Abstract
Acute lymphoblastic leukemia (ALL) is the most common and one of the most treatable cancers in children. Although the majority of children with ALL are now cured, 10%-20% of patients are predicted to relapse and outcomes with salvage therapy have been disappointing, with approximately only one-third of children surviving long-term after disease recurrence. Several prognostic factors have been identified, with timing of recurrence relative to diagnosis and site of relapse emerging as the most important variables. Despite heterogeneity in the elements of salvage therapy that are delivered in trials conducted internationally, outcomes have been remarkably similar and have remained static. Because most intensive salvage regimens have reached the limit of tolerability, current strategies are focusing on identifying new agents tailored to the unique biology of relapsed disease and identifying methods to develop these agents efficiently for clinical use. Recently, high-resolution genomic analyses of matched pairs of diagnostic and relapse bone marrow samples are emerging as a promising tool for identifying pathways that impart chemoresistance.
Introduction
Overall survival (OS) rates for children with newly diagnosed acute lymphoblastic leukemia (ALL) now approach 90% after treatment with multiagent chemotherapy tailored to established risk factors.1 In contrast, outcomes for children with relapsed ALL are far inferior and have changed little over time despite efforts to intensify treatment approaches.2–9 Although only 10%-20% of patients fail frontline therapy, given the number of cases of ALL each year, relapsed ALL accounts for more deaths from cancer in children than any other malignancy.7,10
Most contemporary treatment regimens for relapsed ALL use common approaches. Reinduction regimens frequently include many of the same drugs used in frontline ALL therapy except with increased dose intensity or alternative schedules. After remission reinduction, recommendations for continuation therapy include ongoing intensive chemotherapy with or without radiation therapy or hematopoietic stem cell transplantation (HSCT). Decisions regarding optimal postremission therapy in relapsed ALL are frequently based on well-established prognostic features, including the timing and site of disease recurrence and the disease immunophenotype. Recently, many studies have also allocated therapy based on early minimal residual disease (MRD) response at the end of the reinduction phase of treatment because this has been shown to be correlated with longer-term outcomes.5
Because the OS rates for relapsed ALL have plateaued at 35%-40% with traditional approaches,2,8–11 there is a need for alternative treatment options. Although many challenges exist, the knowledge gained from recent genomic discoveries and the development of classes of agents with mechanisms of action that are distinct from traditional cytotoxics, such as monoclonal antibodies (mAbs) targeting leukemic cells, offer promise for improvements in outcome. This review highlights current outcomes and treatment strategies for relapsed ALL in children, including some of the contemporary challenges and questions and potential future directions for therapeutic approaches.
Current outcomes, prognostic factors, and treatment approaches
Relapse outcomes and prognostic factors
Outcomes for children with relapsed ALL have changed little over time despite efforts by many investigators to intensify therapy with approaches that often include HSCT. Several well-established risk factors have been identified in relapsed ALL, with timing of relapse emerging as the most significant predictor in studies conducted internationally.2–4,6,7,9,10,12–14 Although the definitions for “early” versus “late” relapse differ slightly among groups, the earlier relapses occur, the worse the outcome. Within the Children's Oncology Group (COG), early bone marrow (BM) relapse is defined as recurrence within 36 months from initial diagnosis, with subsets of “very early relapse” and “intermediate relapse” defined as those occurring < 18 months and 18-36 months from diagnosis, respectively. Similarly, the Berlin-Frankfurt-Munster (BFM) group has defined early BM relapses as those occurring within 6 months of the completion of frontline therapy and very early relapses as those occurring within 18 months from diagnosis. Late BM relapses are defined as those occurring ≥ 36 months from initial diagnosis within the COG trials or > 6 months after the completion of frontline therapy in the BFM trials (Table 1). Other groups have also used these definitions.5,10,15 Outcomes for relapse are poor and have remained static. Although OS rates for relapse, including BM and extramedullary relapses at all times relative to initial diagnosis, typically range from 35%-40%,2,8–11 inferior outcomes have been observed for medullary relapses, especially when they occur early, with overall long-term survival rates of approximately 25% (Table 2).3,4,8,10,11 Moreover, reported outcomes have remained remarkably similar among various groups despite differences in components of salvage regimens, allocation to HSCT, frontline therapy, and supportive care. In an updated analysis of longer-term event-free survival (EFS) rates for children with early and late BM relapse treated in the COG AALL01P2 study, which accrued 124 eligible patients 1-21 years of age from 2003-2005,6 the 5-year EFS rates overall were 29% ± 4.6%; for relapses occurring < 18 months, 18-36 months, and 36+ months from initial diagnosis, they were 11% ± 7%, 24% ± 9%, and 40% ± 7%, respectively (Figure 1).
Definitions for timing of relapse

*The early-relapse group in the COG studies is separated into very early (< 18 mo from diagnosis) and intermediate (18 to < 36 mo from diagnosis) subgroups.
Outcomes for BM relapse according to time to recurrence

*Outcomes for an intermediate subgroup with BM relapses occurring 18 to < 36 mo from diagnosis are reported for the COG studies.
Outcomes based on timing of relapse. Outcomes after first isolated or combined relapses of B-lymphoblastic leukemia in children and adolescents treated in COG study AALL01P2. The overall 5-year EFS probabilities are depicted for very early, intermediate, and late medullary relapses (unpublished data).
Outcomes based on timing of relapse. Outcomes after first isolated or combined relapses of B-lymphoblastic leukemia in children and adolescents treated in COG study AALL01P2. The overall 5-year EFS probabilities are depicted for very early, intermediate, and late medullary relapses (unpublished data).
Additional prognostic variables include the site of relapse and disease immunophenotype. Far better outcomes are observed in children with isolated extramedullary disease than recurrences involving the BM, with EFS rates generally 50% or less for early isolated extramedullary relapses and approximately 75%-80% in those with late isolated extramedullary disease recurrences: rates approximating those seen in some subsets of newly diagnosed patients.4,16,17 Especially favorable 10-year EFS rates of 93% ±6% were reported for children with late isolated testicular relapses after treatment with chemotherapy and radiotherapy on the ALL-REZ BFM 90 trial.9 Patients with T-cell relapses at any site have dismal outcomes that are inferior to those observed with recurrences of B-lymphoblastic disease, and T-cell recurrences tend to occur early.2,4,7,9,10,18
Elegant genomic studies of matched pairs of BM samples from individual children at diagnosis and relapse have demonstrated the acquisition of new genetic alterations at relapse, often involving pathways involved in cell proliferation and B-cell development.19–22 Another important observation from backtracking experiments has been that the presence of genetic alterations at relapse can also be detected in minor subpopulations at initial diagnosis, suggesting that the relapse clone is selected for during treatment.21 A question that has followed and has been debated is whether the intensity of prior therapy affects outcomes such that children who relapse after less intensive frontline therapy are more readily salvaged. Recent evidence suggests that this is not the case. In a report of postrelapse survival rates in 1961 children according to treatment era at initial diagnosis (trials conducted from 1988-1995 vs 1996-2002), with treatment intensity increasing over time, the postrelapse outcomes were nearly identical.4 In another recent analysis of the outcomes of 272 pediatric patients who relapsed after treatment for National Cancer Institute (NCI)–defined high-risk ALL on the CCG-1961 trial, which compared standard versus augmented postinduction therapy, there were no differences in outcome based on the intensity of the protocol therapy that was administered.11 The 3-year postrelapse survival rates were 36.4% ± 5.7% versus 39.2% ± 4.1% (P = .72) among those treated with augmented versus standard-intensity frontline regimens, respectively. These observations support a model in which relapse emerges from a drug-resistant subclone present at initial diagnosis that is selected for during treatment independently from the nature of the frontline therapy delivered.11
With the growing recognition of the unique biological features of ALL in adolescents and young adults,23 the prognostic importance of age has also been analyzed, and there is evidence to suggest this may portend outcome in relapsed ALL as well. The 3-year postrelapse survival rates for children, adolescents, and young adults after treatment in the CCG-1961 trial for NCI-defined high-risk ALL were recently analyzed according to age at diagnosis. OS rates after BM relapse were 48.6% ± 5.3%, 35.4% ± 5%, and 14.7% ± 6.8% (P = .001) respectively, for patients 1-9, 1-15, and 16-20 years of age. Although the details of salvage therapy were not reported, these outcomes suggest that age at initial diagnosis is a prognostic factor in relapsed ALL, just as it is for newly diagnosed disease.
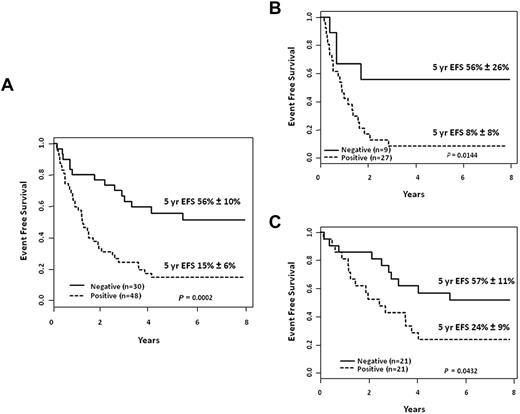
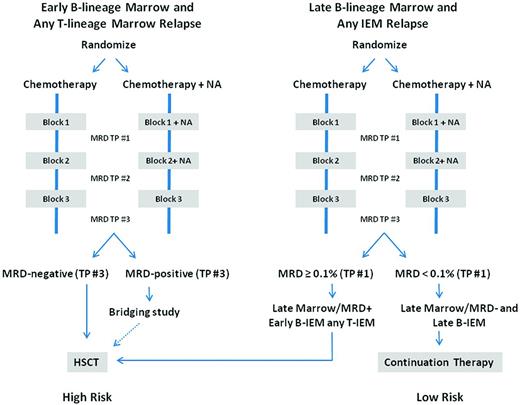
The prognostic significance of MRD response at relapse has also been assessed in several studies.6,24,25 MRD responses at the end of the first block of reinduction chemotherapy and the kinetic patterns of disease regression over time in the COG AALL01P2 study were strongly correlated with outcomes6 and, when combined with timing of relapse, could be used to identify 3 different patient risk groups (Figure 2). The COG is adopting a risk-classification algorithm (Figure 3), which will incorporate MRD response in addition to timing, site, and immunophenotype; a similar stratification system was used in the UKALL R3 relapse trial.5
Outcomes according to MRD response at the end of reinduction treatment block 1. EFS probabilities for pediatric patients with first isolated or combined BM relapses of B-lymphoblastic leukemia in morphological CR2. (A) 5-year EFS probabilities in all patients according to MRD response using flow cytometry–based assays (negative < 0.01%; positive ≥ 0.01%). (B) Analysis includes patients with early BM relapses (< 36 months from diagnosis) only. (C) Analysis includes patients with late BM relapses (≥ 36 months from diagnosis) only (unpublished data).
Outcomes according to MRD response at the end of reinduction treatment block 1. EFS probabilities for pediatric patients with first isolated or combined BM relapses of B-lymphoblastic leukemia in morphological CR2. (A) 5-year EFS probabilities in all patients according to MRD response using flow cytometry–based assays (negative < 0.01%; positive ≥ 0.01%). (B) Analysis includes patients with early BM relapses (< 36 months from diagnosis) only. (C) Analysis includes patients with late BM relapses (≥ 36 months from diagnosis) only (unpublished data).
Proposed site-, timing-, and response-based risk allocation at relapse. Shown is the proposed classification schema for the upcoming COG comprehensive therapeutic trial for children, adolescents, and young adults with relapsed ALL (J. Whitlock, P. Brown, written communication, May 7, 2012). NA indicates novel agent; TP, time point; and IEM, isolated extramedullary.
Proposed site-, timing-, and response-based risk allocation at relapse. Shown is the proposed classification schema for the upcoming COG comprehensive therapeutic trial for children, adolescents, and young adults with relapsed ALL (J. Whitlock, P. Brown, written communication, May 7, 2012). NA indicates novel agent; TP, time point; and IEM, isolated extramedullary.
Current treatment approaches
Current treatment approaches for relapsed ALL begin with reinduction therapy in an attempt to induce a second complete remission (CR2). Overall reinduction (CR2) rates for BM relapse are approximately 85% and vary according to timing.3,6,9,10 CR2 rates for late BM relapse typically approach 95%, whereas those for early relapse range from 70%-85%, and are frequently < 50% for very early relapses. Historically, most remission reinduction failures have resulted from resistant disease. In addition, salvage regimens have reached their limit of tolerability, in many cases with toxic death rates ranging from 4%-5%.6,7,9 After the induction of CR2, options for ongoing continuation therapy are frequently risk based. Several studies have demonstrated a survival advantage for HSCT in CR2 for children with early BM recurrences, and the comparability of several different stem cell sources has expanded this option for many children.26 Optimal postremission therapy for children with late BM relapse has been debated. Several studies, including a large retrospective analysis,27 have shown comparable outcomes with both HSCT and chemotherapy for late BM relapse.9 Recent data suggest that MRD responses may identify those patients with late BM relapse or intermediate-risk disease who might benefit from HCST versus chemotherapy alone.5
Outcomes for children with late (> 18 months from initial diagnosis) isolated extramedullary disease have been very good with chemotherapy and site-directed radiation without HSCT. Because disease recurrences are not truly isolated by strict definition, as evidenced by the fact that most children with extramedullary relapses have detectable disease in the BM as well, intensive systemic therapy is essential for preventing later BM recurrences. Although this approach has been quite successful for late extramedullary recurrence, the outcomes for early extramedullary recurrences have been inferior, with EFS of < 50%, and HSCT in CR2 has also been considered for these patients.7 Within the COG, recommendations for treatment of high-risk relapse include remission reinduction therapy with the study of novel agents, followed by HSCT in CR2 with the best available donor. Treatment with intensive chemotherapy with radiation is recommended for low-risk extramedullary disease. In future trials, recommendations for the treatment of children with late BM relapse will be stratified according to MRD response at the end of the first treatment block. Bridging therapy strategies are also being developed for patients with BM relapses and detectable MRD at the end of the third treatment block (Figure 3).
Second or greater BM relapses are very challenging to treat, with response rates to salvage therapy estimated to be only in the range of 40%.28 In a report from the Austrian BFM Study Group, the median duration of complete remission after second relapse was reported to be 13 months, with 10-year EFS rates of only 9% ± 3% and 6% ± 6% after second and third relapses, respectively.10 Treatment with novel therapies is often recommended for this group, with the consideration for HSCT in those achieving a durable remission. Some agents currently being investigated for children with multiply relapsed disease and approaches that can be considered for agent prioritization are described below.
Treatment challenges/questions
Improving remission reinduction rates for children with early BM relapse
One of the greatest challenges in treating relapsed ALL is overcoming treatment resistance, which is reflected early on in therapy by the inferior remission reinduction rates for early BM relapses, regardless of the chemotherapy regimen that is used. Furthermore, among those patients who achieved a CR2 in COG protocol AALL01P2, 75% of those with early BM relapses had detectable MRD.6 The overall outcomes are also dismal for patients who do not achieve a CR2 with an initial attempt.3,6 In an effort to improve CR2 rates, the COG adopted the approach of combining a new agent with an established reinduction platform, looking for an improvement in CR2 rate as well as MRD response, compared with historical controls treated with the platform alone. The first new agent to be combined with chemotherapy in this manner was the mAb epratuzumab, which targets CD22.29 The phase 2 portion of this study tested epratuzumab on 2 dosing schedules (once weekly for 4 doses and twice weekly for 8 doses) in combination with the same reinduction chemotherapy platform in children and young adults with first, early (< 36 months from diagnosis) BM relapses of CD22+ ALL. Although the rates of CR2 did not differ compared with a historical control population treated with chemotherapy alone, MRD responses in those who achieved remission were significantly more favorable in those who received epratuzumab (42% MRD− compared with 25% MRD− among historical controls, P < .01%), suggesting biological activity of this agent in this population.30 Analyses are also under way to determine whether the addition of epratuzumab affected longer term outcomes. A study with a similar design combining bortezomib with a 4-drug reinduction platform is also in progress within the COG.
Application of MRD assessments in relapsed ALL
Early treatment response as assessed by measurements of MRD has been established as a powerful prognostic marker in relapsed ALL. Recent trials have demonstrated that the presence of MRD at the end of the first reinduction course or block of therapy correlates with inferior outcomes6,24,25 ; similarly, the presence of MRD before HSCT portends poor outcomes.31 In relapsed ALL trials in which MRD has been measured at serial time points, the kinetic pattern of disease regression has also been shown to be highly prognostic and has supported trial designs in which several blocks or courses of reinduction chemotherapy are delivered to increase the durability of remission.6 In the recently completed COG AALL01P2 study, 70% of children who achieved a morphological CR but had detectable MRD after the first treatment course had further MRD regression with ongoing chemotherapy courses. Given its power as a prognostic tool, MRD has several potential applications in relapsed ALL trial design and therapy, including its integration into risk classification schemes as depicted in Figure 3.
An emerging and important question in relapse trial design is whether MRD can be validated as a surrogate for outcome to prioritize agents for further study more efficiently. Recent trials such as COG ADVL04P2, in which epratuzumab was combined with a conventional chemotherapy platform, have been designed to look for improvements in CR2 rates and MRD responses using the historical comparator of the identical chemotherapy regimen alone.29 In that trial, MRD responses in those who achieved remission were significantly more favorable in those who received epratuzumab (42% MRD− compared with 25% MRD− among historical controls, P < .01%).30 Analyses are presently under way to determine whether the addition of epratuzumab affected longer-term outcomes. Conflicting results, however, were observed in the Medical Research Council (MRC) UKR3 trial, in which reinduction therapy with mitoxantrone was superior to that with idarubicin, yet no differences in end of reinduction MRD were observed.5 This study differed from others in that it assessed 2 different drugs with distinctive properties within a multidrug regimen rather the addition of a single agent to an established platform. Given the complexities of using historical controls, the upcoming planned relapse trial within the COG will consist of randomized studies of an established platform with or without the addition of a new agent, looking for improvements in CR2 and MRD rates as a measure to define new agent activity and, potentially, to select more efficiently candidate agents for future study (Figure 3).
Identifying new agents to study
Given the poor outcomes for relapsed ALL, new approaches are needed to improve chances for cure for this population and also to identify promising new agents that could be used in other subgroups of patients with high-risk ALL in the future. Because responses to single-agent therapy have been poor, integrating new agents in combination with established chemotherapy platforms has been a therapeutic approach that has been explored. Given the toxicity associated with most relapse chemotherapy regimens, ideal candidate new agents are those that uniquely target blasts and have favorable safety profiles. mAbs directed to cell surface antigens expressed by leukemic blasts (eg, CD19, CD22, and CD52) are among the agents of promise.32 mAb-based therapeutic approaches in pediatric ALL encompass several categories of agents, including unconjugated Abs, Abs conjugated to immunotoxins that are selectively delivered to target cells, and bispecific Abs that are directed to 2 antigens and recruit immunologically active cells to leukemic blasts. Studies with targeted immunological therapies, including epratuzumab, blinatumomab, inotuzumab ozogamicin, and moxetumomab pasudotox, among others, are in progress.29,33–36 There are several additional agents that are presently being investigated by the COG in relapsed ALL in children because they target key biological pathways; these include the proteasome inhibitor bortezomib and JAK kinase, aurora kinase, and mammalian target of rapamycin (mTOR) inhibitors.
Based on the premise that understanding the underlying biological pathways responsible for drug resistance will provide rational opportunities for both prevention of relapse and effective salvage therapy, several investigators have studied the evolution of genetic lesions from diagnosis to relapse that lead to drug resistance. Alterations in cytogenetic abnormalities from diagnosis to relapse in ALL have been recognized for many years.37 More recently, the ability to identify alterations in cancer genomes has been revolutionized by the completion of the human genome project and the development of high-throughput technologies. Efforts have been under way to identify global genetic and epigenetic changes that characterize disease progression using analyses of DNA copy number abnormalities, gene expression, DNA methylation, and next-generation sequencing in matched diagnosis/relapsed ALL BM samples. These studies have been undertaken by several groups of investigators, and heterogeneous genetic alterations have been observed to evolve from diagnosis to relapse.19–22,38,39 Relapse-specific alterations in genes involved in regulation of the cell cycle and apoptosis, DNA replication repair, nucleotide biosynthesis, and B-cell development have been identified, and several appear to converge on pathways such as the WNT and MAPK pathways.20 Furthermore, biologically distinct pathways have been implicated in the development of early versus late recurrences.19,20,39
In addition to their application in relapsed ALL, comprehensive genomic assays have also defined new biological subtypes and key pathways that are altered in newly diagnosed high-risk B-lymphoblastic leukemia, including JAK2 mutations, CRLF2 alterations, IKZF deletions or mutations, and “BCR-ABL–like” gene-expression signatures.40–48 These discoveries have important therapeutic implications because they could potentially lead to the development of targeted frontline therapies directed at kinase signaling, similar to the successful paradigm of combining tyrosine kinase inhibitors with chemotherapy in Philadelphia chromosome–positive ALL.49 It is hoped that approaches such as these might be successful in preventing more relapses from occurring in the future.
There are recent examples of the translation of genomic discoveries into the clinical care of children with relapsed ALL. Using microarray-based gene-expression studies comparing diagnosis/relapse patient pairs, survivin (BIRC5) emerged as one of the top genes that was up-regulated at relapse.19 Survivin is also an attractive target due to its selective expression in tumors, its role in preventing apoptosis, and the availability of novel agents to silence its activity. Preclinical work in ALL cell lines and xenograft models using shRNA and EZN-3042, a third-generation locked nucleic acid oligonucleotide targeting survivin mRNA, confirmed that down-regulation of survivin induced apoptosis and, when paired with chemotherapy, increased chemosensitivity was observed.50,51 Based on these preclinical studies and preliminary results from a phase 1 trial of EZN-3042 in adults with relapsed or refractory solid tumors and lymphoma,52 a phase 1 trial for pediatric patients with second or greater BM relapses was initiated through the Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL) consortium (www.ClinicalTrials.gov identifier NCT01186328) to determine the safety and tolerability of administering EZN-3042 in combination with a traditional reinduction chemotherapy platform. This trial was recently completed due to lack of further clinical development of this agent and final analyses are under way.
Genome-wide methylation assays in matched diagnosis/relapse pairs of BM samples have demonstrated a distinctly higher CpG methylation level at relapse relative to initial diagnosis, implicating epigenetic dysregulation in the acquisition of chemoresistance at relapse. Capitalizing on the knowledge of microarray technology, the concept of the Connectivity map database53 has been used to identify drugs that would mimic reversal of the relapse gene-expression signature and therefore functionally restore chemosensitivity in leukemic blasts. Vorinostat, a histone deacetylase inhibitor emerged as a top candidate drug that could potentially endow a chemosensitive profile. Based on these observations, preclinical studies were undertaken to examine the effects of epigenetic agents in inducing chemosensitivity in leukemic blasts. These studies demonstrated that vorinostat not only reprograms the aberrant gene expression profile of relapsed blasts by epigenetic mechanisms, but also is synergistic when applied before chemotherapy.54 Furthermore, incorporation of the DNA methyltransferase inhibitor decitabine led to reexpression of genes shown to be preferentially methylated and silenced at relapse. Combination pretreatment with vorinostat and decitabine resulted in even greater cytotoxicity compared with each agent individually with chemotherapy in primary patient samples and cell lines.54 As an extension of preclinical work, a multi-institutional phase 1 clinical trial is currently open within the TACL consortium to evaluate the feasibility and safety of using these epigenetic agents in combination with chemotherapy in children with second or greater ALL BM relapse (www.Clinicaltrials.gov identifier NCT01483690).
Conclusions
Although the progress in treating relapsed ALL has lagged significantly behind that in newly diagnosed disease, additional prognostic factors such as MRD response are now refining risk allocation in current trials. In addition, several new agents are being developed, including immunological therapies and other agents that selectively target pathways that are dysregulated in recurrent disease. Recent discoveries from high-resolution genomic profiling offer promise for greatly enhancing our understanding of the evolution of resistant disease and identifying additional pathways that can be targeted therapeutically. These discoveries also have identified new groups of high-risk disease in the frontline setting so that treatment strategies at diagnosis can potentially be optimized to prevent recurrence. Challenges for the future will include defining methods to prioritize new agents and implementing trial designs that can efficiently assess their activity so those that show promise can be developed further in both the relapse and frontline settings.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Elizabeth A. Raetz, MD, NYU Langone Cancer Institute and School of Medicine, 522 First Ave, Smilow 1206, New York, NY 10016; Phone: 212-263-9908; Fax: 212-263-9978; e-mail: elizabeth.raetz@nyumc.org.