with standard agents (warfarin or LMWH), which are amenable to laboratory monitoring and dose adjustments.
Finally, there are practical issues related to the way healthcare systems operate that affect NOAC utilization, particularly for the initial treatment of symptomatic DVT entirely in the outpatient setting. These include the need for preauthorization of NOACs by some insurance companies (with variable copayments) and the lack of physician familiarity with NOACs both in emergency departments and among practitioners responsible for follow-up. Over time, these issues will be addressed and it is likely that NOACs will replace heparin and vitamin K antagonists as standard therapy for the majority of patients with an initial venous thrombotic event.
Antidotes and laboratory monitoring
There is no specific antidote available to reverse the anticoagulant effect of dabigatran, but an approach using a monoclonal antibody is in clinical development.35 Approaches for managing serious bleeding and monitoring the drug's anticoagulant activity have been published.36 Hemodialysis is effective in removing ∼ 60% of the dabigatran in the blood over 2 to 3 hours and can be used to treat dabigatran toxicity. Rivaroxaban and apixaban are both highly protein bound and therefore cannot be removed by hemodialysis. There is no specific antidote available to reverse the anticoagulant effect of factor Xa inhibitors, but one is in development using a modified recombinant form of factor Xa.37
Although the new oral anticoagulants have been developed without the need for laboratory monitoring, assays to assess drug levels will be helpful when major bleeding occurs to determine whether it results from high drug levels or another etiology (eg, bleeding from a discrete anatomic site, coagulopathy resulting from liver disease, or disseminated intravascular coagulation) and before some surgical procedures (eg, neurosurgery, cardiac surgery).
For dabigatran, assays using the ecarin clotting time or a dilute thrombin time have been developed and appear to be sufficiently sensitive.38 Although thrombin time determinations are widely available, they are too sensitive for detecting clinically relevant plasma drug concentrations. However, a normal measurement provides assurance that a significant drug concentration is no longer present. Dabigatran prolongs the partial thromboplastin time (PTT), but the effects are not dose dependent; although a prolonged PTT may indicate the presence of dabigatran, it will not provide an exact level of anticoagulant activity. For therapeutic doses of oral factor Xa inhibitors, the prothrombin time (PT) is more sensitive than the PTT; however, the results are dependent on the PT reagent used in the assay and apixaban has less effect on the PT than rivaroxaban. Anti-factor Xa chromogenic assays with appropriate calibrators will provide sensitive and specific assays for measuring drug concentrations of oral direct factor Xa inhibitors.38 NOAC levels will need to be interpreted in relation to timing of drug administration and its pharmacokinetics.
Until specific antidotes for NOACs become available, prothrombin complex concentrates, factor eight inhibitor bypass activity, or recombinant factor VIIa are available for overcoming their anticoagulant effect in the management of severe bleeding episodes.39,40 However, there is little clinical experience in humans with respect to the efficacy or safety of any of these agents in managing life-threatening hemorrhage. If NOACs are prescribed appropriately, the current lack of a specific antidote may not be a great drawback in most bleeding situations because there will be relatively few circumstances in which a reversal agent will be required.
NOAC use in chronic kidney disease
Approximately 80%, 33%, and 25% of dabigatran, rivaroxaban, and apixaban, respectively, are renally eliminated as active drug. Therefore, renal function must be assessed before prescribing a NOAC and this is best done by calculating creatinine clearance using the Cockcroft-Gault formula, which incorporates body weight (as opposed to other formulas that provide estimates for a body surface area of 1.73 m2, the normal mean value for young adults). All of the trials evaluating dabigatran etexilate or rivaroxaban excluded patients with a creatinine clearance < 30 mL/min, whereas the ARISTOTLE trial excluded patients with creatinine clearance < 25 mL/min. In patients with a creatinine clearance of 30 to 49 mL/min, the dose of rivaroxaban was reduced to 15 mg, whereas no adjustments were made in the RE-LY trial for either the 150 or 110 mg doses of dabigatran etexilate. Only the 150 mg dose of dabigatran was approved in the United States,41 whereas both doses were approved in many countries, allowing physicians some latitude with respect to dose selection. Based on pharmacokinetic considerations, a reduced dose of 75 mg BID was approved by the FDA for patients with atrial fibrillation and a creatinine clearance of 15 to 29 mL/min in the absence of efficacy and safety data. The FDA also approved rivaroxaban at a dose of 15 mg daily for atrial fibrillation patients with a creatinine clearance of 15 to 29 mL/min. In ARISTOTLE, the dose of apixaban was reduced by 50% in patients with any 2 of the following: advanced age, low body weight, or impaired renal function. The FDA also extended the label for the apixaban dose of 2.5 mg BID for patients with a creatinine clearance of 15 to 24 mL/min. Many clinicians with expertise in anticoagulant therapy were surprised by these decisions by the FDA, particularly given the current absence of a specific antidote or effective approaches to reverse the anticoagulant effects of the NOACs. Therefore, many believe that NOACs should not be prescribed at any dose in patients with a creatinine clearance < 25 to 30 mL/min.
Adherence, anticoagulation clinics, and medication costs
Perhaps the most significant concern with the NOACs is medication adherence. Issues specific to vitamin K antagonists have led to the development of anticoagulation clinics (so-called Coumadin clinics) in many parts of the world, which play a very important role in optimizing the safety and efficacy of vitamin K antagonists. Warfarin's long half-life of ∼ 40 hours is an advantage for patients who occasionally miss doses of medication compared with one of the NOACs. Furthermore, the twice-daily dosing schedules of some NOACs may be more difficult for some patients to adhere to than a daily regimen; conversely, the impact of a missed dose may be greater with NOACS that are taken daily. Ongoing medication adherence will be extremely important to attain good clinical outcomes with NOACs, especially in atrial fibrillation patients who have not experienced symptoms of cerebral ischemia and generally require indefinite anticoagulation. The lack of a requirement for regular coagulation monitoring may eliminate the initial and continuing education currently provided to patients on vitamin K antagonists along with the early detection of medication nonadherence. Healthcare systems are now grappling with how to handle the adherence issue with the NOACs. One approach is to have patients on NOACs be followed by anticoagulation or thrombosis management clinics. Reports of thrombotic and hemorrhagic complications after the introduction of dabigatran etexilate in New Zealand42 and Denmark43 sound a cautionary note regarding the many nuances related to safely prescribing NOACs, particularly in frail elderly patients with renal dysfunction and patients transitioning from warfarin. This will be an added burden for clinics that are oversubscribed and already unable to cover the costs of managing patients on warfarin. However, with better care coordination and bundled payment systems, there should be an impetus for incorporating patients on NOACs into these clinics to optimize outcomes (including reduced need for hospitalizations and emergency department visits). Pharmacists/nurses/physicians with special expertise in anticoagulation management can provide the oversight to ensure that NOACs are prescribed appropriately based upon an individual patient's history and clinical characteristics. In a recent editorial, Cushman recommended that institutions adopt protocols for NOAC use in acute VTE that address 6 key components: patient preference, patient selection, drug interactions, compliance, follow-up, and monitoring.44
In the United States, the much higher drug costs of NOACs must be taken into consideration. Even among patients with insurance that covers drugs, prescribers should ascertain how much patients pay out of pocket (copayment) compared with warfarin. High drug costs can contribute to reduced medication adherence: patients are known to not fill prescriptions they regard as too expensive or skip/split doses to make the medication last longer.45 Although warfarin is an inexpensive generic medication, regular INR monitoring and caring for patients with treatment-related hemorrhagic complications is a substantial cost for healthcare systems.
The vitamin K antagonists will remain an important anticoagulant option. They remain the medication of choice for patients with mechanical heart valves.46 It should also be noted that vitamin K antagonists have been widely prescribed for more than 50 years and have minimal, if any, long-term effects on organ systems and physiologic processes other than blood coagulation. There is no such experience with NOACs.
Disclosures
Conflict-of-interest disclosure: The author has consulted for Instrumentation Laboratory, Boehringer Ingelheim, Pfizer, Bristol-Meyers Squibb, Janssen Pharmaceuticals, Bayer Healthcare, and CSL Behring. Off-label drug use: Dabigatran and apixaban for the prevention and treatment of VTE.
Correspondence
Kenneth A. Bauer, MD, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215; Phone: 617-667-2174; Fax: 617-738-1450; e-mail: kbauer@bidmc.harvard.edu.