Abstract
Chronic pulmonary complications, including pulmonary hypertension (PH), are common in sickle cell disease (SCD), especially in adults with sickle cell anemia (SCA). The underlying pathophysiology is complex and variable, involving multiple biological systems. Recent emphasis has been placed on the pleotropic biological factor nitric oxide (NO). An elevated tricuspid regurgitant velocity (TRV) appears to have limitations in specificity in SCA, but may indicate the presence of PH, a diagnosis confirmed by right heart catheterization. TRV has been used in recent clinical trials to identify or define subjects with PH for enrollment into PH-specific interventions; these include sildenafil, which enhances NO-induced vasorelaxation. Results from a controlled trial show no benefit and an unexpected increase in adverse events, emphasizing the biological complexities of SCA. Management remains principally supportive, includes recognition and treatment of comorbidities, and may incorporate individualized PH-specific strategies (despite recent trials) based on appropriate diagnostic testing. Ultimately, therapy is likely to be multimodal and tailored to the processes identified to be the most contributory in a given individual. Based on the relative prevalence of the conditions, routine screening for asthma in children with SCD and by Doppler echocardiography to measure TRV as an initial screen for PH in adults with SCA may be warranted. Data are limited regarding the clinical utility of screening in other forms of SCD and the pediatric population. This article offers an individual perspective on practical and challenging clinical considerations.
Introduction
HbS-containing red blood cells (sRBCs) induce multiple pathobiological changes while circulating intact and after hemolysis occurs, far beyond a simplistic notion of “log-jamming” in the microvasculature. Complex and interrelated effects on vascular stasis and tone, endothelial function and remodeling, and regulation of inflammatory and oxidative responses affect acute and chronic organ function.1,2 Recent emphasis has been placed on pleotropic biological factors such as nitric oxide (NO) as potential therapeutic target.3
Chronic pulmonary complications are common in patients with sickle cell anemia (SCA), a term hereafter used to refer to HbSS and HbSβ° thalassemia, and are associated with an increased risk of mortality. An elevated tricuspid regurgitant velocity (TRV) is common in children and adults with SCA and is associated with an increased risk of mortality.4–8 Because an elevated TRV may be an indication of pulmonary hypertension (PH), recent research efforts and clinical trials in SCA have focused on interventions targeted at PH. Unexpected findings from clinical recent trials offer clinicians the opportunity to review updated concepts regarding pathophysiology as they consider the management of chronic lung disease and PH in sickle cell disease (SCD).
SCD and chronic pulmonary complications
Chronic sickle lung disease was initially characterized by longitudinal assessment in a small cohort of patients with SCA.9 Progression from mild restrictive changes through the development of hypoxemia during stable periods to severe pulmonary fibrosis and cor pulmonale was observed, and could be predicted by the total number of acute chest syndrome events, sickle cell crisis with chest pain, and the presence of avascular necrosis. A subsequent 4-decade observational study noted the prevalence of chronic lung disease, most often with cor pulmonale in adults, to be 16%.10 Restrictive physiology has been observed in up to 90% of SCA patients at a mean age of 30 years11 ; thoracic imaging shows pulmonary fibrosis with areas of infarction in some.12 Autopsy series document the presence of plexiform lesions, medial hypertrophy, and intimal and subintimal proliferation/fibrosis of the pulmonary vasculature in 12%-14% of SCA patients.13,14 These data support a role for progressive changes in the vasculature and lung tissue of individuals with SCD. Chronic lung disease and PH have also been observed in individuals with SCA without a history of acute chest syndrome,4–6,11 most often in adults with evidence of other chronic organ injury.6,10,15 This suggests that simple interruption of overt acute clinical episodes may be only partially beneficial in chronic sickle lung disease and PH unless underlying chronic pathologic processes are addressed.
Pathobiological processes in SCA that may contribute to chronic lung injury and PH
Vasculopathic changes
Sickle vasculopathy has been characterized by both functional and structural abnormalities and has been extensively reviewed elsewhere.1–3 Many biological processes, including inflammation, oxidative and reperfusion injury, coagulation activation, and angiogenesis, are affected in SCA as a consequence of direct injury from intact sRBCs, hemolysis, or as part of the host response to these stimuli. Several functional vascular changes may impair flow and have particular impact on the development of PH.1–3 Impairment of endothelial-dependent vasodilation and impaired vasorelaxation with administration of NO and NO-generating agents, often referred to as “NO resistance,” limits adaptive responses. Enhanced adhesion of intact sRBCs (even when not “sickled”) and leukocytes to vascular endothelium slows flow and contributes to vessel wall activation and injury. Elevated markers of coagulation activation and prothrombic changes on the surface of endothelial cells are common and may predispose to local thrombosis. Whereas each of these dysfunctions interacts with the others, any one of them in isolation variably contributes to aberrant flow in the pulmonary vasculature and PH, creating clinical heterogeneity. Histopathologic (structural) changes have been observed, including endovascular remodeling, with intimal hypertrophy and endothelial lesions, and in situ thrombosis,1,13,14,16 resembling changes seen in idiopathic pulmonary arterial hypertension (PAH) in other populations.
Limited NO bioavailability and hemolysis
Several reviews detail the processes in SCA that may lead to the underproduction of NO, failure of delivery to appropriate vessel or tissue compartments, consumption, or scavenging by plasma free hemoglobin.1–3,17,18 A link among markers associated with hemolysis, NO scavenging, and elevated TRV has been reported in some but not all cohorts of pediatric and adult patients with SCD.2,18,19 Intravascular hemolysis also results in the release of heme, iron, and other oxidants, which may contribute to vascular injury.1,2,19 Several lines of clinical research suggest a potential role of NO in the pathobiology of SCA. Short-term administration of arginine, the substrate for NO, leads to improvement in NO levels and metabolites in NO pathway, potentially enhanced by coadministration of hydroxyurea.3,17,20,21 Intravenous administration of arginine resulted in a 50% reduction in opioid use in a randomized, controlled pediatric inpatient SCA study.22 After a promising pilot study, however, inhaled NO failed to improve the time to crisis resolution in a multicenter study of hospitalized children and adults with SCD.23 Oral arginine administered for 5 days reduced pulmonary artery systolic pressure estimated by echocardiogram in subjects with SCA.24 Variable results were reported in 2 longer-term (12-week) oral arginine supplementation studies: one showed no difference in arginine or NO levels in a multicenter SCA pediatric cohort,25 and another demonstrated improvement in arginine levels but no improvement in abnormal TRV or functional capacity in an adult cohort with SCA on hydroxyurea.26 The inconsistent impact on biological and clinical outcomes may reflect a need to better understand how to manipulate NO biology, or it may indicate that intervention directed at a single (albeit pleotropic) factor may be insufficient to render significant clinical benefit.1 However, endothelial dysfunction resulting in impaired vasodilation, responsive to manipulation of NO biology, is a recognized mechanism of disease in PH in other populations and likely contributes to the pathophysiology of PH for a subset of SCA patients.
Parenchymal injury
Sickle vasculopathy, inflammatory and oxidative processes, and reperfusion injury and infarction may all contribute to parenchymal lung injury in SCD. Other types of lung disease, including chronic obstructive pulmonary disease, restrictive lung disease, and interstitial fibrosis, lead to PH27 ; parenchymal changes reported in chronic sickle lung disease resemble these conditions and may contribute to the development of PH in SCA.
Comorbidities
Asthma.
Studies of pulmonary function tests (PFTs) in infants and children with SCA demonstrated a lower airway obstructive pattern preceding the development of the restrictive lung disease more commonly described in adults.28 Airway hyperreactivity (AHR) is reported in 40%-77% of SCA patients, and the clinical diagnosis of asthma in 2%-50%.29 Recent investigation suggests that AHR and clinical asthma may not be strongly correlated in children with SCA.30 Further studies are needed to understand whether AHR associated with elevated lactate dehydrogenase levels30 represents a unique and independent pulmonary process in children with SCA. Obstructive changes on PFTs and a clinical diagnosis of asthma are associated with a higher rate of hospitalization for acute chest syndrome and vasoocclusive crisis and a 2.36-fold increased risk of death compared with children without these findings.28,29 Segregation analysis suggests that asthma, which disproportionately affects African-American children, is a comorbidity rather than a consequence of SCD.31 Features of AHR and asthma, including elevation of inflammatory mediators (leukotrienes, interleukins, and soluble vascular adhesion molecules) and transient hypoxia, ventilation-perfusion mismatch, and reperfusion injury, may contribute to the development of vasculopathy and chronic lung injury.28,29
Thromboembolic disease.
Autopsy series demonstrate acute and chronic thrombi in the macro- and/or microvascular pulmonary circulation of 24%-38% of SCD patients.13,14 Data regarding the prevalence of antemortem pulmonary thrombosis in SCD are limited. Abnormal ventilation-perfusion scans have been reported in up to 50% of asymptomatic adult SCD patients, with “high probability” results in 12% and up to 33% of those with an elevated TRV (> 2.5 m/s).32,33 Markers of coagulation activation are detected in patients with SCA,16 likely stimulated by multiple factors that promote tissue factor elaboration.1 However, available data do not demonstrate a statistical difference in coagulation activation markers between SCD patients with and without elevated TRV.34,35
Anemia and left ventricular dysfunction.
Sustained moderate to severe anemia creates a hyperdynamic state with an elevated cardiac output (CO), leading to left ventricular remodeling and diastolic dysfunction. These cardiac changes have been observed in children and adults with SCA, are correlated with a relative systemic hypertension, and predict mortality in adults independently of, but additive to, elevated TRV.4,5,8,19,36
SCD and PH
Definition and classification of PH
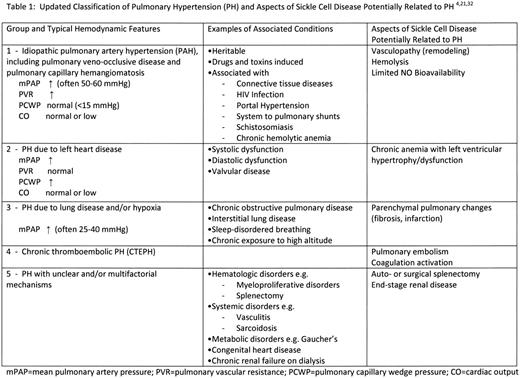
The recent 4th World Symposium on Pulmonary Hypertension designated a mean pulmonary artery pressure (mPAP) of > 25 mmHg as “manifest PH” and values of 21-25 mmHg as “borderline.”27 The newest classification of PH is noted in Table 1. SCA and other chronic hemolytic anemias were placed in group 1 based on the potential for a high rate of NO consumption associated with hemolysis, although it was noted that the mechanisms leading to PH in SCA have not been fully elucidated and that it was difficult to interpret the modest elevations of PAP and pulmonary vascular resistance (PVR) in the setting of the hyperdynamic circulation.27 As noted in Table 1, different features of SCA and chronic lung disease may contribute to PH, potentially placing a given individual with SCA in any one of the diagnostic groups, including group 5 (unclear or multifactorial mechanisms).
Diagnosis of PH
Disease detection by echocardiogram.
Determination of TRV is a relatively inexpensive, noninvasive method for estimating systolic PAP. A systematic review and meta-analysis of the diagnostic accuracy of echocardiography yielded a summary sensitivity of 83% and a specificity of 72% for detecting a subsequent diagnosis of PH.37 The 4th World Symposium designated a systolic TRV of > 2.8 m/s by echocardiogram to be highly indicative of a “manifest pulmonary hypertension” and 2.5-2.8 m/s as “borderline.”27 Cohort studies have demonstrated that 30%-35% of adults with SCA have a TRV > 2.5 m/s, and an estimated 9%-10% have values > 2.9 m/s.4,5 Whereas up to 30% of some pediatric SCA cohorts have an elevated TRV, this finding is not associated with the same short-term risk of mortality that is observed in adults.4,7 A large pediatric cohort of 310 subjects with any form of SCD found an elevated TRV of > 2.6 m/s in 11% and only 0.3% with a TRV of > 3 m/s.8 Multiple factors may affect TRV in SCA. Acute but transient elevation in TRV has been observed during uncomplicated pain episodes in the absence of acute chest syndrome,38 which may reflect transient systematic changes (eg, and increase in hyperdynamic state or worsened anemia). Left-sided heart disease with diastolic dysfunction and a chronic hyperdynamic state may increase TRV without intrinsic pulmonary vascular disease.8,19 These factors may limit the specificity of echocardiogram as a test for “manifest” PH in SCA. Longitudinal studies are needed to draw conclusions about the predictive value of an elevated TRV for progression to PH, especially in pediatric and young adult SCD populations.
RHC.
Right heart catheterization (RHC) is necessary to confirm the diagnosis of PH, to distinguish hemodynamic mechanisms, and to perform vasoreactivity testing to guide therapy.27 The diagnosis of PH requires a mPAP > 25 mmHg. PAH (group 1) is characterized by elevated PVR in the presence of a normal pulmonary capillary wedge pressure (PCWP). In classic PAH, mPAP values of 50-60 mmHg, increased PVR, and normal or low CO are typical.27,39 Small cohorts of SCA adult patients have undergone RHC.4,6,15,32,40 Even with a TRV ≥ 3.0 m/s, RHC most often demonstrates modest elevations in mPAP (28-40 mmHg) and PVR in the setting of an increased CO. In ∼ 40%, an elevated PCWP is detected, indicating left-sided cardiac dysfunction. In ∼ 10%, a hyperdynamic state is described, characterized by a normal or modestly elevated mPAP and normal PVR and PCWP; this is the more common finding in children as estimated by noninvasive testing.8 A single-center cohort of 86 adult subjects with SCD and an elevated TRV underwent RHC; 56% demonstrated PH with a similar distribution of hemodynamic characteristics and a 3.55-fold risk of mortality over 4 years compared with those without PH.41 A prospective multicenter study evaluated 403 consecutive adult patients with SCD using echocardiogram.42 Of these subjects, 96 (24%) had a TRV > 2.5 m/s. Subsequent RHC demonstrated a normal mPAP in 72 (75%). The most common findings associated with an elevated mPAP were an elevated PCWP or a hyperdynamic state with an elevated CO and normal PVR. This study may have captured a less severely ill population, in contrast to prior cohorts and to interventional trials in which recruitment was directed at identifying eligible subjects. However, for the subset (6%) identified with PH in this multicenter study, a 2-year mortality of 12.5% was reported, which was correlated with mPAP determined by RHC.
PH assessment: 6MWD and BNP.
The 6-minute walk distance (6MWD) is used to assess restriction on cardiac and pulmonary performance, and is a predictor of mortality in patients with PAH, chronic obstructive pulmonary disease, and chronic heart failure.27,43 6MWD may be affected by many factors, including age, body height, fitness, and comorbidities such as musculoskeletal disease that alter the ability to walk.27 Despite potential limitations, the 6MWD is accepted by the US Food and Drug Administration (FDA) as a surrogate end point in studies of PH, with a change of 30-40 meters considered clinically significant.27,43 Complications of SCA, including severe anemia, avascular necrosis, stroke, and musculoskeletal pain, may limit the utility of the 6MWD as a measure of cardiopulmonary restriction associated with PH. However, in a small series of SCA patients without such apparent limitations and after correction for anemia, the 6MWD was correlated with TRV and mPAP by RHC.32 These subjects also had restrictive changes on PFTs and abnormalities on perfusion lung scanning, underscoring the potential multifactorial nature of PH and lung disease in SCA. Plasma levels of brain natriuretic peptide (BNP) reflect both right and left ventricular stretch and pressure and are correlated with TRV, 6MWD, and mPAP in SCA patients with PH.4,5,15,27,33 Elevated levels of BNP may indicate the presence of PH, but cannot be used to diagnose or distinguish between types and are associated with severity and prognosis.4,27,37
PH interventional clinical trials in SCD
Observational studies of PH-specific therapies
Several small, uncontrolled clinical studies have explored the potential benefit of PH-specific intervention for subjects with SCA and PH, most often confirmed by RHC. Anecdotal success with intravenous prostanoid analogs has been reported.4 Endothelin receptor antagonists (bosentan and ambrisentan), directed at the reduction of endothelin-1–induced vasoconstriction, were used as monotherapy or in combination with sildenafil for 6 months, resulting in a 40-m increase in 6MWD in a retrospective report of 14 patients with SCD.44 Phosphodiesterase type 5 inhibitors including sildenafil prevent the breakdown of cGMP in the pulmonary vasculature, enhancing NO-mediated vasorelaxation.3,45 Sildenafil is approved by the FDA for use in mild to moderate PAH, with data to support some potential benefit in other forms of PH and in heart failure.45 A pilot study of sildenafil in 12 SCA patients with a mean TRV > 3.0 m/s and mPAP of 35 mmHg demonstrated a mean improvement in 6MWD of 78 meters after 6 months.46 These data and anecdotal experience prompted major interventional trials for PH in SCA.
Randomized controlled PH trials: ASSET-1 and ASSET-2
The Randomized, Placebo-Controlled, Double-Blind, Multicenter, Parallel Group Study to Assess the Efficacy, Safety and Tolerability of Bosentan in Patients with Symptomatic Pulmonary Arterial Hypertension Associated With Sickle Cell Disease sought to enroll subjects with SCA and PAH (ASSET-1) or “mixed vasculopathy” PH (ASSET-2) documented by RHC.40 Subjects underwent RHC if determined to be eligible by a TRV ≥ 2.5 m/s, dyspnea on exertion, and a 6MWD of 150-450 m. The primary end point was change from baseline in PVR (ASSET-1) or 6MWD (ASSET-2) after 16 weeks of treatment with bosentan or placebo. A total of 26 subjects were enrolled in both studies before they were closed due to slow enrollment. RHC confirmed moderate elevation in mPAP (range 29-40 mmHg), PVR (range 122-320 dynes/s/cm5 ), and elevated CO (range 6.6-9.1 L/min). 6MWD was correlated with CO (P = .006). On multivariate regression analysis, 55% of the total variation in 6MWD was explained by hemodynamic parameters. Whereas not statistically significant, the administration of bosentan was associated with a greater reduction in PVR but less improvement in 6MWD compared with placebo.
Randomized controlled PH trials: walk-PHaSST
The Treatment of Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Treatment (Walk-PHaSST) study sought to enroll subjects with any form of SCD with PH defined by an elevated TRV ≥ 2.7 m/s and a 6MWD of 150-500 m.15 Subjects were stratified by TRV into 2 groups: those with TRV of 2.7-2.9 m/s and those with ≥ 3.0 m/s. RHC was required in the TRV ≥ 3.0 m/s group; all subjects were randomized to sildenafil or placebo regardless of findings at RHC. The primary end point was change from baseline 6MWD after 16 weeks of treatment. The study was stopped early by the National Heart, Lung, and Blood Institute after the enrollment of 74 subjects at the recommendation of the Data and Safety Monitoring Board. Significantly more sildenafil-treated subjects (46%) experienced a serious adverse event compared with placebo (22%); the most frequent was hospitalization for SCD pain episodes. In addition, estimation of results by futility analysis suggested no observed improvement in the primary efficacy measure (6MWD) with sildenafil.15 Of 720 subjects screened for possible enrollment, 626 (87%) had a detectable TRV. Of all subjects screened, 31% had a detectable TRV that was > 2.7 m/s and 11% had a TRV ≥ 3.0 m/s. The mean 6MWD was low in all groups, including those with TRV < 2.7 m/s. Twenty-five percent had both a TRV ≥ 2.7 m/s and a 6MWD of 150-500 m/s. RHC findings at baseline were available for 24 subjects with a screening TRV ≥ 3.0 m/s and low 6MWD. Subjects with SCA comprised 85% of these subjects; 14% had HbSC disease. The mPAP was 28 ± 11 mmHg at baseline, with a PVR of 157 ± 122 dynes/s/cm,5 and CO of 8.6 L/min. PH, defined by a mPAP of > 25 mmHg on RHC, was diagnosed in 13 of these 24 subjects (54%). Therefore, 46% of subjects with a TRV of 3.0 m/s and 6MWD between 150 and 500 meters did not meet the RHC definition of PH.
Practical considerations for the clinician: one hematologist's view
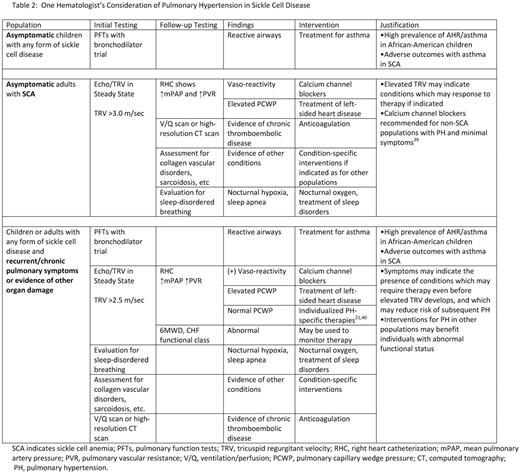
Chronic lung disease and PH appear to contribute significantly to the morbidity and mortality of adults with SCA and perhaps other forms of SCD. Evidence-based treatment algorithms are available for PAH.47 SCD–specific guidelines from the American Thoracic Society regarding pulmonary hypertension are anticipated in 2011, and in late 2011 or early 2012, SCD management guidelines for primary care providers are anticipated from the National Heart, Lung and Blood Institute. Table 2 outlines the author's proposed approach considering available data and experience and despite recent negative trials. Based on the available clinical data and observations, the author offers answers to several specific questions below.
Should all patients with SCA be screened for PH with an echocardiogram?
A screening test is used to detect disease in an asymptomatic individual. A major goal of screening is to detect disease at a stage when treatment can be more effective than it would be after symptoms develop. Echocardiogram is the recommended initial test to detect PH27 ; application to SCA patients will identify ∼ 30% with an elevated TRV.4,5,7,15 However, echocardiogram alone will not fulfill the goal of detection, because a significant percentage of individuals with SCA and an elevated TRV (perhaps 50% or more15,42 ) will not have not have PH. Expensive and invasive testing, including RHC, is necessary to confirm the presence of PH. It could be argued that the prevalence (6%-16%) and associated mortality of PH justifies screening echocardiogram and subsequent testing of asymptomatic adults with SCA. However, there is less justification for routine screening of asymptomatic children, a population with a much lower prevalence of PH. Prospective studies are needed to determine whether children with an elevated TRV in the absence of RHC-proven PH eventually develop a form of PH and if such early detection permits the effective interruption of that progression. Until this is known, the risks of routine screening and subsequent invasive diagnostic testing in asymptomatic children may outweigh benefits. A similar concern is considered for individuals with other forms of SCD (eg, HbSC disease), who appear to have a low prevalence of PH and also may have fewer risk factors for the development of PH (eg, less hemolysis, less anemia, and generally more benign course of disease). Even if an elevated TRV was highly correlated with PH and screening facilitated early diagnosis, there are no studies demonstrating benefit or improved outcomes from PH-specific therapy in asymptomatic patients. Guidelines for the treatment of PAH in non-SCA patients are based on functional class (ie, symptoms).47 Use of the 6MWD and elevated BNP levels as an indication for intervention in the absence of other symptoms has not been validated in SCA, nor has it been proven to improve long-term outcomes or reduce mortality. Detection and treatment of specific conditions identified in the course of evaluation (eg, asthma and thromboembolic disease) may be of intrinsic value, although the impact on the development of chronic sickle lung disease and PH is unknown.
For specific individuals with any form of SCD at any age with pulmonary symptoms, history of recurrent acute chest syndrome, or evidence of other severe chronic organ damage, an echocardiogram performed during steady state may be a reasonable first step in an the initial clinical evaluation to detect PH. A high level of suspicion for chronic lung disease and PH should be maintained and routinely considered when assessing clinical history and physical examination.
Can an echocardiogram be used to diagnose PH or guide PH-specific therapy in SCA?
Available data suggest that the specificity of TRV may range from 54% in adults with limited 6MWD9 to 25% in an unselected cohort42 ; there are no data documenting the specificity in children and there are challenges with reproducibility.48 Therefore, RHC is necessary for the confirmation and characterization of PH. A significant subset of individuals may have an elevated PCWP, indicating the contribution of left-sided heart disease, and another subset may be in a hyperdynamic state.4,5,15,40,42 A thorough evaluation for possible causes of PH and comorbidities should be undertaken (Table 2 gives an example) in consultation with an expert in PH and lung disease. In the absence of SCA-specific data, individualized treatment will be guided by this evaluation and is recommended as for other PH patients with similar features.27,39,47 Data and outcomes from studies using elevated TRV as a definition for PH without RHC confirmation and characterization, especially those which investigate PH-specific therapies, should be interpreted with a great deal of caution.
Can sildenafil and other PH-specific treatments be used in SCD?
Anecdotal experience suggests that individuals with SCD and PH may benefit from PH-specific therapies, including endothelin-1 receptor antagonists (eg, bosentan) and phosphodiesterase-5′ inhibitors (eg, sildenafil).4,44,45 This experience was gained in selected SCD patients with known PH and demonstrates improvement in hemodynamic parameters and functional outcomes. The apparent lack of benefit of sildenafil in the Walk-PHaSST study may have stemmed in part from the inclusion of subjects using TRV and 6MWD to define PH rather a diagnosis confirmed by RHC. Nearly half of the subjects with TRV > 3.0 m/s who underwent RHC did not have PH (mPAP > 25 mmHg),15 and it is likely even fewer of the subjects with a TRV of 2.7-2.9 m/s would meet RHC criteria. Conversely, the mean 6MWD was abnormal in all groups, raising concern about its use an outcome measure in SCA, although post hoc analysis did not reveal an impact of reported pain interfering with walking. These factors may have significantly limited the ability to discern an effect of a phosphodiesterase type 5 inhibitor on PH in SCA. Because the study was closed before full enrollment, the impact of sildenafil compared with placebo on the subset of subjects with RHC-confirmed PH (and between different hemodynamic groups) could not be assessed.
The increase in hospitalizations for SCD pain in Walk-PHaSST was unexpected and of great interest. The potential improvement in NO bioavailability with sildenafil might have been expected to have the opposite effect: improvement in the overall course of disease. However, a growing body of evidence implicates NO in the development of central sensitization, a phenomenon that may worsen the pain experience.15 The full explanation for the observed increase in serious adverse events in Walk-PHaSST is unclear and further analysis is under way.
As was the case before the Walk-PHaSST and ASSET studies, the clinician is left to consider therapies that have benefited other PH populations if an individual with SCA has proven PH with similar features. The potential for side effects must always be weighed against benefit. In the setting of documented severe PH with classical findings on RHC, an individual with SCA may elect to risk increased pain in an effort to achieve symptomatic and functional relief of PH-related symptoms. The use of disease-modifying therapy such as hydroxyurea or transfusion could be considered for worsening SCD–related symptoms associated with sildenafil, although the benefit of this approach is unproven in this setting.
Is an elevated TRV an indication to start disease-modifying therapy for SCA?
One major goal of screening is to identify risk factors that may increase the likelihood of developing a disease, and then using this knowledge to modify risk factors or to introduce other interventions to prevent disease. A commonly stated justification for a routine screening echocardiogram in SCA is the increase in short-term mortality associated with an elevated TRV that is observed in adults. The risk is for all-cause mortality, not necessarily death related to chronic pulmonary disease or PH. An elevated TRV may therefore be an overall marker of severity of SCA and perhaps its underlying vasculopathy. The observation that there is no significant short-term mortality associated with an elevated TRV in children with SCA complicates this picture. There are as yet no longitudinal data available to demonstrate that this finding in childhood independently predicts for adverse outcomes or premature mortality in adulthood. Even if a valid marker of current disease severity or future progression, there are no controlled trials using intervention based on an elevated TRV to demonstrate improvement in outcomes, mortality, or even consistent improvement in TRV itself. In many cohorts reported in the literature, a significant portion of subjects with SCA report current or past use of hydroxyurea and/or chronic transfusion therapy at the time PH is documented. The impact of these disease-modifying therapies on PH itself, on short-term mortality, or on disease progression if introduced at an earlier stage remains unproven. The apparent lack of correlation between TRV and fetal hemoglobin,6,32 for example, emphasizes the need for controlled trials to determine the benefit of hydroxyurea and related interventions. Therefore, the determination of the clinical utility of detecting an elevated TRV, beyond the indication to proceed with diagnostic testing for PH, will require interventional studies using this parameter for the initiation of disease-modifying therapy.
Conclusion
In summary, TRV is a useful screening modality to indicate when further diagnostic testing for PH is required for adults with SCA, or for those with any form of SCD at any age with cardiopulmonary symptoms or risk factors for PH. RHC confirmation of PH is necessary; TRV alone is insufficient to guide therapeutic intervention. Although a predictor of early mortality in adults with SCA, prospective studies of intervention directed at SCD based solely on TRV are needed before recommendations based on high-quality evidence can be made.
Disclosures
Conflict-of-interest disclosure: NIH funding as an investigator for the Walk-PHaSST study. Off-label drug use: inhaled NO for the treatment of vaso-occlusive crisis.
Correspondence
Kathryn L. Hassell, Professor of Medicine, Division of Hematology, Director, Colorado Sickle Cell Treatment and Research Center, University of Colorado-Denver School of Medicine, 13121 E 17th Ave, Box C-222, Aurora, CO 80045; Phone: (303) 724-9070; Fax: (303) 724-9161; e-mail: kathyrn.hassell@ucdenver.edu.