Abstract
Venous thromboembolism (VTE) is a common condition that can lead to complications such as postphlebitic syndrome, chronic pulmonary artery hypertension, and death. The approach to the diagnosis of has evolved over the years and an algorithm strategy combining pretest probability, D-dimer testing, and diagnostic imaging now allows for safe, convenient, and cost-effective investigation of patients. Patients with low pretest probability and a negative D-dimer can have VTE excluded without the need for imaging. The mainstay of treatment of VTE is anticoagulation, whereas interventions such as thrombolysis and inferior vena cava filters are reserved for special situations. Low-molecular-weight heparin has allowed for outpatient management of most patients with deep vein thrombosis at a considerable cost savings to the health care system. Patients with malignancy-associated VTE benefit from decreased recurrent rates if treated with long-term low-molecular-weight heparin. The development of new oral anticoagulants further simplifies treatment. The duration of anticoagulation is primarily influenced by underlying cause of the VTE (whether provoked or not) and consideration of the risk for major hemorrhage. Testing for genetic and acquired thrombophilia may provide insight as to the cause of a first idiopathic deep vein thrombosis, but the evidence linking most thrombophilias to an increased risk of recurrent thrombosis is limited.
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third most common cardiovascular disorder, having an estimated annual incidence of 0.1% and affecting 2% to 5% of the population during their lifetimes. Approximately 20% of patients with PE will die before diagnosis or on the first day. For those surviving more than 1 day, up to 11% may die in the first 3 months even with adequate therapy, although many of these patients succumb to comorbidities associated with VTE (eg, cancer) rather than from PE itself.1 Long-term complications of VTE include postphlebitic syndrome (after DVT) and chronic thromboembolic pulmonary hypertension (after PE), which develop in up to 40% and 1% to 4% of cases, respectively. Anticoagulant therapy effectively treats symptoms and decreases the risk of recurrent VTE; however, its use increases the risk of major hemorrhage, which may be fatal in up to 25% of cases.2 Given the potential for poor outcomes of patients with VTE and the risks of major hemorrhage associated with anticoagulant therapy, it is key that timely, accurate diagnostic strategies are available to correctly diagnose VTE when present and to safely rule it out when absent. This review provides an overview of the management of suspected VTE, including diagnosis and initial treatment.
Clinical diagnosis
Common symptoms of DVT are unilateral calf or thigh pain, leg swelling, or redness. In general, only 10% to 20% of patients investigated for DVT actually have the disease. In the majority of cases, PE is suspected due to dyspnea and pleuritic chest pain either alone or in combination.3 Patients with massive PE may experience syncope associated with findings of hemodynamic collapse. At the other extreme, patients with PE involving only segmental or subsegmental pulmonary arteries may have minimal or no symptoms.4 Many patients have one or more well recognized risk factors such as recent surgery or hospitalization, cancer, previous VTE, or obesity.
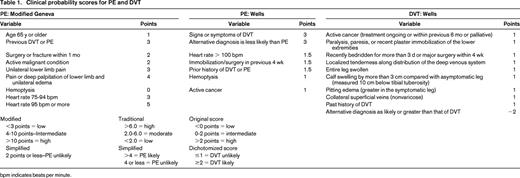
All patients with clinically suspected VTE should undergo a careful clinical examination considering signs and symptoms of VTE, risk factors for this diagnosis, and whether there are other potential explanations for their symptoms. Although, in isolation, none of the symptoms or signs of VTE are diagnostic, it has been well established that clinical prediction rules incorporating signs, symptoms, and risk factors can be accurately applied to categorize patients as low, moderate, or high probability (Table 1) or “likely” or “unlikely” to have DVT or PE. Once the assessment is complete, if the clinician believes VTE is a diagnostic possibility, he or she should assign a pretest probability of VTE to decide on the best diagnostic strategy. Pretest probability is useful because Bayes theorem indicates that with a reasonably sensitive and specific test, the lower the pretest probability, the more likely a positive test result will be falsely positive, whereas with a high pretest probability, the more likely a negative test result will be falsely negative. Concordant results are likely to be true.5 For DVT, more than 14 studies have demonstrated the reproducibility of the Wells model and, for PE, more than 52 studies and 55 000 patients confirm that the Wells rule or the revised Geneva rule are acceptable for PE, although one study suggested superiority of the Wells rule.6-8 These studies were management studies proving the safety for clinical use, and at least one demonstrated that the Wells rule can be safely used by trainees.9 The PE meta-analysis suggests that a gestalt approach can be used, but these results should be carefully interpreted because clinicians often disagree on the pretest probability of PE using gestalt.10 Experience seems to influence the assessment and gestalt assessment tends to categorize fewer patients into the useful categories of high or low11 when the 3-category classification is used. Furthermore, most studies reporting gestalt estimation were conducted at centers with ample experience in the use of the proven clinical prediction, thereby perhaps overestimating their “gestalt” abilities. The revised Geneva and Wells rules have recently been simplified and retrospective analysis suggests that these new models may be effective, but prospective validation is required.12 Other clinical prediction rules exist, but they have been studied less.
D-dimer
D-dimer is a degradation product of a cross-linked fibrin blood clot that is typically elevated in patients with acute VTE, but also by a variety of nonthrombotic disorders including recent major surgery, hemorrhage, trauma, pregnancy, or cancer. D-dimer is a diagnostic (not screening) test and assays validated in VTE patients generally have sensitivities in the mid-90% range and specificities in the mid-40% range.7,13 Given these properties, the value of the D-dimer resides with a negative test.
Imaging tests
Compression ultrasonography is the diagnostic imaging test of choice for DVT. Lack of compressibility of a venous segment is the most sensitive and specific diagnostic criterion for a first episode of DVT. The addition of Doppler (including color flow) can be useful to accurately identify vessels and if there is doubt as to the compressibility of a particular segment. For PE, although many diagnostic imaging tests such as conventional contrast pulmonary angiography, thoracic ultrasound, and magnetic resonance angiography are proposed for the diagnosis of PE, ventilation-perfusion (V/Q) lung scans and computerized tomographic pulmonary angiography (CTPA) currently are the most widely used and evaluated tests for the diagnosis. A recent randomized controlled study showed that when the two techniques are compared, CTPA diagnoses approximately 33% more PE; however, it does not appear that patients in whom PE is excluded with V/Q lung scans are any more likely to return with consequences of undetected VTE than patients in whom PE was ruled out by CTPA. For most clinicians, CTPA has become the preferred diagnostic test because of its higher sensitivity and simpler reporting system. However, there are concerns that widespread use of CTPA has resulted in increased numbers of patients being diagnosed with PE, some of whom have minimal symptoms and minor thrombi involving only segmental and subsegmental vessels. Carrier et al evaluated the rate of subsegmental PE in patients managed with CTPA and showed that the prevalence rose as newer multislice CT scanners became available and that interobserver disagreement was higher for defects detected in subsegmental vessels than for larger PE.14,15 CTPA has other disadvantages compared with V/Q scanning, including radiation and contrast dye exposures. Planar V/Q is still a reliable diagnostic test for PE. V/Q scan has a very high negative predictive value and should be used particularly when a low radiation dose is desirable (eg, in young patients and females).16 The use of V/Q scanning is supported by the results of management studies demonstrating that strategies relying on V/Q scanning and CTPA are similarly effective to rule out PE. Initial studies have shown single photon emission computed tomography technology to have similar diagnostic accuracy as multislice CTPA.
Ultrasound may be used as the initial test in patients with suspected PE and complements the management of patients evaluated with V/Q scans. A recent study showed that the specificity of compression ultrasound in patients studied for PE was 99% and the sensitivity was 39%.17 In patients with symptoms of DVT, the prevalence is up to 40%. Ultrasound is a particularly useful in the initial assessment for PE in patients with symptoms of DVT when diagnostic imaging for PE is not widely available or when CTPA is contraindicated, such as in patients with chronic kidney disease or contrast allergies.
The widespread availability of CTPA and ultrasound, coupled with the potentially fatal nature of VTE, has resulted in a dramatic increase in the number of patients tested for PE and DVT, and therefore with a lower rate of positive tests but without a concomitant reduction in mortality. This is unfortunate because many studies in the past 15 years have proven that algorithm-based strategies enable rapid and safe exclusion of DVT or PE before diagnostic imaging. Although initially driven by the need to eliminate unnecessary invasive imaging and for patient convenience, the need to use these strategies is now of even greater importance due to overutilization of imaging tests.
Diagnostic algorithms
The ideal diagnostic strategy uses the benefits of clinical prediction rules and D-dimer and imaging tests in a stepwise approach. The first step is using a validated clinical prediction rule to determine pretest probability. If the prediction rule suggests a low, moderate, or unlikely probability of VTE, a negative VTE-validated D-dimer test rules out VTE and negates the need for diagnostic imaging. All other patients require imaging. Note that it is recommended that a negative D-dimer result should not be used to exclude VTE in patients who are high pretest probability because of the higher false negative rate in this subgroup.
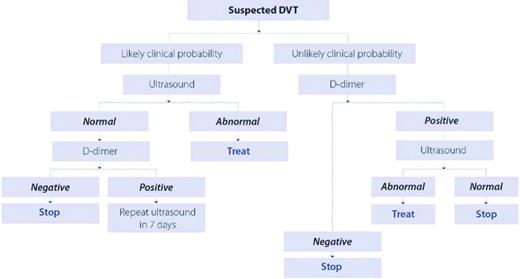
Patients who require imaging for DVT can be managed with whole-leg ultrasound, in which a negative result rules out DVT,18 or by compression ultrasonography limited to the proximal venous system. With the latter approach, select patients require a repeat (serial) test 1 week later as outlined in Figure 1. Randomized trials have not demonstrated an advantage to the whole-leg approach, which will diagnose isolated calf vein DVT that may not require anticoagulant treatment. No study has used clinical probability in conjunction with whole-leg ultrasound, but data suggest a false negative rate of 2.5% in those at high probability for DVT.19
The ideal strategy for diagnosing DVT in patients with a prior DVT in the symptomatic leg is still a subject of debate. Because ultrasound abnormalities may persist indefinitely with DVT, the criterion of vein compressibility may not distinguish patients with acute recurrent DVT from patients with chronic findings. It is helpful to recognize that acute DVT is usually occlusive, not echogenic, and it tends to be continuous. If the ultrasound reveals thrombosis that is echogenic, nonocclusive, or discontinuous, then chronic DVT should be considered. Comparison with previous ultrasound results may be helpful. Increase in clot diameter by 4 mm or evidence of new areas of thrombosis not previously seen on ultrasonography are strongly suggestive of recurrence.20
It should be noted that clinical prediction rules for DVT and PE were developed and validated predominantly in outpatients and pregnant women were not evaluated in these studies. A prediction tool for DVT, yet to be validated, exists for pregnant women.21 Furthermore, the utility of the D-dimer test in hospitalized patients who often have other comorbidities (infection, postoperative) is lower because the D-dimer is rarely negative. Finally, if DVT is not a diagnostic possibility, a D-dimer test should not be done because positive results may redirect a clinician away from investigating the true cause of the leg symptoms toward unnecessary investigation for DVT.
For PE, the use of CTPA in combination with clinical assessment has a high positive and negative predictive value.22 Physicians can consider positive CTPA results as diagnostic if the pretest probability is high or the PE is located in a segmental vessel or larger, but not when the pretest clinical probability is low or unlikely or if the PE is located in the subsegmental arteries. In the latter cases, the results should be reviewed with a radiologist to consider a false-positive result because anticoagulation could potentially lead to more harm than benefit.
The appropriate use of V/Q in a validated diagnostic algorithm provides similar outcomes as multislice CTPA.23 CTPA produces fewer nondiagnostic test results than V/Q, but more auxiliary tests may be required to exclude false positives in patients diagnosed with subsegmental PE. A recent diagnostic study showed that managing patients with ultrasound and V/Q scanning avoids the need for CTPA in 89% of patients. Ultrasound may be used as the initial test in patients with suspected PE and complements the management of patients evaluated with V/Q scans or single-slice CTPA. Our suggested algorithm for the diagnosis of PE is illustrated in Figure 2.
Diagnostic management of patients with suspected PE. DD indicates D-dimer; and US, ultrasound.
Diagnostic management of patients with suspected PE. DD indicates D-dimer; and US, ultrasound.
Clinical assessment and D-dimer testing have the further advantage of enabling the management of patients presenting with suspected VTE at times when radiographic imaging is not routinely available. Patients in whom there is a moderate or high clinical suspicion of VTE may receive an injection of low-molecular-weight heparin (LMWH) in doses designed to treat acute VTE. Diagnostic imaging can then be arranged on an elective basis the following day. Because LMWH is a safe and effective therapy for patients with proven VTE, it also provides adequate protection for patients with suspected VTE. Patients at low risk by either clinical diagnostic models or with use of a sensitive D-dimer test may have diagnostic imaging delayed for a 12- to 24-hour period without the need for anticoagulant coverage.
Treatment
The goal of the therapy for VTE is to prevent the extension of thrombus, PE, and to relieve symptoms in the short term while preventing recurrent events in the long term. Extensive research evaluating the risk of recurrent VTE has established guidelines for the duration of anticoagulation, and this will be focus of another chapter in this publication. LMWH has changed the landscape of treatment DVT and PE by enabling home treatment and by providing an alternative long-term anticoagulant in those populations in whom warfarin is less effective, ineffective, or contraindicated. The following pertains to the treatment of PE and proximal lower extremity DVT, because there is little evidence to formulate recommendations for isolated calf DVT.
Initial therapy must involve therapeutic doses of either unfractionated heparin or LMWH, fondaparinux or rivaroxaban. Rivaroxaban and the new oral anticoagulants will be discussed by Dr Bauer in another chapter. Initial therapy with vitamin K antagonists (VKAs) alone is unacceptable.24 The ease of administration and efficacy of LMWH make this the preferred anticoagulant over IV or subcutaneous unfractionated heparin whether treatment is given as an outpatient or an inpatient. In a meta-analysis comparing the effectiveness of fixed-dose LMWH with adjusted-dose unfractionated heparin, significantly fewer deaths, major hemorrhaging, and recurrent VTE occurred with LMWH.25 Therefore, the current standard for initial treatment is to administer once-daily weight-adjusted LMWH until the international normalized ratio (INR) from the concomitant VKA therapy is therapeutic. This is generally 5 to 10 days. It remains unknown whether twice-daily dosing of LMWH is superior to once-daily dosing of LMWH. A meta-analysis suggested fewer hemorrhages and recurrences with twice-daily dosing, but the 95% confidence interval on the odds ratio crossed 1.0.26 Because LMWH is predominantly renally excreted, in patients with significant renal dysfunction, unfractionated heparin is the parenteral anticoagulant of choice.
Inpatient versus outpatient treatment
Early studies evaluating the outpatient treatment of DVT determined this practice to be safe and effective in most patients, with an improved quality of life and cost savings to the health care system.27 Outpatient treatment of PE is more controversial, although it has been the practice in many centers in Canada for more than 15 years28 and recent studies,29,30 including a randomized trial, may increase the comfort level for this strategy.
Long-term treatment of VTE
For the majority of patients with VTE, oral VKAs such as warfarin are very effective for the long-term prevention of recurrent thrombosis and, as will be discussed, the new oral anticoagulants can be also be used. The duration of long-term treatment varies depending on risk (the recent American College of Chest Physicians [ACCP] guidelines provides an excellent summary of this topic31 ) and can be divided into 5 categories as follows.
(1) First VTE that occurs in the context of a transient risk factor (such as surgery or trauma) has a very low risk of recurrence and 3 months duration is adequate. (2) Patients with malignancy have a higher incidence of recurrent thrombosis and bleeding complications while receiving anticoagulation therapy. Long-term anticoagulation with LMWH instead of warfarin appears to be more effective at preventing recurrent venous thrombosis without a statistically significant increase in bleeding risk. It is our practice to treat all patients with active malignancy with at least 6 months of LMWH if there is adequate renal function. The use of LMWH rather than VKAs also facilitates the management of these complex patients who often undergo procedures (biopsy, line insertion, etc) and who have periodic thrombocytopenia due to chemotherapy. Because the risk of recurrence is high (3-fold higher in cancer vs noncancer patients), extended treatment with anticoagulation is recommended as long as the cancer is felt to be active and bleeding risk is not high (1B evidence from ACCP31 ). We generally wait 6 months after cure or complete remission before stopping therapy. (3) Recent data suggest that factor V Leiden, prothrombin gene mutation, protein C, protein S, antithrombin deficiency, and increased factor VIII levels do not sufficiently alter recurrence risk to be necessary for decisions about duration of therapy unless patients have combined or homozygous genetic defects or very strong family history of VTE associated with the thrombophilic defect. Patients with persistently elevated antiphospholipid antibodies determined by either ELISA or clotting assays have a 2-fold higher relative risk of recurrence within 4 years after stopping anticoagulation and therefore are generally treated indefinitely.32 (4) After a second VTE, the risk of further thromboembolic events after the discontinuation of anticoagulation is felt to be excessive if only 6 months of oral anticoagulation is given. Therefore, we generally recommend continuing anticoagulation in this situation with yearly visits to assess bleeding risk, which enables a risk-benefit evaluation to determine whether anticoagulation should continue. If the bleeding risk is very high, then indefinite therapy may not be ideal. However, no study has looked at risk of recurrent VTE if both events occurred during a transient risk period. In this situation, a shorter duration of anticoagulation may be adequate (3-6 months), but other factors may influence this decision. (5) First VTE that occurs in the absence of temporary or identifiable ongoing risk factors for thrombosis (unprovoked) will be the topic of the chapter by Dr Agnelli in this publication. Decisions on the need for indefinite therapy must be made because recurrence risk may be significant.
Intensity of anticoagulation
The standard intensity of oral anticoagulation with VKAs is determined by an INR of 2-3. In patients with antiphospholipid antibody-related thrombosis, it has long been felt that higher intensity anticoagulation is needed to prevent recurrence, but randomized controlled trials found that standard anticoagulation is as effective as high-intensity treatment even in this subgroup of patients.33 Therefore, high-intensity anticoagulation is not recommended in any patient with VTE. Maintaining good INR control will decrease the risk of developing post-phlebitic syndrome. There has also been debate on the usefulness of a reduced intensity regimen of anticoagulation (INR 1.5-1.9) long-term to prevent recurrent thrombosis while reducing the risk of bleeding. A large randomized trial has shown that low-intensity anticoagulation is less effective at preventing recurrent thrombosis and does not lead to a lower risk of bleeding.34 Therefore, low-intensity therapy is not recommended, but is more effective than no therapy.35
Upper extremity DVT
Upper extremity DVT (UEDVT) can be subdivided into catheter-related and non-catheter-related thrombosis. There is a risk of PE with this condition, so treatment with anticoagulation is generally recommended. Thrombolytic therapy as initial therapy for acute UEDVT has been used with some success, but no randomized controlled trials comparing thrombolytic therapy with anticoagulation alone have been performed. A more detailed discussion of UEDVT is beyond the scope of this article, and we refer the reader to a review addressing this topic.31
Pregnancy
The treatment of VTE during pregnancy deserves special mention because treatment with oral anticoagulation is generally avoided during pregnancy due to the teratogenic effects in the first trimester and the risks of fetal intracranial bleeding in the third trimester. LMWH is the treatment of choice for VTE during pregnancy. However, there is no consensus as to what the appropriate dose should be and whether anti-Xa levels need to be monitored. If acute DVT occurs near term, interrupting anticoagulation may be hazardous because of the risk of PE, and a temporary inferior vena cava filter must be considered. This topic was well reviewed recently.36,37
Other interventions for VTE Treatment
Although anticoagulation is the mainstay of treatment of DVT, thrombolysis and inferior vena cava filters are 2 interventions that deserve mention. The addition of systemic thrombolysis to standard anticoagulation leads to earlier patency of an occluded vein, but does not affect the rate of PE. There is a definite increase in major hemorrhage, including intracranial hemorrhage, with thrombolytics. Catheter-directed thrombolysis has also been reported to increase bleeding complications. It is unclear whether the earlier recanalization seen with thrombolytics translates into lower rates of postthrombotic syndrome long term. Thrombolysis for DVT is not generally recommended except in the case of massive DVT leading to phlegmasia cerulea dolens and threatened limb loss. The role of catheter-directed thrombolysis and clot desiccation is being evaluated in the ongoing ATTRACT randomized controlled trial, which should provide further guidance about its role for patients with proximal DVT.
Systemic administration of thrombolysis for PE has now been the subject of 2 well performed randomized trials (one pending publication), but it does not appear to result in a mortality reduction and due to higher risk of significant hemorrhage should likely be reserved for those with hemodynamic compromise or deterioration on standard anticoagulant therapy.38
Inferior vena cava filter placement in addition to anticoagulation has not been found to prolong survival in patients with DVT. Although it prevents PE, the insertion of a filter increases the risk of recurrent DVT.39 A retrievable filter is indicated when there is a contraindication to anticoagulation therapy (recent hemorrhage, impending surgery) in patients with newly diagnosed proximal DVT. It remains to be determined whether a retrievable filter in patients at higher risk of death (eg, those with limited cardiopulmonary reserve) will lead to a reduction in PE-related mortality.
Post-phlebitic syndrome is a frequent complication of DVT that has received relatively little attention. It is a major public health issue and an under-researched area. It is unclear who is at highest risk and how best to prevent and treat this complication. Some data suggest a potential benefit from the use of graduated compression stockings to prevent this complication after DVT, but findings from a recent randomized placebo-controlled trial suggest otherwise.40
Disclosures
Conflict-of-interest disclosure: P.W. has received honoraria from BMS, Pfizer, Bayer, and Boehringer Ingelheim. D.A. is on the board of directors or an advisory committee for, has received research funding from, has consulted for, and has been affiliated with the speakers' bureaus for Bayer Pharma, Pfizer Pharmaceuticals, and Boehringer Ingelheim. Off-label drug use: None disclosed.
Correspondence
Philip Wells, Suite M1857, 501 Smyth Road, Ottawa Hospital, General Campus, Ottawa, ON K1H 8L8, Canada; Phone: 613-737-8755; Fax: 613-737-8851; e-mail: pwells@toh.on.ca.