Abstract
After more than a decade of treatment of chronic myeloid leukemia (CML) patients with the BCR-ABL tyrosine kinase inhibitor imatinib, and despite the impressive clinical results of this targeted therapeutic, many questions remain unresolved. One major question is how to cure CML, and the next step for the future will be to address this key issue. CML is a good model of cancer. The fact that the majority of CML patients who respond very well but discontinue tyrosine kinase inhibitors later show evidence of molecular recurrence focuses attention on the need for further research on leukemic stem cells. The challenge now is to understand why, after stopping treatment, the leukemia recurs in some patients but not in others. If we win this battle, this progress will certainly benefit the treatment and management of other leukemias and solid tumors and will validate this new topic.
CML is a model disease
Chronic myeloid leukemia (CML) is frequently taken as a model of cancer for many reasons. It is a hematopoietic stem cell disease characterized by the clonal expansion of terminally differentiated myeloid cells. The notion that cancer initiation or progression involves stem-like cells has been reinforced in recent years by the outstanding amount of work in solid tumors focusing on cancer stem cells.1 CML presents as a chronic myeloid disorder most commonly progressing from a chronic phase (CP-CML) through an accelerated phase to a myeloid/lymphoid blast crisis, which also illustrates the molecular “multi-hit” theory of oncogenesis.2 However, the main reason for choosing CML as a model relates to the discovery of a constitutively active BCR-ABL tyrosine kinase, the functional result of a reciprocal translocation between chromosomes 9 and 22 that generates the famous Philadelphia chromosome.3,4 Not only is BCR-ABL a cancer marker, it is also the causative lesion in CML, leading to the development of the first tyrosine kinase inhibitor (TKI), imatinib.5 As a result, CML is also a model of targeted therapy for human malignancies. The dramatic clinical success of imatinib and second-generation TKIs has changed the outcome for CML patient profoundly. Studies from different centers and different countries have demonstrated that survival in CML has improved significantly in the past 20 years since the introduction of imatinib. For example, the M.D. Anderson Cancer Center recently analyzed 1148 CP-CML patients and showed that the 8-year survival was ≤ 15% before 1983, 42%-65% from 1983-2000, and 87% since 2001.6 Therefore, the projections for the next decade, taking into account the recent progress with second-generation TKIs, are for the life expectancy of CML patients to be close to that observed in the general population.7
CML today is definitely a true model of the success of targeted therapies and, until recently, the recommendation was to continue TKI treatment permanently. However, with the emerging problems of long-term tolerability of TKIs, particularly for young patients, variable compliance with treatment, fertility and pregnancy, the economic burden of these expensive drugs, and, last but not least, patients' requests in our daily practice, the issue of treatment cessation became of the utmost importance. Therefore, CML now continues to be more than ever a model of hematology and cancer from the point of view of curability. By asking the question “Is going for a cure possible?”, we set ourselves on the path to finding the answer that encompasses both clinical research and philosophical considerations on the issue of cure.
What is the definition of cure?
Cure comes from the Latin “cura,” which means care, as in “take care.” Cure means disappearance of the disease or disappearance of all signs of the disease and return to a “normal” healthy state. The concept that a patient can only be considered cured of leukemia if every last leukemia cell has been eradicated from his or her body evolved in recent years from the lessons drawn from the use of TKIs. In fact, this idea had already started with allogeneic hematopoietic stem cell transplantation (allo-HSCT), which was regarded until recently as the only curative treatment for CML. We may cure CML patients, but we may never know whether all leukemic cells have been completely eradicated. We could also take the example from microbiology and infectious diseases that persistence of bacteria does not necessarily imply relapse. This distinction is what led John Goldman to propose some years ago the definition of “operational cure.”8 This type of definition allows for the fact that, using an ultrasensitive PCR technique, a low level of BCR-ABL transcripts can be found in the blood of normal individuals.9,10
Before IFN-α was supplanted by imatinib as first-line treatment, we showed that it could be stopped after a complete cytogenetic response (CCyR) was reached, and that the rate of persistent CCyR depended on the time elapsed between the achievement of CCyR and treatment discontinuation.11 In very rare instances, some patients achieved a so-called complete molecular remission (CMR) defined by the absence of detectable BCR-ABL transcripts using qualitative RT-PCR. At that time, the sensitivity of the detection of minimum residual disease was not so well defined. However, in our center, we proposed discontinuation of IFN-α in 21 patients with adeep sustained molecular response (more than 2 years) and followed up on this cohort of rare patients recently.12 The median follow-up after discontinuation of IFN-α was 8 years (mean, 9; range, 5-18). Nine of these 21 patients clearly had persistence of leukemic cells with a level of BCR-ABL transcripts close to that of major molecular response (MMR) after discontinuing IFN-α without definite CML relapse. Moreover, among the 12 patients with sustained CMR confirmed by quantitative RT-PCR (qRT-PCR; 4.5-log reduction), 1 relapsed and progressed suddenly after 12.6 years of IFN-α discontinuation and was treated with allo-HSCT.12 Therefore, it seems that the persistence of leukemic cells at low levels in patients after discontinuation of IFN-α treatment does not automatically lead to CML relapse. This means that one must be careful, because rare late relapses may occur even in patients with undetectable residual disease.
Allo-HSCT has been and is still considered as the sole treatment able to cure CML. This belief is based on the evidence that allo-HSCT can offer long-term freedom from cytogenetic or hematologic recurrence of the disease without the need for maintenance therapy.12,13 For a long time, CML was the best indication for allo-HSCT because the GVL effect mediated by donor-derived T lymphocytes was more prominent than in other malignant conditions treated by allografting.14 Somewhat surprisingly, there are reports of positive results of qRT-PCR for BCR-ABL a long time after transplantation, a finding that does not automatically imply relapse, because no other signs of disease recurrence were observed.15,16 In that situation, most of the patients were apparently cured even though complete eradication of all leukemic cells was not achieved.
This pattern of residual disease investigated by sensitive molecular assays after allo-HSCT and IFN-α illustrate nicely the concept of “operational cure.”8 A similar pattern is now emerging from the long-term follow-up of TKI-treated patients who stop treatment after achieving a good and sustained molecular remission.
What are the results of studies on cessation of imatinib?
Before 2007, molecular relapses with fast kinetics were reported in patients who stopped imatinib on their own, especially if a deep and sustained response had not been reached.17–20 The combination of these data and of our previous work on cessation of IFN-α suggests that the duration of response, especially the duration of CMR, is of major importance in planning imatinib discontinuation strategies. We reported a pilot study in which imatinib was withdrawn in 12 CML patients treated and maintained in CMR for at least 2 years. In that study, the sensitivity of qRT-PCR was considered to be between a 4.5- and a 5-log reduction. After a median follow-up time of 18 months, 50% of patients remained off-therapy without confirmed reappearance of peripheral blood BCR-ABL transcripts.21 Recently, we updated the analysis and showed that 50% of patients still have an undetectable level of BCR-ABL transcripts after a median follow-up time of 6 years (range, 4-8). All patients in that study had been treated previously with IFN-α.
Those preliminary results prompted us to start a multicenter study (registered with www.ClinicalTrials.gov as number NCT00478985) entitled the “Stop Imatinib” (STIM) trial (Figure 1). We included prospectively 100 CP-CML patients on imatinib therapy with undetectable peripheral blood BCR-ABL transcripts for 2 years (with an assay sensitivity close to a 5-log reduction). Fifty-one percent of the patients had been previously treated with IFN-α, and the other half were treated upfront with imatinib only. Molecular relapse, which was arbitrarily defined as 2 positive qRT-PCR results over a period of 1 month showing a significant increase (1 log) in BCR-ABL transcripts, was a trigger for imatinib rechallenge. An interim analysis yielded promising results, with a 12-month molecular relapse-free survival rate of 41%.22 A recent update of that study showed that the overall probability of maintaining CMR at 36 months was 39% (95% confidence interval, 29-48); 3 cases of late relapse were observed at months 19, 20, and 22, respectively.23 Most patients who experienced molecular relapses did so within 6 months from imatinib cessation and remained responsive to retreatment with imatinib, as we had observed in the pilot study.
Very comparable results were obtained in the CML8 study conducted by the Australasian Leukaemia & Lymphoma Group (ALLG), who enrolled 35 patients with BCR-ABL transcripts undetectable for at least 2 years on imatinib (as determined by a qRT-PCR assay with a sensitivity of at least 4.5-log). At the last analysis, the molecular relapse-free survival was 36% (Ross et al23 and D. Ross, personal communication).
A nationwide survey was conducted in Japan, and 50 patients were identified who discontinued imatinib for at least 6 months, 43 of whom were analyzed. Molecular relapse was detected in 19 patients, and the CMR rate after imatinib discontinuation was estimated to be 47%.24
Recently, Yhim et al from South Korea reported 14 CML patients who had front-line treatment with imatinib who had stopped treatment while in CMR. The probability of CMR persistence at 1 year was 28.6%. However, half of the patients were considered to be high risk according to the Sokal score and none of the patients was strictly in CMR throughout the entire 2-year period preceding interruption of the drug.25
In addition to these studies, there are sporadic cases of patients who stopped imatinib; a total of almost 300 patients have been reported in the literature26–28 (Table 1).
Different studies or case reports on imatinib discontinuation
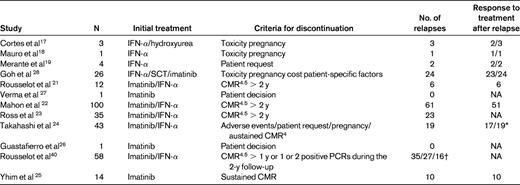
The total number corresponds to 298 patients.
NA indicates not applicable.
*Two patients had shown sustained MMR (<0.1%) for 98 months or near MMR (0.175%) for 24 months, respectively, with no therapy.
†Different criteria of molecular recurrence were used.
These clinical reports are consistent on several points. First, the different studies demonstrate the proof of concept for stopping imatinib in good-responder CML patients, in contrast to what was claimed previously.17 Molecular relapses with fast kinetics were reported in patients who stopped imatinib shortly after a CMR was obtained,20 but larger studies such as the STIM trial suggest that the duration of responses, especially CMR, is of major importance in planning imatinib discontinuation strategies. Most of the molecular relapses or molecular recurrences (which is probably a more appropriate term) occurred within the first few months of imatinib discontinuation. It is clearly necessary to perform molecular assessments using qRT-PCR to detect early those patients who exhibit a fast molecular relapse to retreat them as soon as possible. That is what we have done in the STIM study and this policy probably prevents harmful events such as disease evolution and blast crisis. Therefore, methodical monitoring with qRT-PCR revealed that peripheral blood BCR-ABL transcripts could be detected intermittently. Actually, on rare occasions, fluctuations in BCR-ABL levels could be detected after discontinuation without confirmation of a molecular relapse. In the STIM study, molecular relapse was defined as positivity of BCR-ABL transcripts in qRT-PCR, followed by a 1-log increase at a second analysis point.22 Interestingly, in the Japanese study, 2 patients experienced molecular recurrences after sustaining MMR or near MMR for an extended period with no therapy.24
In the Australian study, Ross et al used genomic DNA-based PCR as a monitoring tool, allowing the detection of BCR-ABL gene rearrangements at a level of around 1- to 2-log below the detection limit of conventional (mRNA) qRT-PCR.23 The BCR-ABL gene was detected in most patients before and after imatinib withdrawal, even in those who maintained undetectable corresponding transcripts. However, there was no link between detection of BCR-ABL by genomic DNA-based PCR and relapse. Likewise, using the STIM samples, we reported that a more sensitive qRT-PCR to assess CMR does not allow the prediction of relapse after discontinuation of imatinib.29 All of these data reinforce the concept of “operational cure.”8
Which category of patients may most benefit from treatment discontinuation?
If we want to design a study that would allow safe treatment discontinuation, it would probably be necessary to obtain a level of residual disease as low as possible. Jamieson et al reported that CML can progress as a result of additional molecular events occurring, not in a stem cell, but in a more differentiated progenitor or granulocyte-macrophage precursor.30 If this is generally valid, it means that evolution of the disease does not result from the transformation of a stem cell but, rather, of more differentiated cells that are believed to be much more sensitive to TKIs than leukemic stem cells (LSCs).31 The key question in this case relates to the level of residual disease that would be required to ensure that the granulocyte-macrophage compartment is eliminated by TKIs. We know that it requires at least CMR in imatinib-treated patients and we know that CMR does not mean complete eradication of leukemic cells. However, we still need to define CMR, including the lowest level of disease compatible with discontinuation of therapy. This is the reason that we recently attempted to standardize and define CMR by taking into account the sensitivity of the qRT-PCR technique. A CML working group of the European LeukemiaNet agreed on a consistent definition: CMR is subdivided in MR,4 MR4.5, and MR,5 with the superscript numbers referring to the log-reduction from the baseline that can be measured by the qRT-PCR assay used by individual laboratories.32
It seems certain that an MMR (ie, a 3-log reduction) is not enough to reach a safe level to stop treatment. The STIM study used a 5-log reduction (actually, a > 4.7-log reduction) and the Australian study used a 4.5-log reduction for their definition of CMR. If one takes into account the concept of operational cure mentioned in the previous section, it might not be necessary to increase the sensitivity of BCR-ABL detection to decide the future strategy of TKI discontinuation.
The other important criterion of discontinuation strategies is the duration of response, especially CMR. In the both the ALLG CML8 and STIM trials, a sustained CMR for at least 2 years was used as the criterion. The validation of this criterion was recently reinforced using mathematical models confirming a biphasic dynamic of BCR-ABL transcript decline with a 2-slopes model of imatinib response: the α slope corresponded to the rapid initial decrease in BCR-ABL transcript levels (ie, cycling cells) after the start of treatment and the β slope corresponded to the longer-term BCR-ABL dynamics (ie, less-proliferative cells).33
Other mathematical models with 3 slopes have been proposed in which the first slope represents the turnover rate of leukemic differentiated cells, the second slope represents the turnover rate of leukemic progenitor cells, and the third slope represents the behavior of immature leukemic cells, potentially stem cells. This model suggests that long-term TKI therapy may reduce the abundance of LSCs in some patients and fits quite well with what we have observed regarding the probable heterogeneity of the LSCs and the CML itself.34,35
In the STIM study, several potential factors for prediction of molecular relapse were assessed retrospectively. For example, the probability of remaining in stable CMR after discontinuation was significantly better for the low Sokal risk group compared with the intermediate or high Sokal risk groups. Using multivariate analysis and logistical regression at 8 months, Sokal risk and imatinib therapy duration were confirmed as 2 independent prognostic factors for prediction of molecular relapse after imatinib cessation. Despite the low number of patients, Yhim et al's report also confirmed that high Sokal risk was associated with a higher rate of molecular relapse after imatinib discontinuation.25 Finally, based on results from the STIM trial, Deininger proposed a hypothetical model of CML persistence and recurrence versus extinction in which the eradication of the leukemic clone depends on some inherent feature or features of the disease (which could be reflected in the Sokal score), on the duration of therapy, or both.36
The identification of other predictive factors of molecular recurrence depends on the power of the statistical analysis, which requires the analysis of a larger homogeneous cohort of patients. In France, we have started the STIM2 trial, which includes CP-CML patients treated upfront with imatinib as a single agent and with a sustained CMR for at least 2 years; we plan to recruit 200 patients. Clearly, the identification of patients who would benefit most from discontinuation of imatinib remains a key issue. It is also one of the objectives of an European Stop Kinase Inhibitors (EURO SKI) trial that will start soon. The criteria for discontinuation are less strict than in the STIM studies: duration of TKI treatment before enrollment of at least 3 years, at least CMR4 within the past year, and no PCR results > 0.01% during the same period.
How can we increase the number of patients who will reach the criteria required for discontinuing treatment?
Last year, Melo proposed 3 hypothetical models of “operational cure” by TKIs in CML: stem cell depletion, stem cell exhaustion, and immunologic suppression.37 If we want to improve the results of a discontinuation strategy, it should be necessary to find therapeutics that can comply with each of these 3 models.
Patients treated with imatinib who are eligible for treatment interruption are rare. During the past 6 months of the STIM trial enrollment, we included 30 patients from approximately 300 newly diagnosed cases of CML in France, so candidates for discontinuation might represent 10% of all patients. The second generation of TKIs, nilotinib and dasatinib, which are used in newly diagnosed CP-CML patients (in the front-line studies DASISION and ENESTnd) produced faster and deeper rates of molecular response compared with imatinib.38,39 Therefore, discontinuation of therapy could be proposed in a larger proportion of CP-CML patients. However, the current experience with dasatinib or nilotinib discontinuation is still limited. Rea et al and Ross et al from 2 independent studies reported on the outcome of 4 patients with imatinib-resistant CML who were rescued by dasatinib and obtained very low or undetectable BCR-ABL transcripts.40,41 These patients stopped dasatinib against medical advice or because of severe side effects, and the combined data suggest that long-term dasatinib-free disease control was achieved. In an ongoing pilot study, dasatinib or nilotinib cessation is currently being proposed to patients with undetectable peripheral blood BCR-ABL transcripts for at least 2 years (defined as consistently undetectable BCR-ABL transcript levels as determined by a qRT-PCR test with a 4.5-log sensitivity). In that study, having drawn lessons from imatinib discontinuation, treatment rechallenge is proposed when a patient loses his or her MMR. Thus far, 33 patients have stopped dasatinib or nilotinib and, at the most recent median follow-up of 9 months, the rate of treatment-free MMR by 6 months was 72.8%; however, using the same criteria as used in the STIM study, the rate of molecular recurrence seems very similar.42 One of the key lessons from this study is the confirmation that some of these patients remained off therapy with only intermittently detectable BCR-ABL transcripts. Consequently, a low level of detectable residual disease after TKI withdrawal may not automatically herald CML relapse and may not preclude the possibility of remaining treatment-free under close molecular monitoring conditions. Using a cohort of patients different from the STIM study entitled “according to STIM,” loss of MMR was validated as a trigger for reintroducing TKIs and consequently could be a more accurate criterion for restarting imatinib and other TKIs than loss of CMR.43
Another solution is to combine imatinib with other potential effective drugs to treat CML. The use of recombinant IFN-α is back “in fashion” as a result of its success in deepening molecular responses when combined with imatinib in patients with newly diagnosed CP-CML. In the French SPIRIT trial, the addition of pegylated IFN-α2a to imatinib therapy for chronic-phase CML patients resulted in significantly higher rates of molecular response (4-log reduction).44 Burchert et al also reported results of IFN-α maintenance therapy after induction therapy with imatinib plus IFN-α in CML,45 which induced stable long-term molecular remissions and could be helpful in increasing the number of patients able to stop TKIs, particularly if immunologic responses are achieved.
Although the mechanism underlying the antileukemic effect of IFN-α in CML is unknown, a single-arm phase 2 study started by the French Fi-LMC group is designed to evaluate pegylated IFN-α2a in association with nilotinib in newly diagnosed CP-CML patients.
Having said already that we still do not know if LSCs are really the true enemies because progression to AP may occur predominantly at the level of a more committed progenitor cell, a large number of publications have focused on targeting the LSCs.46,47 These investigators have clearly been motivated by the important work showing that, at least in vitro, quiescent LSCs are insensitive to TKIs despite kinase inhibition.48,49 Several strategies have been proposed to target the LSCs. For example, JAK/STAT, JAK2 kinase, the protein phosphatase 2A (PP2A), arachidonate 5-lipoxygenase gene (ALOX5), histone deacetylases (HDACs), Sirtuin 1 (SIRT1), and BCL6 are among the most relevant targets for such a strategy.50–54 However, 2 of the most important pathways for self-renewal of CML LSCs are the Wnt-β-catenin and the Hedgehog (Hh) pathways.55,56 Targeting of the Hh pathway in solid tumors has been attempted by smoothened homolog (SMO) inhibitors.57 These drugs (LDE225 and BMS-833923) are currently being combined with TKIs in phase 1 and 2 trials to investigate if such combination can eliminate LSCs and improve the curability of CML.
Finally, the economic implications of TKI treatment cessation are also very important. In the STIM study, accounting for the cost of imatinib and the number of months without treatment in the total study population, the savings were estimated at approximately 4.5 million Euros at last analysis.29 A formal and rationally designed medico-economic study taking into account different aspects of dealing with CML as a chronic disease, including the quality-of-life parameter, is currently in progress. It is likely that the cost of the increased frequency of molecular monitoring in the first 24 months after therapy cessation will be offset largely by the substantial savings for no drug expenditure, particularly beyond that period when regular PCR testing can be spaced out.
Conclusion
Although CML is undoubtedly a heterogeneous disease, it is nevertheless considered a unique and simple model of cancer with a putative one-step molecular hit driving the leukemic cells. The story and the results of the imatinib cessation studies illustrate that the model is not as simple as we imagined at the beginning of the imatinib era. The subset of patients with molecular remission leading to cessation of treatment is itself probably heterogeneous, as exemplified by the variable sequence of events after imatinib is stopped in CMR patients. This suggests that other aspects of disease variability have to be taken into account. Is going for cure in CML justifiable? It depends on the balance between the potential benefit of TKI discontinuation and continuation on the survival and life expectancy for patients with CML, which is at a level never reached before. A long-term follow-up of the different cessation studies is necessary to answer this fascinating question.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Novartis Pharma; has consulted for Bristol-Myers Squibb, Novartis Pharma, and Pfizer; and has received honoraria from Bristol-Myers Squibb and Novartis Pharma. Off-label drug use: None disclosed.
Correspondence
Professor François-Xavier Mahon, Laboratoire d'Hématologie, CHU de Bordeaux Laboratoire Hématopoïèse normale et pathologique, Université Victor Ségalen Bordeaux 2, 146 rue Léo Saignat, Inserm U1035, 33076 Bordeaux Cedex, France; Phone: 33-57-57-15-24; Fax: 33-56-93-88-83; e-mail: francois-xavier.mahon@u-bordeaux2.fr.

